Abstract
Overexpression of human epidermal growth factor receptor-2 (HER2) in metastatic breast cancer (MBC) is associated with poor prognosis. This single-arm open-label trial (EGF109491; NCT00508274) was designed to confirm the efficacy and safety of lapatinib in combination with capecitabine in 52 heavily pretreated Chinese patients with HER2-positive MBC. The primary endpoint was clinical benefit rate (CBR). Secondary endpoints included progression-free survival (PFS), time to response (TTR), duration of response (DoR), central nervous system (CNS) as first site of relapse, and safety. The results showed that there were 23 patients with partial responses and 7 patients with stable disease, resulting in a CBR of 57.7%. The median PFS was 6.34 months (95% confidence interval, 4.93–9.82 months). The median TTR and DoR were 4.07 months (range, 0.03–14.78 months) and 6.93 months (range, 1.45–9.72 months), respectively. Thirteen (25.0%) patients had new lesions as disease progression. Among them, 2 (3.8%) patients had CNS disease reported as the first relapse. The most common toxicities were palmar-plantar erythrodysesthesia (59.6%), diarrhea (48.1%), rash (48.1%), hyperbilirubinemia (34.6%), and fatigue (30.8%). Exploratory analyses of oncogenic mutations of PIK3CA suggested that of 38 patients providing a tumor sample, baseline PIK3CA mutation status was not associated with CBR (P = 0.639) or PFS (P = 0.989). These data confirm that the lapatinib plus capecitabine combination is an effective and well-tolerated treatment option for Chinese women with heavily pretreated MBC, irrespective of PIK3CA status.
Keywords: Lapatinib, capecitabine, HER2, metastatic breast cancer
Human epidermal growth factor receptor-2 (HER2) is a member of the epidermal growth factor receptor (EGFR) family of transmembrane cell surface receptors, which possess intrinsic tyrosine kinase (TK) activity[1]. Amplification of the HER2 gene or its product is observed in 17% to 30% of human breast cancers and is associated with poor prognosis, enhanced risk of disease progression, and reduced progression-free survival (PFS) and overall survival (OS)[2]–[7].
Inhibition of HER2, for which there are currently 2 approved therapeutic approaches, is now a validated means of improving clinical outcomes in patients with HER2-overexpressing breast cancer. One approach involves trastuzumab, a humanized monoclonal antibody directed against the HER2 extracellular domain[8]. When added to chemotherapy, trastuzumab has been shown to improve overall response rates and prolong the median time to tumor progression (TTP), the median duration of response (DoR), and the median OS for patients with HER2-overexpressing metastatic breast cancer (MBC)[9]. A second approach involves lapatinib, an orally active small molecule TK inhibitor with activity against both HER2 and EGFR, which has clinical activity as monotherapy or in combination with chemotherapy, hormonal therapy, or trastuzumab in patients with HER2-positive MBC[2],[10]–[15].
The pivotal phase III study EGF100151 tested the efficacy of lapatinib plus capecitabine compared with capecitabine alone in HER2-positive patients with locally advanced breast cancer or MBC who had prior treatment with anthracyclines, taxanes, and/or trastuzumab[11],[12]. Following a planned interim analysis, this study demonstrated that the addition of lapatinib to capecitabine significantly prolonged TTP [median 8.4 vs. 4.4 months, hazard ratio (HR) = 0.49; 95% confidence interval (CI): 0.34–0.71; P < 0.001][11]. Thus, the study was halted early and crossover to the combination therapy was offered to women receiving capecitabine monotherapy. A follow-up analysis confirmed that the lapatinib plus capecitabine prolonged TTP versus capecitabine monotherapy (HR = 0.57; 95% CI: 0.43–0.77; P < 0.001)[12].
The treatment of breast cancer in Asia is largely similar to that in Western countries; however, several distinct factors in Asia impact clinical decisions, including cost considerations, agent availability due to regulatory approval, and ethnic differences[16],[17]. Despite these factors, the treatment and dosing schemes used in Asia are largely grounded in clinical data generated from research in Western nations[16]. For example, approximately 90% of the patients randomized to treatment in the pivotal EGF100151 study were Caucasian.
Accordingly, the present trial (EGF109491) was initiated as a single-arm, open-label study to evaluate the efficacy and safety of lapatinib plus capecitabine in Chinese women with HER2-positive advanced breast cancer or MBC. Exploratory analyses of oncogenic mutations in the PIK3CA gene were also conducted. Activating mutations residing in 2 hotspots in exons 9 and 20 of PIK3CA led to aberrant activation of phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathways[18],[19]. In addition, activating PIK3CA mutations are associated with resistance to trastuzumab treatment in vitro [20] and a shorter TTP[20] and lower response rates[21] to trastuzumab-based therapy in HER2-positive breast cancer patients, suggesting that PIK3CA mutations could be a resistance marker for trastuzumab-based therapy. Although one report suggests a PIK3CA mutation could also serve as a mechanism of resistance to lapatinib efficacy[22], other in vitro [23] and clinical [24],[25] studies contradict this notion. To further test this hypothesis, exploratory analyses were performed to establish the frequency of PIK3CA hotspot mutations in exons 9 and 20 in Chinese patients enrolled in EGF109491 and to determine if the presence of a PIK3CA mutation impacts the clinical benefit of lapatinib plus capecitabine.
Patients and Methods
Study design and endpoints
EGF109491 was a single-arm open-label trial in which patients received lapatinib plus capecitabine. This study was conducted in accordance with good clinical practice and all applicable regulatory requirements, as well as the guiding principles of the Declaration of Helsinki. The primary objective of the study was to determine the clinical benefit rate (CBR), which was defined as the percentage of patients achieving either a complete or partial tumor response (CR or PR), or stable disease (SD) for at least 24 weeks. Secondary endpoints included PFS, defined as the time from treatment initiation until disease progression or death from any cause; time to response (TTR), defined as the time from treatment initiation until first response (CR or PR); DoR, defined as the time from first response (either PR or CR) until disease progression or death due to breast cancer; central nervous system (CNS) as first site of relapse; and safety.
Patient eligibility
Eligible patients included in the trial were women, 18 years of age or older, with pathologically confirmed invasive stage MIB/MIC or stage IV breast cancer with at least 1 measurable lesion according to response evaluation criteria in solid tumors (RECIST) criteria v1.0. Institutional review board approval was obtained, and each patient provided written informed consent. Documentation of HER2 overexpression by immunohistochemistry (3+) or HER2 amplification by fluorescence in situ hybridization was also required by the local or central laboratory. Patients must have received prior therapy with a taxane and/or an anthracycline and could have received prior trastuzumab. Additional inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1, life expectancy of at least 12 weeks, and left ventricle ejection fraction (LVEF) within the institutional range of normal as assessed by echocardiogram.
The main exclusion criterion was a history of other malignancies. Exceptions were made for patients who were disease-free for 5 years, those with a history of completely resected non-melanoma skin cancer, or those with successfully treated in situ carcinoma. Also excluded were patients receiving prior capecitabine (unless 6 months had elapsed since the last capecitabine dose and patients had no remaining capecitabine-related toxicity) and/or EGFR or HER2 inhibitory therapies except trastuzumab, and those taking concurrent anti-cancer therapy (including investigational and herbal remedies and Chinese traditional medicines for cancer) while on study medication, as were those with a known history of angina, arrhythmias, or congestive heart failure.
Treatments
All eligible patients received lapatinib (1250 mg once daily) and capecitabine (2000 mg/m2 per day) on days 1 to 14 every 21 days. Dose reductions and delays for lapatinib- and/or capecitabine-related toxicities were permitted. For capecitabine, standard recommendations for dosage modifications were followed for the management of adverse events (AEs). Lapatinib was withheld for up to 14 days for grade 2 hematologic toxicity or any grade 3 or 4 toxicity. Lapatinib was permanently discontinued if grade 3 or 4 interstitial pneumonitis or cardiac dysfunction occurred. A dose reduction was permitted for lapatinib to 1000 mg.
Assessments
Efficacy and safety assessments were performed every 6 weeks for the first 36 weeks, then every 12 weeks and at the end of treatment. Additional safety assessments were performed on all patients every 3 weeks and at the end of treatment. Patients withdrawing from treatment who had not progressed were assessed every 12 weeks until progression during follow-up. Disease progression was assessed using the modified RECIST criteria v1.0 that included radiological scans and medical photographs.
Exploratory biomarker analysis
Sample DNA extraction
Three 10-µm sections were cut from each paraffin-embedded tumor block. Slices were deparaffinized twice with 1 mL of xylene, vortexed, and centrifuged. The supernatant was removed and then washed twice with 1 mL of 100% ethanol. The samples were resuspended in 180 µL lysis buffer containing proteinase K (Merck KGaA, Darmstadt, Germany) and were incubated overnight at 56°C with gentle shaking. DNA was extracted from each sample using a spin column [GTPure, Gene Tech (Shanghai) Company Limited, Shanghai, China] according to the manufacturer's instructions. The final concentration of each DNA sample was adjusted to 50 ng/µL, and samples were stored at –20°C.
Polymerase chain reaction (PCR) amplification
Briefly, DNA fragments were amplified using PCR with primers specific to the regions of interest (codons 539, 542, 545, and 546 of exon 9 and codons 1043, 1044, 1047, and 1049 of exon 20) in the PIK3CA gene (Table 1). Each PCR mix contained 1× reaction buffer, 2 mmol/L MgCl2, 5 pmol each of the forward and reverse primers, 10 nmol each of dNTP, 1 U HotStar Taq DNA polymerase (TaKaRa, Otsu, Shiga, Japan), and 100 ng genomic DNA in a 50 µL volume. The mixtures were denatured for 3 min at 95°C and underwent 50 thermal cycles for 15 s at 95°C, 20 s at 56°C, and 30 s at 72°C, as well as final extension at 72°C for 5 min.
Table 1. Primers used for polymerase chain reaction and DNA sequencing.
| Primer | DNA sequence (5′ to 3′) |
| PIK3CA-9-F1 | AACAGCTCAAAGCAATTTCTACAC |
| PIK3CA-9-R1 | Biotin-GGTATGGTAAAAACATGCTGAGAT |
| PIK3CA-9-S1 | AAGCAATTTCTACACGAG |
| PIK3CA-20-F1 | GACATTGCATACATTCGAAAGAC |
| PIK3CA-20-R1 | Biotin-GTTTAATTGTGTGGAAGATCCAA |
| PIK3CA-20-S1 | AGGCTTTGGAGTATTTCAT |
Mutation detection by pyrosequencing
Single-stranded DNA (ssDNA) was prepared with the vacuum prep workstation [QIAGEN China (Shanghai) Co Ltd, Pudong, Shanghai, China] from 30 µL biotinylated PCR product using Streptavidin Sepharose High Performance beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Sequencing primer (0.4 µmol/L, Table 1) was annealed to the ssDNA template and pyrosequencing was performed using the Pyro Gold Reagent kit and the PyroMark ID system [QIAGEN China (Shanghai) Co Ltd, Pudong, Shanghai, China] according to the manufacturer's instructions.
Identification of mutations
All sequencing pyrograms were analyzed first by the SNP model using PyroMark ID software, which supports quality assurance for the sequencing results. Peaks passing the quality threshold for the assay were highlighted in blue, whereas peaks that were highlighted in yellow required further evaluation before obtaining an acceptable result. Therefore, the blue peaks were used to report the genotype directly and the yellow peaks underwent further assessment. Samples containing over 10% of allele frequency were classified as containing a mutation.
Statistical methods
The intention-to-treat (ITT) population consisted of all enrolled patients and was used for the analysis of efficacy data. The safety-analysis population, defined as all enrolled patients who received at least 1 dose of lapatinib, was used for the analysis of safety, demographic characteristics, and baseline disease status. In this study, all enrolled patients received at least 1 dose of lapatinib, so the ITT and safety populations were identical. The final analysis was performed after all patients had been followed for 24 weeks at minimum or had died or had withdrawn earlier in the treatment period.
The primary objective of the study was to test the null hypothesis that the investigator-assessed CBR was less than 17% versus the alternative hypothesis that the CBR was greater than 35%. A minimum of 50 evaluable patients were required to test the hypothesis with a 0.05 level of significance and 90% power. If at least 13 of the 50 patients had a demonstrated CBR, the null hypothesis would be rejected.
PI3KCA mutation status was tested for association with CBR using the Fisher exact test. For assessing PI3KCA mutation status and its association with PFS, mutation status was coded as a binary variable (0 = mutation negative, 1 = mutation positive) and modeled using Cox proportional hazards regression. The assumption of proportional hazards was tested using mutation status with a mutation status-by-time interaction term in the Cox model. Since the interaction term was not statistically significant, the proportional hazards assumption was valid. Statistical tests were considered significant with a Wald Chi-square P < 0.05. Analyses were conducted with available data using SAS version 9.2.2.
Results
Patients and treatments
The cutoff date was August 22, 2008, at which time 52 eligible patients had been enrolled from 9 centers in mainland China and Hong Kong. Nearly all (98.1%) patients had stage IV disease, most (78.8%) had extensive disease with visceral and/or nonvisceral metastases, and more than half (57.7%) had an ECOG PS of 0 (Table 2). Just less than half of the patients were estrogen receptor-positive and/or progesterone receptor-positive, and 50.0% of patients were negative for both. Nearly all patients (92.3%) had received chemotherapy for advanced or metastatic disease, 57.7% had received at least 2 chemotherapy regimens, and 36.5% of patients were trastuzumab-naive (Table 3). Most patients with prior exposure to trastuzumab (90.9%) had received it for MBC, and the median duration of trastuzumab exposure was 7.2 months. Consistent with the standard capecitabine dosing regimen (i.e., 2 weeks on, 1 week off), the median duration of exposure was 170 days for lapatinib and 112 days for capecitabine.
Table 2. Baseline demographic characteristics of 52 patients treated with lapatinib plus capecitabine.
| Characteristic | No. of patients (%) |
| Age (years) | |
| Median | 50 |
| Range | 26–71 |
| Menopausal | 24 (46.2) |
| Stage of disease | |
| Stage IIIB/IIIC | 1 (1.9) |
| Stage IV | 51 (98.1) |
| Hormone receptor status | |
| ER+ and/or PR+ | 25 (48.1) |
| ER– and PR– | 26 (50.0) |
| Unknown | 1 (1.9) |
| ECOG PS | |
| 0 | 30 (57.7) |
| 1 | 22 (42.3) |
| Metastatic sites | |
| Visceral and/or nonvisceral | 41 (78.8) |
| Nonvisceral only | 11 (21.2) |
Data are presented as number of patients, with the percentage in parentheses, except for age. ER+, estrogen receptor–positive; PR+, progesterone receptor–positive; ER–, estrogen receptor–negative; PR–, progesterone receptor–negative; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Table 3. Prior anti-cancer treatments of 52 patients treated with lapatinib plus capecitabine.
| Anticancer therapies before lapatinib | No. of patients (%) |
| Treatment for advanced or metastatic disease | 48 (92.3) |
| 1 regimen | 18 (34.6) |
| 2 regimens | 10 (19.2) |
| ≥ 3 regimens | 20 (38.5) |
| Capecitabine | 15 (28.8) |
| ≥ 1 anthracycline, taxane, or trastuzumab containing regimen | |
| Anthracycline | 44 (84.6) |
| Taxane | 48 (92.3) |
| Trastuzumab | 33 (63.5) |
| Neoadjuvant | 0 (0) |
| Adjuvant | 7 (21.2) |
| Metastatic | 30 (90.9) |
| Trastuzumab exposure duration (months) | |
| Median | 7.2 |
| Range | 1–40 |
Data are presented as number of patients, with the percentage in parentheses, except for trastuzumab exposure duration.
Efficacy
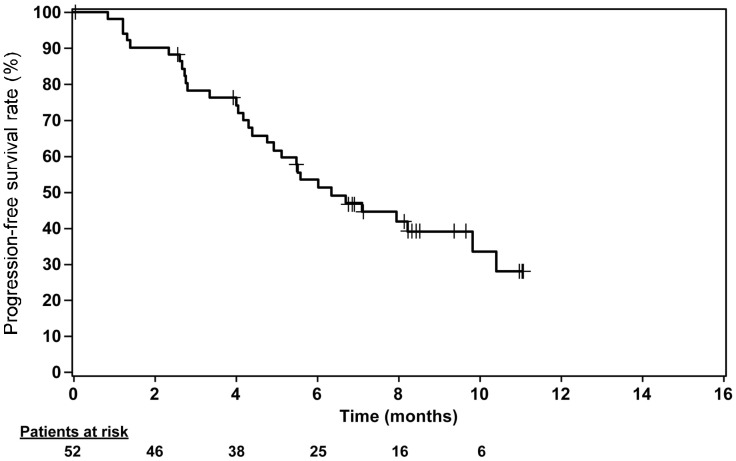
There were 23 patients with PR, 7 patients with SD for at least 24 weeks, and 0 patients with CR, resulting in a CBR of 57.7% (Table 4). The median PFS was 6.34 months (95% CI, 4.93–9.82 months) (Figure 1). The median TTR and DoR were 4.07 (range, 0.03–14.78 months) and 6.93 months (range, 1.45–9.72 months), respectively. There were 13 (25.0%) patients who had new lesions as disease progression. Among them, 2 (3.8%) patients had CNS disease reported as first site of relapse.
Table 4. Investigator-assessed response rates by RECIST criteria v1.0 and PFS for 52 patients treated with lapatinib plus capecitabine.
| Endpoint | No. of patients (%) |
| Best response rate | |
| CR | 0 (0) |
| PR | 23 (44.2) |
| SD ≥ 24 weeks | 7 (13.5) |
| PD | 4 (7.7) |
| Unknown | 2 (3.8) |
| CBR (CR + PR + SD ≥ 24 weeks) | |
| % (95% CI) | 57.7 (43.2–71.3) |
| PFS | |
| Median, months (95% CI) | 6.34 (4.93–9.82) |
| 6-month rate, % (95% CI) | 53.4 (39.4–67.4) |
| Median time to response (months) | 4.07 |
| Median duration of response (months) | 6.93 |
| First site of relapse | |
| Any new lesion(s) | 13 (25.0) |
| CNS disease as site of first relapse | 2 (3.8) |
Data are presented as number of patients with the percentage in parentheses, except for CBR, median PFS, median time of response and median duration of response. CBR, clinical benefit rate; CR, complete response; PR, partial response; SD, stable disease; CI, confidence interval; PD, progressive disease; PFS, progression–free survival; CNS, central nervous system.
Figure 1. Kaplan-Meier curve for progression-free survival (PFS) of 52 patients with HER2-positive metastatic breast cancer treated with lapatinib plus capecitabine. Median PFS was 6.34 months (95% confidence interval, 4.93–9.82 months).
Safety
A total of 49 patients (94.2%) experienced at least 1 AE during the study. The most common toxicities (i.e., an incidence rate greater than 10%) were palmar-plantar erythrodysesthesia (PPE, 59.6%), diarrhea (48.1%), rash (48.1%), hyperbilirubinemia (34.6%), and fatigue (30.8%) (Table 5). The maximum grades of these AEs were mainly grade 1 or 2. The only grade 3 or 4 AEs were rash (3.8% grade 3), hyperbilirubinemia (3.8% grade 3), fatigue (1.9% grade 3), and neutropenia (1.9% grade 3, 3.8% grade 4). Notably, there were no instances of interstitial pneumonitis or LVEF decrease. A total of 3 (5.8%) patients experienced severe AEs. One patient experienced severe hemoptysis and was subsequently found to have multiple lung metastases on chest X-ray. A second patient experienced grade 4 neutropenia that resolved subsequent to treatment discontinuation, though this patient was ultimately withdrawn from the study due to progressive disease. A third patient developed grade 4 thrombocytopenia 14 days after beginning treatment. This event resolved subsequent to a transfusion, but 20 days after beginning treatment, the same patient developed grade 4 nonfebrile leucopenia and neutropenia. Although both events resolved with treatment, the patient subsequently died with multiple organ failure. The events were considered related to treatment. A total of 18 patients (34.6%) experienced hyperbilirubinemia, including 2 patients who had pre-existing liver metastases and developed grade 3 events. In both instances, hyperbilirubinemia was transient and subsequently returned to within normal range. These events were considered to be possibly related to lapatinib.
Table 5. Summary of adverse events experienced by at least 10% of 52 patients treated with lapatinib plus capecitabine.
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
| PPE | 25 (48.1) | 6 (11.5) | 0 | 0 | 31 (59.6) |
| Diarrhea | 20 (38.5) | 4 (7.7) | 0 | 0 | 25 (48.1)a |
| Rash | 22 (42.3) | 1 (1.9) | 2 (3.8) | 0 | 25 (48.1) |
| Hyperbilirubinemia | 4 (7.7) | 12 (23.1) | 2 (3.8) | 0 | 18 (34.6) |
| Fatigue | 11 (21.2) | 4 (7.7) | 1 (1.9) | 0 | 16 (30.8) |
| Nausea | 9 (17.3) | 1 (1.9) | 0 | 0 | 10 (19.2) |
| Neutropenia | 0 | 4 (7.7) | 1 (1.9) | 2 (3.8) | 7 (13.5) |
Data are presented as number of patients, with the percentage in parentheses.
aThe grade of diarrhea for one patient (1.9%) was unknown.
PPE, palmar-plantar erythrodysesthesia.
Dose delays and reductions were permitted. A total of 4 (7.7%) and 10 (19.2%) patients required dose reductions in lapatinib and capecitabine, respectively. Eighteen patients (34.6%) required a dose delay for lapatinib. A total of 14 (26.9%) patients were 100% compliant, and the remaining 38 (73.1%) patients were 90% compliant with their lapatinib therapy.
Biomarkers
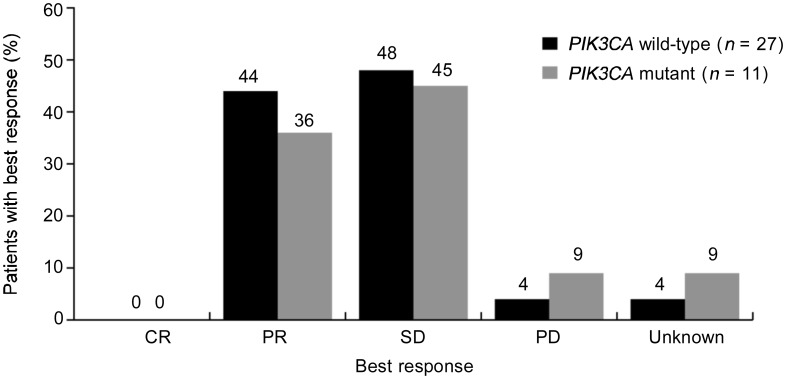
A total of 38 of 52 patients provided tumor samples for additional analyses, and, of those, 11 (28.9%) harbored a mutation in the PIK3CA gene. Of the 11 mutations, 9 resided in the catalytic domain and 2 resided in the accessory domain of the gene. No statistical association with CBR (P = 0.639) or PFS (HR = 1.01, P = 0.989) was observed between patients with PIK3CA wild-type mutations and patients with PIK3CA mutations (Figure 2).
Figure 2. Comparison of best response in patients with wild-type PIK3CA versus mutant PIK3CA. The numbers above each bar indicate the percent of patients with each best response. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Discussion
These data confirm that in an exclusively Chinese population, the combination of lapatinib and capecitabine offers clinical benefit to women with HER2-positive MBC. In the current study, the investigator-assessed CBR (CR + PR + SD for at least 24 weeks) was 57.7%, which is considerably higher than the identically defined independently assessed CBR (27%) reported by Geyer et al.[11] or that (29.3%) by Cameron et al.[12]. The substantive differences in CBR between EGF109491 and EGF100151 may be due to the fact that the CBR calculated in the present study is based on a considerably smaller sample size (52 patients) than EGF100151 (198 patients in the lapatinib plus capecitabine arm). Alternatively, as East Asian ethnicity has been previously associated with improved responses to EGFR inhibitors in other solid tumors[26],[27], it is conceivable that the higher CBR may be related to the fact that the current study was composed exclusively of Chinese patients. The median PFS of 6.34 months observed in the current study also compared favorably with the 6.2-month median PFS (by independent review) reported by Cameron et al.[12].
HER2-positive breast cancers are known to have a higher propensity to develop brain metastases[28]. In the present study, only 2 (3.8%) patients had a CNS lesion as a first progression event. Despite the small study size, these results are consistent with other previously reported data and support the hypothesis that as a small molecule, lapatinib can more easily traverse the blood-brain barrier and may have an advantage relative to trastuzumab in the prevention and/or treatment of CNS metastases[29]. For example, a recent phase II study of 242 patients with HER2-positive breast cancer, progressive brain metastases, and prior trastuzumab therapy showed that lapatinib therapy results in a CNS objective response in 6% of patients, with 21% having at least a 20% volumetric reduction in CNS lesions[30].
The observed safety events of PPE, diarrhea, and rash in the present study are comparable with the published safety profile for the lapatinib and capecitabine combination regimen[11],[12]. Although hyperbilirubinemia was observed in 34.6% of patients, most events were of a low grade and only 1 patient permanently discontinued treatment due to hyperbilirubinemia with a maximum toxicity of grade 2. Because cardiotoxicity is a known concern with HER2 inhibitors[9],[31],[32], it is notable that no cases of LVEF decreases or cardiac failures were observed in this study.
Activating mutations of PIK3CA are common in breast cancer and are associated with poor clinical outcomes[33],[34]. In addition, PIK3CA mutations are a major determinant of resistance to trastuzumab and appear to involve downstream signaling pathways such as PI3K/AKT[20],[33],[35]. Furthermore, preclinical work suggests dysregulation of the PI3K pathway via loss-of-function mutations in phosphatase and tensin (PTEN) homolog or dominant activating mutations in PIK3CA can confer resistance to trastuzumab[20]. In the current work, despite a 29% frequency of PIK3CA mutations, there was no evidence that PIK3CA mutation affected either CBR (P = 0.639) or PFS (P = 0.989) when comparing patients having wild-type tumors with those having mutant-type tumors. Although the conclusions drawn from these data are limited by small sample size and are largely hypothesis-generating in nature, these data will likely boost enthusiasm for additional research in this area. For example, these findings suggest a potential benefit of lapatinib and capecitabine therapy regardless of the PIK3CA genotype and may represent an important clinical option for patients who have developed resistance to trastuzumab.
Conclusions
In summary, the present study, EGF109491, demonstrates that the combination of lapatinib plus capecitabine is active and well-tolerated in a population of Chinese women with HER2-positive advanced breast cancer or MBC who have progressed following other treatments, including an anthracycline and/or a taxane, with or without trastuzumab.
Acknowledgments
We thank the patients and the study investigators for their participation. All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. Editorial support in the form of the development of the first draft of the manuscript, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, referencing, and graphic services was provided by Brad Imwalle, PhD, Peter Sciavolino, PhD, Catherine Kitto, and Tim Reilly at Ogilvy CommonHealth Scientific Communications, Parsippany, New Jersey, USA.
Funding for this study was provided by GlaxoSmithKline.
Footnotes
Conflicts of Interest: Winnie Yeo is an advisory board member and received honoraria from GlaxoSmithkline. Beth Newstat, Anne-Marie Martin, and Alka Preston are employees and shareholders of GlaxoSmithkline. Hai-Dong Chi and Li Wang are employees of GlaxoSmithkline China. Bing-He Xu, Ze-Fei Jiang, Daniel Chua, Zhi-Min Shao, Rong-Cheng Luo, Xiao-Jia Wang, Dong-Geng Liu, and Shi-Ying Yu have no conflicts of interest.
Unique Trial Number: NCT00508274
Trial Registration Date: July 26th, 2007
References
- 1.Pegram M. Can we circumvent resistance to ErbB2-targeted agents by targeting novel pathways? [J] Clin Breast Cancer. 2008;8(Suppl 3):S121–S130. doi: 10.3816/cbc.2008.s.008. [DOI] [PubMed] [Google Scholar]
- 2.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer [J] J Clin Oncol. 2009;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 3.Zahnow CA. ErbB receptors and their ligands in the breast [J] Expert Rev Mol Med. 2006;8(23):1–21. doi: 10.1017/S146239940600010X. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Berezov A, Wang Q, et al. ErbB receptors: from oncogenes to targeted cancer therapies [J] J Clin Invest. 2007;117(8):2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badache A, Goncalves A. The ErbB2 signaling network as a target for breast cancer therapy [J] J Mammary Gland Biol Neoplasia. 2006;11(1):13–25. doi: 10.1007/s10911-006-9009-1. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer [J] Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu Oncogene [J] Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 8.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice [J] N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 [J] N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell KL, Burstein HJ, Sledge GW, et al. Updated survival analysis of a randomized study of lapatinib alone or in combination with trastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy [C] Abstract presented at: 32nd Annual San Antonio Breast Cancer Symposium; December 14–17, 2009; San Antonio, TX.
- 11.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer [J] N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 12.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses [J] Breast Cancer Res Treat. 2008;112(3):533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 13.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer [J] J Clin Oncol. 2008;26(34):5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer [J] J Clin Oncol. 2008;26(18):2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 15.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer [J] J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 16.Chow LWC, Young-Hyuck IM. Current treatment of locally advanced and metastatic breast cancer in the Asia-Pacific region: challenges and limitations [J] Asia Pac J Clin Oncol. 2009;4(s3):S14–S23. [Google Scholar]
- 17.Lal S, Wong ZW, Jada SR, et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients [J] Pharmacogenomics. 2007;8(6):567–575. doi: 10.2217/14622416.8.6.567. [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells [J] Cancer Cell. 2005;7(6):561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers [J] Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 20.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer [J] Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Vorkas PA, Agelaki S, Poumpouridou N, et al. PI3K pathway activity and response to first-line chemotherapy in combination with trastuzumab in patients with HER2-positive metastatic breast cancer [C] Abstract presented at: 32nd Annual San Antonio Breast Cancer Symposium; December 14–17, 2009; San Antonio, TX.
- 22.Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235 [J] Cancer Res. 2008;68(22):9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albertson D, Chin K, Devries S, et al. Genomic approaches to breast cancer subset identification and treatment [J] Breast Cancer Res Treat. 2007;106(Suppl 1):S10. Abstract 31. [Google Scholar]
- 24.Migliaccio I, Gutierrez MC, Wu MF, et al. PI3 kinase activation and response to trastuzumab or lapatinib in HER-2 overexpressing locally advanced breast cancer (LABC) [C] Abstract presented at: 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX.
- 25.Toi M, Iwata H, Fujiwara Y, et al. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies [J] Br J Cancer. 2009;101(10):1676–1682. doi: 10.1038/sj.bjc.6605343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer [J] N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma [J] N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 28.Melisko ME, Glantz M, Rugo HS. New challenges and opportunities in the management of brain metastases in patients with ErbB2-positive metastatic breast cancer [J] Nat Clin Pract Oncol. 2009;6(1):25–33. doi: 10.1038/ncponc1243. [DOI] [PubMed] [Google Scholar]
- 29.Moy B, Goss PE. Lapatinib: current status and future directions in breast cancer [J] Oncologist. 2006;11(10):1047–1057. doi: 10.1634/theoncologist.11-10-1047. [DOI] [PubMed] [Google Scholar]
- 30.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer [J] Clin Cancer Res. 2009;15(4):1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 31.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer [J] Breast. 2004;13(3):173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Perez EA, Koehler M, Byrne J, et al. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials [J] Mayo Clin Proc. 2008;83(6):679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 33.Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery [J] Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 34.Garcia S, Dales JP, Charafe-Jauffret E, et al. Overexpression of c-Met and of the transducers PI3K, FAK and JAK in breast carcinomas correlates with shorter survival and neoangiogenesis [J] Int J Oncol. 2007;31(1):49–58. [PubMed] [Google Scholar]
- 35.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance [J] Breast Cancer Res. 2006;8(6):215–222. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]