Abstract
Recent evidence suggests that the chemokine axis of CXC chemokine ligand-12 and its receptor CXC chemokine receptor-4 (CXCL12/CXCR4) is highly expressed in gynecological tumors and the axis of CXC chemokine ligand-16 and CXC chemokine receptor-6 (CXCL16/CXCR6) is overexpressed in inflammation-associated tumors. This study aimed to determine the relationship between CXCL12/CXCR4, CXCL16/CXCR6 and ovarian carcinoma's clinicopathologic features and prognosis. Accordingly, the expression of these proteins in ovarian tissues was detected by tissue microarray and immunohistochemistry. The expressions of CXCL12/CXCR4 and CXCL16/CXCR6 were significantly higher in epithelial ovarian carcinomas than in normal epithelial ovarian tissues or benign epithelial ovarian tumors. The expression of chemokines CXCL12 and CXCL16 were positively correlated with their receptors CXCR4 and CXCR6 in ovarian carcinoma, respectively (r = 0.300, P < 0.05; r = 0.395, P < 0.05). Moreover, the expression of CXCL12 was related to the occurrence of ascites (χ2 = 4.76, P < 0.05), the expression of CXCR4 was significantly related to lymph node metastasis (χ2 = 4.37, P < 0.05), the expression of CXCR6 was significantly related to lymph node metastasis (χ2 = 7.43, P < 0.05) and histological type (χ2 = 33.48, P < 0.05). In univariate analysis, the expression of CXCR4 and CXCL16 significantly correlated with reduced median survival (χ2 = 4.67, P < 0.05; χ2 = 4.48, P < 0.05). Therefore, we conclude that the chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 may play important roles in the growth, proliferation, invasion, and metastasis of epithelial ovarian carcinoma.
Keywords: Epithelial ovarian carcinoma, chemokine, CXCL12, CXCL16
Epithelial ovarian cancer is the most common ovarian cancer. Because of its occult onset and proneness to intraperitoneal metastasis, the survival rate of patients with epithelial ovarian cancer is relatively low even after combined treatments. The diagnosis and treatment of epithelial ovarian cancer is still a huge challenge in gynecological cancer research. The development of ovarian cancer involves interactions of multiple biological mechanisms, of which imbalance of the chemokine network may become a hot topic in ovarian cancer research.
Chemokines are a family of small basic proteins that play important biological roles by binding to specific receptors on cell surface. Chemokine receptors belong to the G protein-coupled receptor family, members of which possess seven transmembrane segments. According to their expression and function in the immune system, chemokines can be divided into two classes: homeostatic and inflammatory. Homeostatic chemokines are primarily expressed at lymphocyte-homing sites and function to maintain homeostasis and regulate homing and lymphocyte maturation. Inflammatory chemokines are induced by inflammatory cytokines, bacterial toxins, or other pathogens that disrupt homeostasis, and their function is to recruit effector cells and regulate natural and adaptive immune responses.
CXCL12, also known as stromal cell-derived factor 1 (SDF-1), with two isoforms SDF-1α and SDF-1β, belongs to chemokine CXC subfamily, and its major receptor is CXCR4. The CXCL12/CXCR4 interaction constitutes a molecular coupling closely related to cellular signal transduction and cell migration and plays an important role in various processes, such as embryonic and cardiovascular development, formation of bone marrow hematopoietic cells, regulation of immune maturation and responses, inflammation, and HIV infection[1]. CXCL16, a transmembrane protein discovered by Matloubian et al.[2], exists in two forms: membrane-bound form (TM-CXCL16) and soluble form (sCXCL16). TM-CXCL16 is cleaved by membrane integrin proteinases, such as ADAM10 and ADAM17, resulting in the shedding of its chemokine domain off the cellular surface to form SCXCL16, which functions as a chemokine[3]. As a membrane-bound scavenger receptor, CXCL16 is involved in phagocytosis of phosphatidylinositol serine and low-density lipoprotein. CXCR6 is an important receptor for CXCL16. CXCL16/CXCR6 has been found to play a role in leukocyte migration in atherosclerosis, rheumatoid arthritis, inflammatory diseases, and HIV infection[4]. It is also a hot topic in targeted therapy.
Reports confirm that a variety of chemokines participate in tumorigenesis by regulating leukocyte infiltration to activate tumor-specific immune response in host and by promoting tumor angiogenesis and activating various signaling pathways to promote proliferation, invasion, and metastasis of tumor cells[5]. CXCL12/CXCR4 has been found to be involved in a variety of cancers, including cancers of the urinary system [6] (renal cancer, bladder cancer, and prostate cancer) and gastrointestinal tract[7] (esophageal cancer, gastric cancer, and colorectal cancer). Recently, CXCL16/CXCR6 has become a hot topic in cancer research and drawn much attention. Darash-Yahana et al.[4] found that CXCL16 was highly expressed in several inflammation-related tumors, such as ovarian cancer, breast cancer, prostate cancer, colon cancer, and liver cancer, and that CXCL16/CXCR6 could be used as a molecular marker of inflammation-related tumors. However, the role of CXCL16/CXCR6 in ovarian cancer is not well known.
In this study, we investigated the expression of CXCL12/CXCR4 and CXCL16/CXCR6 in epithelial ovarian cancer, and their relationship with clinicopathologic features and prognosis of the patients. The results described herein provide new perspectives for epithelial ovarian cancer therapy.
Materials and Methods
Patients and samples
Samples were collected from patients treated in the Affiliated Hospital of Qingdao University Medical College between January 2004 and June 2007. Informed consents were obtained from all patients. None of the patients underwent chemotherapy or other adjuvant treatments before surgery. We collected 22 specimens of normal ovarian tissue, 26 specimens of benign epithelial ovarian tumor, 10 specimens of borderline epithelial ovarian tumor, and 56 specimens of epithelial ovarian cancer. Of the 56 specimens of epithelial ovarian cancer, 23 were at stage I–II and 33 at stage III–IV; 40 were serous adenocarcinomas, 9 mucinous adenocarcinomas, 3 clear cell carcinomas, 2 endometrial adenocarcinomas, 1 Brenner tumor, and 1 malignant mixed mesenchymoma; 11 were well or moderately differentiated and 45 were poorly differentiated; 31 had ascites; 24 had lymph node metastasis. Patients were 17 to 75 years old (median, 51.5 years); 38 were ≥ 50 years old. All samples were diagnosed by the same pathologist. Ten days after surgery, patients were treated once with PT regimen (cisplatin plus paclitaxel) and then with PT or PC regimen (cisplatin plus cyclophosphamide) every 3 to 4 weeks for 6 to 14 cycles (average 8 cycles). The follow-up time was from the date of surgery to June 2010, and the median time was 54 months (16 to 75 months).
Reagents
All primary antibodies were purchased from R&D Systems, Inc., and their working concentrations were 1:25 for mouse anti-human CXCL12 monoclonal antibody (MAB350), 1:80 for mouse anti-human CXCR4 monoclonal antibody (MAB171), 1:10 for goat anti-human CXCL16 antibody (AF976), and 1:40 for mouse anti-human CXCR6 monoclonal antibody (MAB171). The PV9000 immunohistochemical detection kit and DAB color reagent were purchased from Zhongshan Goldenbridge Biotechnologies Co. (Beijing, China).
Preparation of tissue microarray
Paraffin-embedded epithelial tissues (normal ovarian epithelium, benign ovarian tumor, borderline ovarian cancer, and malignant epithelial tumor) were sectioned and stained with HE. After morphologic characteristics were documented and typical pathologic regions were marked, tissues were arranged in a blank recipient paraffin block. The diameter of each tissue spot was 2 mm. The tissue array was sectioned at a thickness of 4 µm and stored at room temperature.
Immunohistochemical examination
The PV-9000 two-step immunohistochemical kit and DAB color reagent were used to detect antigens in the tissue samples according to the manufacturers' instructions. Mouse or goat serum served as negative control.
Two pathologists who were blind of patients' profiles observed the slides. Positive cells showed brown granules in cytoplasm or cell membrane. The samples were scored based on the percentage of positive tumor cells and staining intensity. The positive cell percentage was determined by calculating the percentage of positive tumor cells in total observed cells: if < 10%, 0; 10% to 49%, 1; 50% to 74%, 2; ≥ 75%, 3. The intensity was decided by comparing staining of tumor cells and mesenchymal cells: no staining, 0; light or ambiguous staining, 1; medium staining, 2; and strong staining, 3. The two scores were added to categorize staining: 0 to 1, negative; 2, weakly positive (+); 3 to 4, medium positive (++); 5 to 6, strongly positive (+++)[8].
Statistical analysis
Data were analyzed using the SPSS11.5 package. The expression of CXCL12, CXCR4, CXCL16, CXCR6 among the groups were compared using the Chi-square test. Correlation of protein expression to pathologic features of epithelial ovarian cancer and interrelation of chemokines and receptors were analyzed with Spearman's test. Overall survival was analyzed with Kaplan-Meier method. Results were considered statistically significant when P < 0.05.
Results
Expression of CXCL12, CXCR4, CXCR6, and CXCL16 in ovarian tissues
CXCL12, CXCR4, CXCL16, and CXCR6 were not expressed in normal ovarian epithelium, but were expressed in benign, borderline, and malignant epithelial tumors. The positive rates of CXCL12, CXCR4, CXCL16, and CXCR6 were significantly higher in malignant epithelial tumors than in normal ovarian epithelium, benign, and borderline epithelial tumors (CXCL12: 73% vs. 0, 4%, and 10%; CXCR4: 80% vs. 0, 15%, and 40%; CXCL16: 72% vs. 0, 15%, and 10%; CXCR6: 95% vs. 0, 13%, and 40%, all P < 0.05) (Figure 1).
Figure 1. The expression of CXCL12, CXCR4, CXCL16, and CXCR6 in epithelial ovarian tissues. The chemokines and their receptors were detected using the PV-9000 two-step immunohistochemical kit with DAB staining. The cells with brown granules in cytoplasm or cell membrane were regarded as positive cells. CXCL12, CXCR4, CXCL16, and CXCR6 are not expressed in normal epithelial ovarian tissue, weakly expressed in benign epithelial ovarian tumor and borderline ovarian tumor, and strongly expressed in epithelial ovarian carcinoma.
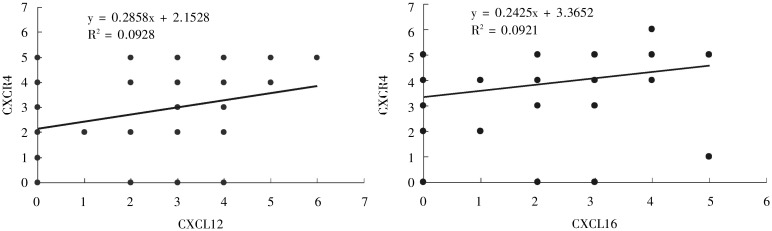
In epithelial ovarian tumors, CXCR4 expression was positively correlated to CXCL12 expression (r = 0.300, P < 0.05); CXCR6 expression was positively correlated to and CXCL16 expression (r = 0.395, P < 0.05) (Figure 2).
Figure 2. Correlations of chemokines CXCL12 and CXCL16 expression to their respective receptors CXCR4 and CXCR6 in epithelial ovarian carcinoma. The correlations were analyzed by the Spearman's test. The plot graphs show a positive correlation between CXCL12 expression and CXCR4 expression as well as between CXCL16 expression and CXCR6 expression in epithelial ovarian carcinoma.
Relationship between CXCL12, CXCR4, CXCL16, and CXCR6 expression and clinicopathologic features of epithelial ovarian cancer
CXCL12 expression in epithelial ovarian cancer was not related to clinical stage, pathologic grade, histological type, lymph node metastasis, and patients' age (P > 0.05), but was related to ascites (P < 0.05). CXCR4 expression was not related to clinical stage, pathologic grade, histological type, ascites, and patients' age (P> 0.05), but was related to lymph node metastasis (P < 0.05) (Table 1).
Table 1. Relationship between the expression of CXCL12 and CXCR4 and the clinicopathologic features of ovarian carcinoma.
| Clinical feature | Cases | CXCL12 |
CXCR4 |
||||||
| + | – | χ2 | P | + | – | χ2 | P | ||
| FIGO stage | 0.265 | 0.607 | 0.451 | 0.502 | |||||
| I, II | 23 | 16 | 7 | 17 | 6 | ||||
| III, IV | 33 | 25 | 8 | 28 | 5 | ||||
| Pathologic classification | 0.339 | 0.560 | 0.083 | 0.774 | |||||
| G1, G2 | 11 | 7 | 4 | 8 | 3 | ||||
| G3 | 45 | 35 | 10 | 37 | 8 | ||||
| Pathologic type | 5.729 | 0.057 | 4.181 | 0.124 | |||||
| Serous carcinoma | 40 | 33 | 7 | 34 | 6 | ||||
| Mucinous carcinoma | 9 | 4 | 5 | 5 | 4 | ||||
| Others | 7 | 5 | 2 | 6 | 1 | ||||
| Lymph node metastasis | 0.024 | 0.877 | 4.371 | 0.037 | |||||
| Yes | 24 | 11 | 13 | 21 | 3 | ||||
| No | 32 | 14 | 18 | 20 | 12 | ||||
| Ascites | 4.755 | 0.029 | 1.156 | 0.282 | |||||
| Yes | 31 | 19 | 12 | 27 | 4 | ||||
| No | 25 | 8 | 17 | 18 | 7 | ||||
| Age (years) | 0.192 | 0.661 | 0.062 | 0.803 | |||||
| ≥ 50 | 38 | 29 | 9 | 29 | 9 | ||||
| < 50 | 18 | 12 | 6 | 15 | 3 | ||||
CXCL16 expression in epithelial ovarian cancer was not related to clinical stage, pathologic grade, histological type, ascites, lymph node metastasis, and patients' age (P > 0.05). CXCR6 expression was not related to clinical stage, pathologic grade, ascites, and patients' age (P > 0.05), but was related to histological type and lymph node metastasis (P < 0.05) (Table 2).
Table 2. Relationship between the expression of CXCL16 and CXCR6 and the clinicopathologic features of ovarian carcinoma.
| Clinical feature | Cases | CXCL12 |
CXCR4 |
||||||
| + | – | χ2 | P | + | – | χ2 | P | ||
| FIGO stage | 1.205 | 0.272 | 0.023 | 0.880 | |||||
| I, II | 23 | 19 | 4 | 22 | 1 | ||||
| III, IV | 33 | 23 | 10 | 30 | 3 | ||||
| Pathologic classification | 0.091 | 0.764 | 2.065 | 0.151 | |||||
| G1, G2 | 11 | 8 | 3 | 8 | 3 | ||||
| G3 | 45 | 28 | 17 | 42 | 3 | ||||
| Pathologic type | 2.268 | 0.322 | 33.48 | 0.000 | |||||
| Serous carcinoma | 40 | 28 | 12 | 38 | 2 | ||||
| Mucinous carcinoma | 9 | 4 | 5 | 9 | 0 | ||||
| Others | 7 | 4 | 3 | 1 | 6 | ||||
| Lymph node metastasis | 1.556 | 0.212 | 7.430 | 0.006 | |||||
| Yes | 24 | 20 | 4 | 21 | 3 | ||||
| No | 32 | 22 | 10 | 17 | 15 | ||||
| Ascites | 0.361 | 0.548 | 0.037 | 0.848 | |||||
| Yes | 31 | 21 | 10 | 29 | 2 | ||||
| No | 25 | 15 | 10 | 24 | 1 | ||||
| Age (years) | 0.291 | 0.589 | 0.348 | 0.555 | |||||
| ≥ 50 | 38 | 26 | 12 | 35 | 3 | ||||
| < 50 | 18 | 11 | 7 | 18 | 0 | ||||
Relationship between CXCL12, CXCR4, CX-CL16, and CXCR6 expression and overall survival of the patients with epithelial ovarian cancer
During follow-up, 9 patients died and 5 were lost. The 3-year survival rate was 93%, and the 5-year survival rate was 70%.
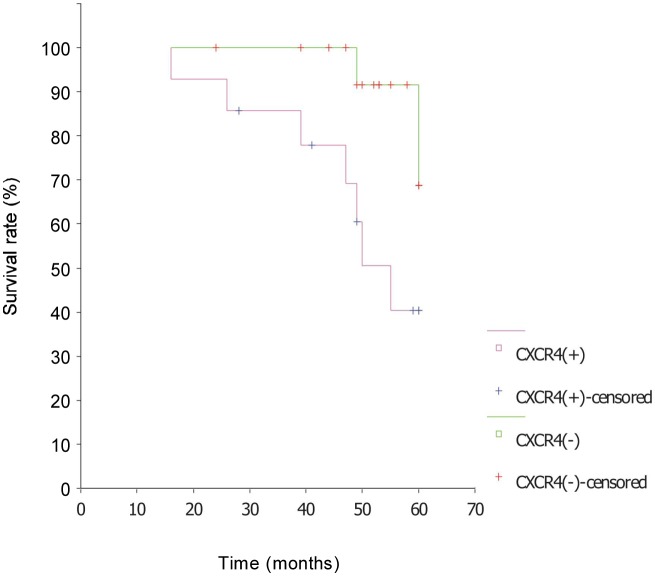
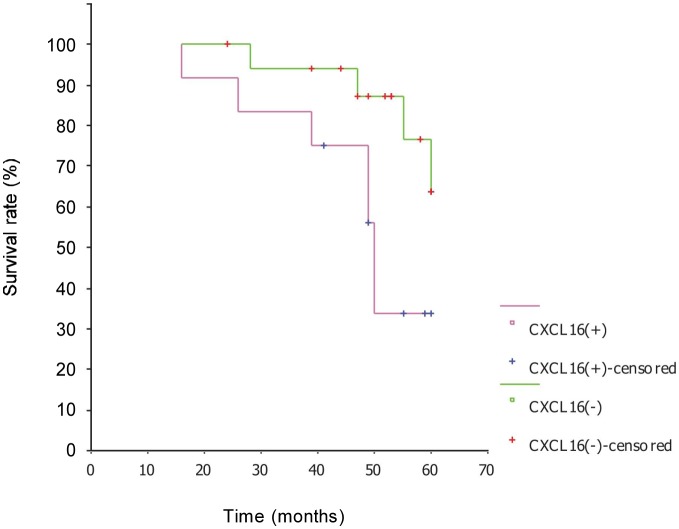
CXCR4 and CXCL16 expressions were closely associated with overall survival in Kaplan-Meier analysis (χ2 = 4.67, P < 0.05; χ2 = 4.48, P < 0.05). The survival rate of CXCR4-negative patients was 87.5% and the median survival was longer than 53 months; the survival rate of CXCR4-positive patients was 50.0% and the median survival was 49 months (Figure 3). The survival rate of CXCL16-negative patients was 77.8% and the median survival was longer than 53 months; the survival rate of CXCL16-positive patients was 41.7% and the median survival was 50 months (Figure 4). The survival time was not related with clinical stage, pathologic grade, histological type, ascites, lymph node metastasis, age, CXCL12 expression, and CXCR6 expression.
Figure 3. Survival curves of epithelial ovarian carcinoma patients with or without CXCR4 expression. The survival rate of CXCR4-positive patients was significantly lower than that of CXCR4-negative patients, indicating that CXCR4 predict a poor prognosis of patients with epithelial ovarian carcinoma.
Figure 4. Survival curves of epithelial ovarian carcinoma patients with or without CXCL16 expression. The survival rate of CXCL16-positive patients was significantly lower than that of CXCL16-negative patients, indicating that CXCL16 also predict a poor prognosis of patients with epithelial ovarian carcinoma.
Discussion
Expression and significance of the CXCL12/CX-CR4 chemokine axis in epithelial ovarian cancer
CXCL12 and CXCR4 are reported to be highly expressed in ovarian cancer, but not in the ovarian tissue of healthy women with or without a family history of ovarian cancer[9]. CXCL12/CXCR4 mediates cell migration and proliferation by activating the PI3K-MAPK-NF-κB pathway[10]. This study showed that CXCL12 and CXCR4 were not expressed in normal ovarian epithelial tissues, but were expressed in malignant epithelial ovarian tumors (73% for CXCL12 and 80% for CXCR4) at rates significantly higher than those in benign and borderline ovarian tumors, suggesting that the CXCL12/CXCR4 chemokine axis is involved in the transformation of epithelial ovarian tumors. The underlying mechanisms may include stimulating growth of tumor cells and stromal cells in an autocrine, paracrine, or exocrine manner[11], inhibiting tumor apoptosis by activating the necrosis factor-κB (NF-κB) pathway.
Peritoneal implantation is the most common type of epithelial ovarian cancer metastasis, followed by lymph node metastasis. In this study, we found that CXCL12 expression was closely related with ascites in patients with epithelial ovarian cancer, indicating that by binding to the receptor CXCR4 on epithelial ovarian cancer cell surface and then activating the G protein-coupled signaling pathway, CXCL12 in ascites may generate a chemokine gradient to facilitate the “homing” of epithelial ovarian cancer cells to the abdominal cavity. CXCL12/CXCR4 may promote tumor angiogenesis by interacting with vascular endothelial growth factor (VEGF), which can create a favorable microenvironment for peritoneal metastasis. Meanwhile, tumor-derived factors such as tumor growth factor-β1 (TGF-β1) can up-regulate CXCL12 expression, which can form a magnifying circuit to promote proliferation and invasion of tumor cells. In this study, we found that lymph node metastasis of epithelial ovarian cancer was closely related with CXCR4 expression. Under the functions of CXCL12 and CXCR4, detached ovarian cancer cells enter into vessels and can achieve directional migration by adhering tightly to lymphatic endothelial cells, whereas cytokines such as NF-κB from immunocytes in lymph nodes can inhibit apoptosis and promote tumor cell growth and proliferation by interacting with CXCR4[12]. Our study showed that the survival rate of CXCR4-negative patients was higher than that of CXCR4-positive patients. The possible reasons may be insensitivity of CXCR4-positive cancer cells to chemotherapy, promotion of tumor cell proliferation and invasion as well as inhibition of cell apoptosis by CXCR4/CXCL12 interaction. However, epithelial ovarian cancer prognosis was not related with any of the classic prognostic factors (such as FIGO stage and lymph node metastasis). This may due to the small sample size in our study, and need to be confirmed by more comprehensive statistical analysis.
Therefore, as a chemokine axis involved in the growth, proliferation, invasion, and metastasis of epithelial ovarian cancer, CXCL12/CXCR4 can be used as a new tumor marker and a new target for cancer therapy. New drugs targeting CXCR4, such as CXCR4 peptide antagonists (T22, T134 and T140), non-peptide antagonist (AMD3100), anti-CXCR4 antibody, PI-3 kinase inhibitor wortmannin and PKC inhibitor GF109203X have been developed[10]. As estrogen can tremendously induce CXCL12 secretion and promote growth of estrogen receptor-α-positive ovarian cancer cells[13], anti-estrogen therapy might be an indirect way to inhibit metastasis of ovarian cancer by suppressing the CXCL12/CXCR4 axis.
Expression and significance of the CXCL16/CXCR6 chemokine axis in epithelial ovarian cancer
The functions of CXCL16/CXCR6 in tumorigenesis have become a hot topic recently. CXCL16/CXCR6 is reported to be expressed in prostate cancer, breast cancer, renal cancer, colorectal cancer, pancreatic ductal carcinoma, nasopharyngeal carcinoma, and malignant melanoma[14]. Our study showed that CXCL16 and CXCR6 were highly expressed in epithelial ovarian cancer tissues and showed a positive correlation between them, whereas they were not or weakly expressed in normal ovarian epithelium and benign ovarian tumors. These results indicate that CXCL16/CXCR6 may function in the growth and invasion of epithelial ovarian cancer.
Darash-Yahana et al.[4] found that CXCL16 and CXCR6 expression was positively related with prostate cancer stage and grade; the more aggressive and malignant the tumors, the higher the levels of CXCL16 and CXCR6 mRNA; CXCL16 and CXCR6 mRNA levels were higher in liver or bone metastatic lesions than in the primary tumor. Our study found that CXCR6 expression was related with lymph node metastasis of epithelial ovarian cancer (P < 0.05), but not related with stage, grade, and ascites, indicating that in some metastatic areas, high CXCR6 expression was related with tumor aggressiveness. In addition, CXCR6 expression was closely related with histological type (P < 0.05). However, when analyzing certain data, CXCR6 was found in 38 out of 40 serous ovarian cancers and 9 out of 9 mucinous ovarian cancers. The difference in group sizes may result in false positive results. The correlation between CXCR6 expression and histological type needs further study.
The function of CXCL16/CXCR6 may vary in different tumors. CXCL16/CXCR6 promotes prostate cancer growth and proliferation, but CXCR6 inhibition stimulates colon cancer cell proliferation[15]. We found that CXCL16-negative patients had a better prognosis than CXCL16-positive patients, suggesting that CXCL16/CXCR6 may lead to malignant progression of ovarian cancer.
Wang et al. [16] have shown that CXCL16/CXCR6 interaction activated the PI3K/PTEN/AKT/mTOR signaling pathway. With the help of ADAMs, CXCL16 was shed from the cell surface to form SCXCL16. For these reasons, antibodies and antagonists of CXCL16/CXCR6 and ADAM10/ADAM17, as well as mTOR inhibitors rapamycin, CCI-779, RAD001, and AP23573 can be used for ovarian cancer therapy.
Acknowledgments
This work was supported by grants from National Natural Science Foundation for Young Scholars of China (No. 30700763); Promotive Research Foundation for Excellent Young and Middle-aged Scientists of Shandong (No. BS2009SW002).
References
- 1.Murdoch C. CXCR4: chemokine receptor extraordinaire [J] Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- 2.Matloubian M, David A, Engel S, et al. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo [J] Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- 3.Hundhausen C, Schulte A, Schulz B, et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes [J] J Immunol. 2007;178(12):8064. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- 4.Darash-Yahana M, Gillespie JW, Hewitt SM, et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers [J] Plos One. 2009;4(8):e6695. doi: 10.1371/journal.pone.0006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Y, Zhou Y, Iribarren P, et al. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease [J] Cell Mol Immunol. 2004;1(2):95–104. [PubMed] [Google Scholar]
- 6.Wang YS, Yang XS, Yang DL. Progress of CXCLI2 and its receptor CXC4 in urinary tract tumors [J] Medical Recapitulate. 2010;16(20):3072–3074. [in Chinese] [Google Scholar]
- 7.Iwasa S, Yanagawa T, Fan J, et al. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis [J] Anticancer Res. 2009;29(11):4751. [PubMed] [Google Scholar]
- 8.Jiang YP, Wu XH, Wu WX, et al. Expressions of chemokine CXCL12 and its receptor CXCR4 and the clinical significance in human epithelial ovarian cancer [J] Tumor. 2006;26(9):851–855. [in Chinese] [Google Scholar]
- 9.Scotton CJ, Wilson JL, Scott K, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer [J] Cancer Res. 2002;62(20):5930–5938. [PubMed] [Google Scholar]
- 10.Lu DY, Tang CH, Yeh WL, et al. SDF-1 alpha up-regulates interleukin-6 through CXCR4, P13K/Akt, ERk, and NF-kappaB-dependent pathway in microglia [J] Eur J Pharmacol. 2009;613(1–3):146–154. doi: 10.1016/j.ejphar.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment [J] Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 12.Baggiolini M. Chemokines and leukocyte traffic [J] Nature. 1998;392(6676):565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 13.Hall JM, Korach KS. Stromal cell-derived factor-1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells [J] Mol Endocrinol. 2003;17(5):792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 14.Deng L, Chen N, Li Y, et al. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer [J] Biochim Biophys Acta. 2010;1806(1):42–49. doi: 10.1016/j.bbcan.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Meijer J, Ogink J, Kreike B, et al. The chemokine receptor CXCR6 and its ligand CXCL16 are expressed in carcinomas and inhibit proliferation [J] Cancer Res. 2008;68(12):4701–4708. doi: 10.1158/0008-5472.CAN-08-0482. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Lu Y, Wang J, et al. CXCR6 induces prostate cancer progression by the AKT/Mammalian target of rapamycin signaling pathway [J] Cancer Res. 2008;68(24):10367–10376. doi: 10.1158/0008-5472.CAN-08-2780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]