Abstract
Castleman's disease is a slowly progressive and rare lymphoproliferative disorder. Here, we report a 55-year-old woman with superior mediastinal Castleman's disease being misdiagnosed for a long term. We found a 4.3 cm mass localized in the superior mediastinum accompanied with severe clinical symptoms. The patient underwent an exploratory laparotomy, but the mass failed to be totally excised. Pathologic examination revealed a mediastinal mass of Castleman's disease. After radiotherapy of 30 Gy by 15 fractions, the patient no longer presented previous symptoms. At 3 months after radiotherapy of 60 Gy by 30 fractions, Computed tomography of the chest showed significantly smaller mass, indicating partial remission. Upon a 10-month follow-up, the patient was alive and free of symptoms.
Keywords: Castleman's disease, radiotherapy, case report
Castleman's disease is a rare lymphoproliferative disorder with a low incidence and very slowly progressive behavior. It is difficult to be diagnosed and patients are usually misdiagnosed. We analyzed the clinical data of a patient with superior mediastinal Castleman's disease hospitalized in the 309 Hospital of the Chinese PLA. It has been 4 years since the onset to the appearance of obvious symptoms and another half a year to definite diagnosis. The patient under went combinatorial antibiotic therapy, anti-tuberculosis treatment for 6 months, and hormone therapy for 2 months, suffering from long-term physical pain and financial burden. This indicates a lack of clinical knowledge on this disease. Thus, we reviewed the literature of Castleman's disease.
Case Report
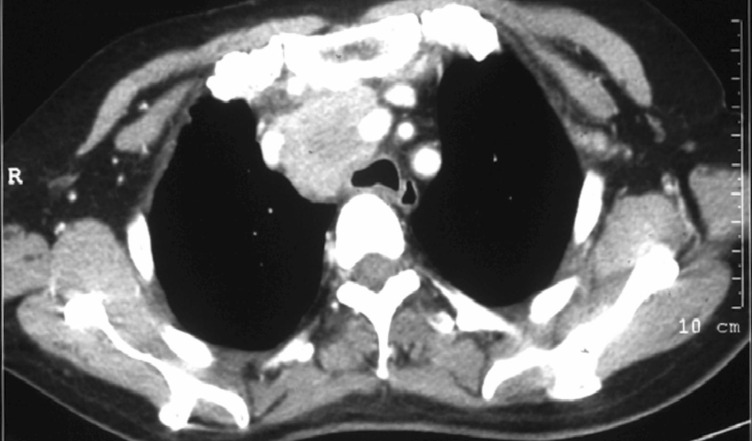
The patient was a 55-year-old woman hospitalized in the Department of Oncology, the 309 Hospital of the Chinese PLA on December 23, 2009 for 4 years of chest pain which was further aggravated for 8 months. Previously, she had paroxysmal dull pain in the chest for 4 years without obvious incentives; unfortunately, no attention was paid to it. The chest pain aggravated since April, 2009, accompanied by cough, white phlegm, hoarse voice, and cough after drinking water. No fever, night sweats, or weight loss was observed. Chest computed tomography (CT) done in another hospital in April, 2009 showed a mass localized in the superior mediastinum with a maximum diameter of 4.3 cm. Routine blood test, erythrocyte sedimentation rate, biochemical test, and tumor marker test were normal, as was abdominal ultrasonography. Thoracic operation was conducted, during which no clear boundary between the mass and surrounding blood vessels was found. As a result, the mass could not be excised and only an incisional biopsy was performed. Postoperative pathologic examination showed reactive hyperplasia of lymph nodes accompanied by charcoal particle deposit. The patient was clinically suspected to have mediastinal tuberculosis lymphadenitis and was given an anti-tuberculosis treatment for almost half a year. No obvious relief occurred and the patient suffered from somasthenia with a weight loss of about 10 kg in 6 months. A chest CT performed in November, 2009 found no obvious change in the superior mediastinal mass (Figure 1).
Figure 1. Computed tomography (CT) of the chest of the 55-year-old woman before radiotherapy. The patient had chest pain for 4 years which was further aggravated for 8 months. A mediastinal tuberculosis lymphadenitis was suspected and the patient was given an anti-tuberculosis treatment for almost half a year. A 4 cm × 4 cm mass in the anterior superior mediastinum with remarkable intensity shows after contrast-enhanced scan.
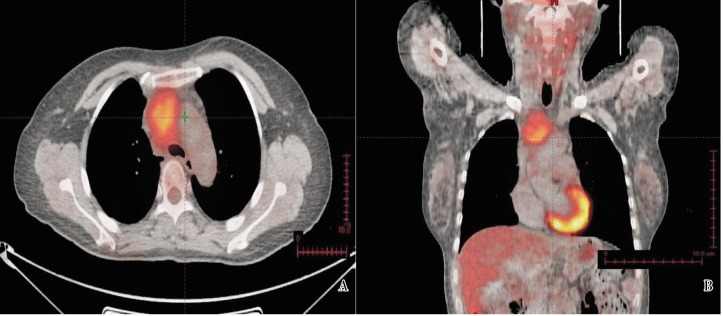
The patient came to the 309 Hospital of the Chinese PLA for further treatment. Results of the tuberculin (PPD) test and MycoDot TM test were both negative. As a result, the Department of Tuberculosis excluded the possibility of tuberculosis and discontinued anti-tuberculosis treatment. The pathologic specimen of the thoracic mass incised in April 2009 was consulted by Departments of Pathology from several hospitals including the Peking Union Medical College Hospital. In the pathologic specimen, there was obvious diffuse infiltration of lymphocytes with blood vessels proliferated and infiltrated into the follicles; immunohistochemical assay for CD20 detected positive staining on the cell capsule inside follicles (Figure 2). Lymph node hyperplasia was then considered, supporting the diagnosis of Castleman's disease. A whole body positron emission tomography (PET)-CT scan was performed on October 30, 2009, showing a soft tissue mass in the superior mediastinum with the maximal diameter of 4.4 cm. The fluorodeoxyglucose (FDG) uptake was slight and the maximum standardized uptake value (SUV) was 2.9. No other abnormality was found. In combination with the medical history, giant lymph node hyperplasia was considered (Figure 3).
Figure 2. Histological examination of the thoracic tumor from the 55-year-old woman. The patient underwent thoracic operation in April 2009 at another hospital, but the mass could not be completely resected. Therefore, a biopsy was performed for histological examination. A, lymphocytes diffuse in the mass and vessels penetrate into the follicles, indicating lymphoid hyperplasia (HE ×20). B, positive staining for CD20 shows on the cell membrane (IHC × 10).
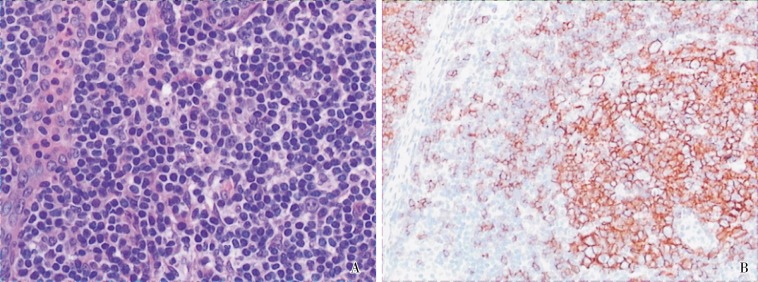
Figure 3. PET-CT scan of the chest of the 55-year-old woman before radiotherapy. A, transverse scan of the chest; B, coronal scan of the chest. PET-CT before treatment shows a 4.4 cm mass in the anterior superior mediastinum with slightly elevated FDG uptake (SUVmax = 2.9).
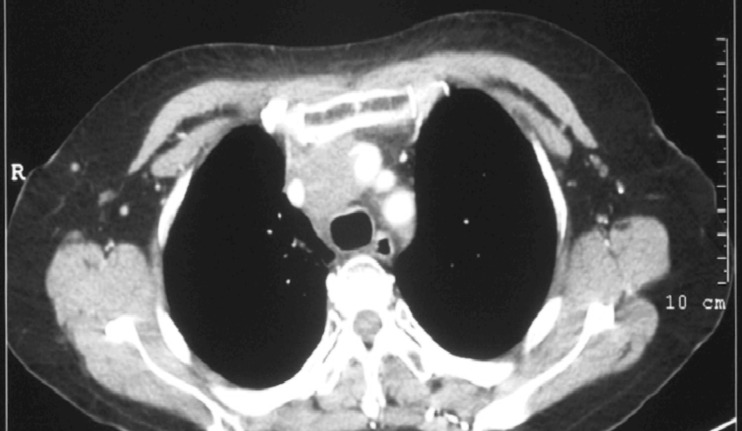
The patient underwent hormone therapy for 2 months with no obvious response, then she was transferred to the Department of Radiotherapy in December, 2009. Three-field intensity - modulated radiotherapy (IMRT) was delivered to the mediastinal mass since December 23, 2009. After radiotherapy of 30 Gy by 15 fractions, symptoms such as chest pain, hoarse voice, and cough after drinking water were markedly relieved, but the chest CT scan showed no obvious shrinkage of the mass. Radiotherapy was continued, and the total dose was increased to 60 Gy by 30 fractions, until February 4, 2010. A chest enhanced CT scan performed on May, 2010 showed that the mass was significantly smaller than before, indicating partial remission (Figure 4). Upon follow-up until December 2010, the patient had no discomfort.
Figure 4. Computed tomography of the chest 3 months after radiotherapy. The mediastinial tumor (2 cm × 3 cm) is obviously smaller than that before radiotherapy. The mass shows slight intensity after contrast-enhancement.
Discussion
The pathogenesis of Castleman's disease is unclear. Recent researches found that this disease was related to the infection of the human immunodeficiency virus (HIV) or the human herpes virus-8 (HHV-8). HHV-8, a human lymphotropic virus, is associated with the onset and development of Kaposi sarcoma. Researches showed that HIV-positive Kaposi sarcoma was usually accompanied by infection of HHV-8. In addition, viral load was related to tumor activity and antiviral treatment was effective for tumor remission. However, as Castleman's disease is rare, pathogenic mechanisms await further confirmation. Diagnosis of this disease primarily depends on histological examination, thus puncture or incisional biopsy should be carried out forthwith. Pathologic types mostly consist of hyaline vascular, plasma cell, and mixed variants.
The clinical manifestation of Castleman's disease includes unicenter and multicenter types, with unicenter type observed most frequently (81%). Characteristics of unicenter type Castleman's disease are as follows[1]: (1) males and females have the same incidence, with a median age of 37 years; (2) hyaline vascular type is the major pathologic type, accounting for 92%; (3) most lesions are in the mediastinum, neck, axillary, and retroperitoneal lymph nodes, and other rare locations include the intra-orbital region, hepatic portal, subcutaneous region, parotid glands, kidney, and pelvic cavity[2]–[7]; (4) the disease progresses very slowly, intact surgical excision could cure the disease and partial excision could significantly relieve symptoms. However, postoperative adjuvant radiotherapy is recommended due to the possibility of relapse after partial excision[8],[9]. Characteristics of multicenter type Castleman's disease are as follows: (1) males and females have the same incidence, with a median age of 53 years; (2) plasma cell and mixed variant types are the major pathologic types, accounting for 89%; (3) it is mainly treated by chemotherapy with adjuvant local radiotherapy. Most of this type is aggressive, accompanied with system symptoms and a poor prognosis, especially the plasma cell type. Median survival is about 27 months with a 5-year survival about 50%. Some cases could transfer to lymphoma, Kaposi sarcoma, and dendritic reticulum cell sarcoma[1],[9].
Surgical and radiotherapy are the principle treatments for unicenter type Castleman's disease (Table 1)[1],[8]–[11]. Chronowski et al.[1] have reported 22 cases of Castleman's disease, admitting between 1988 and 1999. Eight of the 12 patients with unicenter type disease underwent surgical excision (including 2 cases of partial excision) and the other 4 underwent radical radiotherapy. No disease progression-related death occurred. Of the 2 patients who underwent a partial excision, 1 had no systemic symptoms, and the other had mediastinal Castleman's disease accompanied with systemic symptoms such as fever, night sweats, and weight loss. The symptoms were completely relieved after partial excision, with no disease progression observed after a 117-month follow-up. Radiotherapy for Castleman's disease mainly includes radical radiotherapy and preoperative neoadjuvant radiotherapy. Radical radiotherapy is suitable for patients with locally advanced and inoperable disease, patients unable to endure operation due to medical disorders, or those who refuse operation. Vries et al.[12] summarized the data of 32 patients who underwent radiotherapy or radiotherapy-based treatment, including 29 with unicenter type disease. The overall response rate of these 29 patients was 89.6%, with a complete remission rate of 44.8%, a partial remission rate of 44.8%, and a stable disease rate of 10.4%. For most cases, radiotherapy of 40 to 50 Gy could achieve complete or partial remission. Preoperative neoadjuvant radiotherapy is suitable for patients with an obviously shrunk tumor after radiotherapy which could be completely excised by operation. Vries et al.[12] reported a case of pelvic focal type Castleman's disease treated with neoadjuvant radiotherapy. Six weeks after a total radiation dose of 40 Gy by 20 fractions, tumor diameter shrunk from 7.2 cm to 4.7 cm. Complete surgical excision was performed and an Intraoperative radiation boost of 10 Gy was delivered; no sign of recurrence was observed after 27 months. In our study, symptoms of the patient were nearly relieved after 30 Gy by 15 fractions, but CT scan showed no obvious change in the mass; thus, a boost to 60 Gy by 30 fractions was delivered. At 3 months after the completion of radiotherapy, chest enhanced CT showed partial remission of the tumor. During follow-up, the patient had no discomfort, with general status and body weight recovered to normal. The long-term survival awaits further observation.
Table 1. Literature review of surgical operation and radiotherapy for unicentric Castleman's disease.
| Reference | No. of Patients | Histology | Treatment | Response (follow-up) |
| Chronowski et al.[1] | 6 | 5 HV, 1 M | Complete resection | CR (4–74 months) |
| 2 | HV | Partial resection | Symptom-free (31–117 months) | |
| 4 | HV | Radiotherapy | Symptom-free* | |
| Neuhof et al.[8] | 3 | 2 HV, 1 M | Radiotherapy | 1 CR, 2 NR |
| Bowner et al.[9] | 10 | 8 HV, 2 M | Complete resection | CR (9–37 months) |
| 1 | HV | Partial resection | Symptom-free (12 months) | |
| Bucher et al.[10] | 2 | HV | Complete resection | CR (5 years ) |
| Veldhuis et al.[11] | 1 | PC | Radiotherapy | CR |
PC, plasma cell type; HV, hyaline vascular type; M, mixed type; CR, complete remission; PR, partial remission; NR, no response. *Two patients died of other diseases.
Multicenter type Castleman's disease is mainly treated with chemotherapy. Because this disease is lymphoproliferative, the most frequently used clinical regimen is CHOP (cyclophosphamide, adrimycin, vincristine, and prednisolone). Other regimens include CAVD (cyclophosphamide, adrimycin, vincristine, and dexamethasone), MINE (mensa, ifosfamide, mitoxantrone, and etoposide), and nitrogen mustard combined with prednisone. Chronowski et al.[1] reported 9 cases of multicenter type disease which was mainly of mixed variant and plasma cell types. The median age was 53 years, with 5 men and 4 women. Among them, 6 patients had fever, 4 had weight loss. Median follow-up was 65 months. All patients were treated by regimens mentioned above, and 4 (44%) achieved complete remission after chemotherapy. During follow-up, 5 patients lived with tumor, and 4 were disease-free.
In recent years, along with the emergence of molecular targeted agents, rituximab was used to treat multicenter type Castleman's disease. Casquero et al.[13] reported that among 12 patients who were given rituximab, 9 gained complete remission and 3 died of primary disease. Another research showed that the complete remission rate of HIV-positive Castleman's disease which relapsed after combined chemotherapy was up to 95.8% after treatment of rituximab and was 72% at 1 year after treatment [14]. Researches also indicated that the effect of rituximab on CD20-positive multicenter type Castleman's disease was exciting.
Other drugs for this disease include thalidomide, bortezomib, and the antiviral agent ganciclovir. Stary et al. [15] reported a case of HIV-positive multicenter type Castleman's disease treated by thalidomide combined with rituximab achieved complete remission. Complete remission of a case treated by bortezomib combined with rituximab was also reported[16]. Treating Castleman's disease with antiviral therapy is still under exploration.
References
- 1.Chronowski GM, Ha CS, Wilder RB, et al. Treatment of unicentric and multicentrc Castleman disease and the role of radiotherapy [J] Cancer. 2001;92(3):670–676. doi: 10.1002/1097-0142(20010801)92:3<670::aid-cncr1369>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Nistor C, Davidescu M, Ciuche A, et al. A rare case of unicentric plasma cell type Castleman's disease in the mediastinum [J] Pneumologia. 2010;59(1):32–35. [PubMed] [Google Scholar]
- 3.Karami H, Sahebpour AA, Ghasemi M, et al. Hyaline vascular–type Castleman's disease in the hilum of liver: a case report [J] Cases J. 2010;3(3):74. doi: 10.1186/1757-1626-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang JQ, Yang YM, Xiong Y, et al. Surgical treatment and prognosis analysis of localized retroperitoneal Castleman disease: a study of 20 cases [J] Zhonghua Wai Ke Za Zhi. 2009;47(22):1685–1688. [in Chinese] [PubMed] [Google Scholar]
- 5.Naghashpour M, Cualing HD, Szabunio M, et al. Hyaline-vascular castleman disease: a rare cause of solitary subcutaneous soft tissue mass [J] Am J Dermatopathol. 2010;32(3):293–297. doi: 10.1097/DAD.0b013e3181b7269a. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood N, Suresh HB, Swethadri GK, et al. Ultrasound and Doppler findings in a rare case of Castleman's disease of the parotid [J] Dentomaxillofac Radiol. 2010;39(1):54–56. doi: 10.1259/dmfr/77931198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu YC, Huang Y, Yao J, et al. A rare case of Castleman's disease of plasma cell type within kidney [J] Chin Med J (Engl) 2009;122(19):2396–2398. [PubMed] [Google Scholar]
- 8.Neuhof D, Debus J. Outcome and late complications of radiotherapy in patients with unicentric Castleman disease [J] Acta Oncol. 2006;45(8):1126–1131. doi: 10.1080/02841860600812701. [DOI] [PubMed] [Google Scholar]
- 9.Bowne WB, Lewis JJ, Filippa DA, et al. The management of unicentric and multicentric Castleman's disease: a report of 16 cases and a review of the literature [J] Cancer. 1999;85(3):706–717. doi: 10.1002/(sici)1097-0142(19990201)85:3<706::aid-cncr21>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Bucher P, Chassot G, Zufferey G, et al. Surgical management of abdominal and retroperitoneal Castleman's disease [J] World J Surg Oncol. 2005;3:33–41. doi: 10.1186/1477-7819-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veldhuis GJ, van der Leest AH, de Wolf JT, et al. A case of localized Castleman's disease with systemic involvement: treatment and pathogenetic aspects [J] Ann Hematol. 1996;73(1):47–50. doi: 10.1007/s002770050201. [DOI] [PubMed] [Google Scholar]
- 12.de Vries IA, van Acht MM, Demeyere TB, et al. Neoadjuvant radiotherapy of primary irresectable unicentric Castleman's disease: a case report and review of the literature [J] Radiat Oncol. 2010;5(2):7. doi: 10.1186/1748-717X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casquero A, Barroso A, Femández Guerrero ML, et al. Use of rituximab as a salvage therapy for HIV-associated multicentric Castleman disease [J] Ann Hematol. 2006;85(3):185–187. doi: 10.1007/s00277-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 14.Gérard L, Bérezné A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial [J] J Clin Oncol. 2007;25(22):3350–3356. doi: 10.1200/JCO.2007.10.6732. [DOI] [PubMed] [Google Scholar]
- 15.Stary G, Kohrgruber N, Herneth AM, et al. Complete regression of HIV-associated multicentric Castleman disease treated with rituximab and thalidomide [J] AIDS. 2008;22(10):1232–1234. doi: 10.1097/QAD.0b013e3282fa75ce. [DOI] [PubMed] [Google Scholar]
- 16.Sobas MA, Alonso Vence N, Diaz Arias J, et al. Efficacy of bortezomib in refractory form of multicentric Castleman disease associated to poems syndrome (MCD-POEMS variant) [J] Ann Hematol. 2010;89(2):217–219. doi: 10.1007/s00277-009-0795-6. [DOI] [PubMed] [Google Scholar]