Abstract
Osteosarcoma is the most common primary malignant bone cancer in children and adolescents. Emerging evidence has suggested that the capability of a tumor to grow is driven by a small subset of cells within a tumor, termed cancer stem cells (CSCs). Although several methods have been explored to identify or enrich CSCs in osteosarcoma, these methods sometimes seem impractical, and chemotherapy enrichment for CSCs in osteosarcoma is rarely investigated. In the present study, we found that short exposure to chemotherapy could change the morphology of osteosarcoma cells and increase sarcosphere formation in vitro, as well as increase tumor formation in vivo. Furthermore, methotrexate (MTX)-resistant U2OS/MTX300 osteosarcoma cells were larger in size and grew much more tightly than parental U2OS cells. More importantly, U2OS/MTX300 cells possessed a higher potential to generate sarcospheres in serum-free conditions compared to parental U2OS cells. Also, U2OS/MTX300 cells exhibited the side population (SP) phenotype and expressed CSC surface markers CD117 and Stro-1. Notably, U2OS/MTX300 cells showed a substantially higher tumorigenicity in nude mice relative to U2OS cells. Therefore, we conclude that chemotherapy enrichment is a feasible and practical way to enrich osteosarcoma stem cells.
Keywords: Osteosarcoma, chemotherapy, cancer stem cell, methotrexate enrichment
Osteosarcoma is the most common primary malignant bone cancer, primarily arising in children and adolescents. The advent of neoadjuvant chemotherapy has significantly improved the long-term survival rate ranging from 55% to 75% [1],[2]. Nevertheless, a poor response to chemotherapy is usually associated with a poor prognosis[3]. Hence, it is important to identify the chemo-resistant cells for further investigation.
Recent studies indicate that osteosarcoma is driven by a small subpopulation of cells called cancer stem cells (CSCs)[4]. Currently, there are three methods for enriching osteosarcoma CSCs: (1) generating sarcospheres in culture, (2) cell sorting with markers (CD133, ALDH, CD117 combined with Stro-1), and (3) exogenously expressing human Oct4 promoter[5]. According to accumulating evidence, CSCs are responsible for rebuilding the bulk of the tumor after conventional chemotherapy. Therefore, it is reasonable to presume that chemotherapy drugs can be effectively used to identify CSCs within the tumor by eliminating the chemo-sensitive cells. To our knowledge, studies in which chemotherapy drugs have been used for CSC enrichment in osteosarcoma have rarely been discussed.
In this study, we used methotrexate (MTX), an active chemotherapy drug in osteosarcoma[6],[7], to enrich CSCs of osteosarcoma. Our results suggest that MTX-resistant U2OS/MTX300 cells possess properties of CSCs in vitro and in vivo.
Materials and Methods
Reagents and antibodies
Methotrexate, Hoechst 33342, and laminin were from Sigma-Aldrich. Primary antibody Stro-1 and fluorescein (FITC) conjugates were obtained from Santa Cruz Biotechnology. CD117-PE antibody for flow Cytometry was from eBioscience. Dulbecco's modified eagle medium (DMEM)/F12 and N2 medium, as well as fetal bovine serum (FBS) were products of Invitrogen. Human epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were from PeproTech.
Cells and cell culture
The human osteosarcoma cell lines U2OS and MG63 (a gift from Dr. Massimo Serra, Istituti Ortopedici Rizzoli, Bologna, Italy[8]) were cultured in DMEM with 10% FBS, according to the instructions from American Tissue Culture Collection. MTX-resistant variant U2OS/MTX300 cells (a gift from Dr. Massimo Serra, Istituti Ortopedici Rizzoli, Bologna, Italy[8]) were cultured in DMEM with 10% FBS and 300 ng/mL MTX.
Chemotherapy treatment
Human osteosarcoma cell lines U2OS and MG63 were treated with 100 ng/mL or 300 ng/mL MTX for 5 days. Afterwards, dead cells were removed with three phosphate buffered solution (PBS) washes. Then, surviving cells were harvested and subjected to clonogenicity assay, sarcosphere formation assay, and in vivo tumorigenicity assay.
Cell proliferation assay
Cells were seeded in 96-well microplates at a density of 3000 cells/well. Every 24 h until day 7, cells were analyzed with MTT assay as described[9]. Briefly, 20 µL 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazo-liumbromide (MTT) solution (5 mg/mL in PBS) was added to each well and the plates were incubated at 37°C for an additional 4 h. After the medium was aspirated, 180 µL of dimethyl sulfoxide (DMSO) was added to each well. Finally, the absorbance at 490 nm (A490) was measured with a microtiter plate reader.
Clonogenicity assay
U2OS and MG63 osteosarcoma cells, both MTX-naTve and those that survived MTX treatment, were plated in triplicate at 100 cells/well in 6-well plates and then maintained in DMEM medium supplemented with 10% FBS for 14 days. Afterwards, cells were washed three times with PBS, fixed in methanol for 5 min, and dyed with crystal violet for 10 min at room temperature. After that, we washed out the dye and counted the colonies containing more than 50 cells.
Sarcosphere formation assay
Cells were seeded at 2000 cells/well in 6-well ultra-low attachment plates (Corning) and cultured in DMEM/F12 medium mixed with N2 supplement, human EGF (10 ng/mL), and human bFGF (10 ng/mL). After 14 days, the resultant spheroids, also named sarcospheres, were counted under an inverted phase contrast microscope (Nikon).
Flow Cytometry analysis
Flow cytrometry was performed as detailed previously[10]. Briefly, for side population analysis, U2OS and U2OS/MTX300 cells (1 × 106 cells/mL) were labeled with 5 µg/mL Hoechst 33342 at 37°C for 90 min in dark, either alone or in combination with 50 µmol/L verapamil. Afterwards, the cells were washed twice with PBS and kept at 4°C in dark before flow Cytometry (EPICS ALTRA Flow Cytosorter, Beckman Coulter) using dual-wavelength analysis.
To detect CD117+Stro-1+ U2OS and U2OS/MTX300 cells, cells were harvested and incubated with CD117-PE and Stro-1 antibodies on ice for 30 min in dark. For indirect labeling, cells were incubated with primary antibody against Stro-1 and then a secondary FITC-conjugated antibody. Expression of cell markers was determined by comparison with isotype control.
In vivo tumorigenicity assay
All in vivo experiments were approved by the Institutional Review Board of Sun Yat-sen University. After being harvested and counted with trypan blue, cells (5 × 106 to 1 × 105) were resuspended in 200 µL PBS and injected subcutaneously into 6- to 8-week old athymic nude mice (SLAC Animal Center, Shanghai, China). The mice were monitored for up to 60 days, after which they were humanely euthanized.
Statistical analyses
An unpaired Student's t test or one way ANOVA with LSD post-hoc test (SPSS software 13.0, SPSS) was applied for statistical analysis. P values of < 0.05 were considered statistically significant.
Results
Short exposure to the chemotherapy drug MTX changes the cellular phenotype
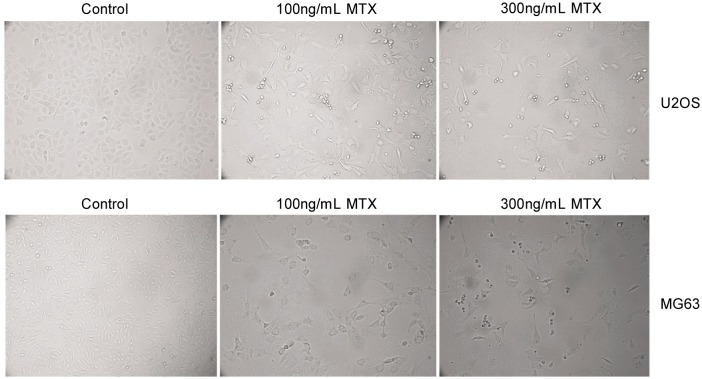
First, we treated U2OS and MG63 cells with MTX (100 ng/mL and 300 ng/mL) for 5 days. Using microscopy, we found that surviving U2OS and MG63 cells had marked morphologic changes, with a shape resembling dendritic-like cells (Figure 1). These cells appeared to connect with each other via the “dendrite” at the end of the cell. Subsequently, these cells were detached with trypsin and plated for the clonogenicity assay. The results showed that clonogenicity was significantly enhanced after MTX treatment for a short time (Table 1). Next, to compare the ability of the MTX-treated cells and their parental cells to generate sarcospheres, cells were grown as described in Materials and Methods section. Both MTX-treated cells and their parental cells were capable of forming sarcospheres, but MTX-treated cells exhibited increased sarcosphere formation compared to parental cells (Table 1). Because MTX-treated cells were found to possess improved capability of colony and sarcosphere formation, we suspected that MTX-treated cells may achieve higher tumorigenic potential. Indeed, as expected, in a subcutaneous tumorigenicity assay, no tumors were developed in 10 mice inoculated with parental U2OS cells, whereas tumors were observed in 2 of 10 mice inoculated with 5 × 106 U2OS cells that survived either 100 or 300 ng/mL MTX treatment and in 1 of 10 mice inoculated with 5 × 105 U2OS cells that survived 300 ng/mL MTX treatment. Hence, these data revealed that short exposure of U2OS and MG63 to MTX changed the cellular phenotype, including changes in cellular morphology, propensity for colony and sarcosphere formation and tumorigenicity.
Figure 1. Short exposure to the chemotherapy drug methotrexate (MTX) changes the cellular morphology.
U2OS and MG63 cells were treated with 100 or 300 ng/mL MTX for 5 days, after which dead cells were washed away with PBS. The remaining cells were observed under phase contrast microscopy. The remaining cells exhibited a shape resembling dendritic-like cells and connected with each other via the “dendrite” at the end of the cell.
Table 1. Colony formation and sarcosphere formation of cells after treatment with methotrexate (MTX).
| Group | Number of colonies | Number of sarcospheres |
| U2OS cells | ||
| Control | 16 ± 5 | 6 ± 2 |
| 100 ng/mL MTX | 23 ± 3a | 15 ± 5a |
| 300 ng/mL MTX | 25 ± 7a | 18 ± 5a |
| MG63 cells | ||
| Control | 45 ± 5 | 11 ± 6 |
| 100 ng/mL MTX | 65 ± 9b | 20 ± 4a |
| 300 ng/mL MTX | 63 ± 4b | 24 ± 4a |
All data are presented as mean ± standard deviation (SD) of 3 experiments. aP < 0.05, bP < 0.01, vs. control cells.
MTX-resistant stable cells have different growth features
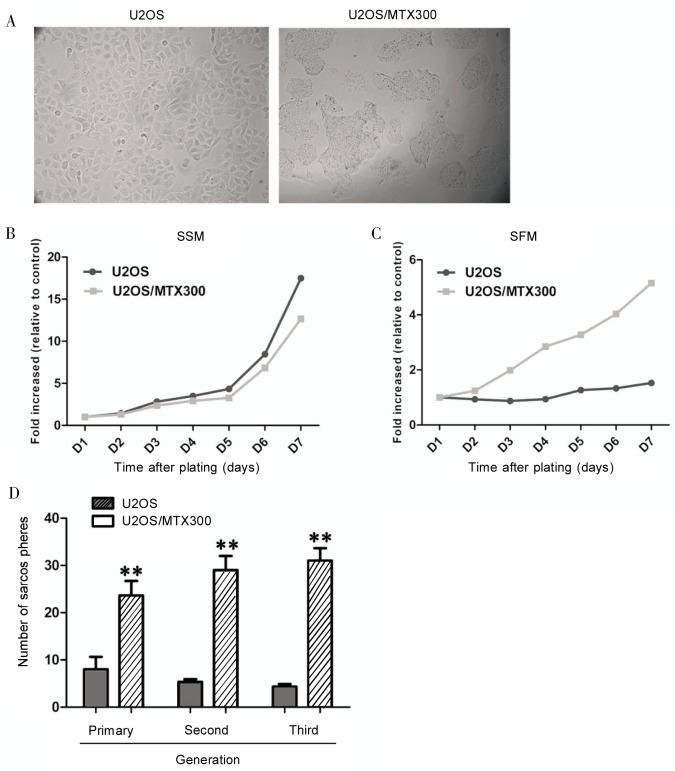
Because short exposure to MTX enhanced the potential for sarcosphere formation and increased tumorigenicity, both key features of CSCs, we investigated whether an MTX-resistant stable cell line U2OS/MTX300 possessed properties of CSCs. We found that U2OS/MTX300 cells were larger in size and grew much more tightly than U2OS cells under phase contrast microscopy (Figure 2A). Next, we used the cell viability assay to evaluate the proliferation of U2OS/MTX300 and U2OS cells in serum-supplemented medium (SSM) or serum-free medium (SFM) with laminin (10 µg/mL). The results revealed that U2OS cells were more adapted to SSM than U2OS/MTX300 cells (Figure 2B). However, U2OS/MTX300 cells exhibited higher proliferating ability in SFM, whereas U2OS cells showed impaired proliferation in SFM (Figure 2C).
Figure 2. MTX-resistant stable cells have different growth features.
A, U2OS/MTX300 cells were larger in size and grew much more tightly than U2OS cells under phase contrast microscopy. B, C, proliferation assay of U2OS/MTX300 cells and U2OS cells in serum-supplemented medium (SSM) and serum-free medium (SFM) with laminin showed that U2OS cells were more adapted to grow in SSM, whereas U2OS/MTX300 cells exhibited higher proliferating ability in SFM. D, sarcosphere formation assay of U2OS/MTX300 and U2OS cells. Columns refer to means and error bars refer to standard deviation (SD) of 3 experiments. **P < 0.01 as indicated.
MTX-resistant stable cells exhibit an increased ability to form clones and sarcospheres
The clonogenicity of U2OS and U2OS/MTX300 cells was evaluated. The clone-forming efficiency of U2OS/MTX300 cells was significantly higher than that of U2OS cells [(34 ± 9)% vs. (16 ± 4)%, P < 0.05].
Next, U2OS and U2OS/MTX300 cells were subjected to the sarcosphere-formation assay to test their self-renewal potential. Although both U2OS and U2OS/MTX300 cells could form sarcospheres, sarcospheres generated from U2OS/MTX300 cells were larger and more numerous (P < 0.01) than those from U2OS cells (Figure 2D). In particular, U2OS/MTX300 cells formed sarcospheres at a frequency of approximately 13%, whereas U2OS cells formed sarcospheres at a frequency of approximately 4 %. Furthermore, sarcospheres from U2OS/MTX300 cells could be passaged for at least 3 generations, whereas those from U2OS cells vanished within 3 passages (Figure 2D). When sarcospheres were passaged to the third generation, U2OS/MTX300 cells had increased sarcosphere-forming ability, but U2OS cells had decreased ability to form spheres. The results above suggested that the MTX-resistant U2OS/MTX300 cell line had an enriched population of self-renewing cells.
MTX-resistant stable cells gain CSC phenotype
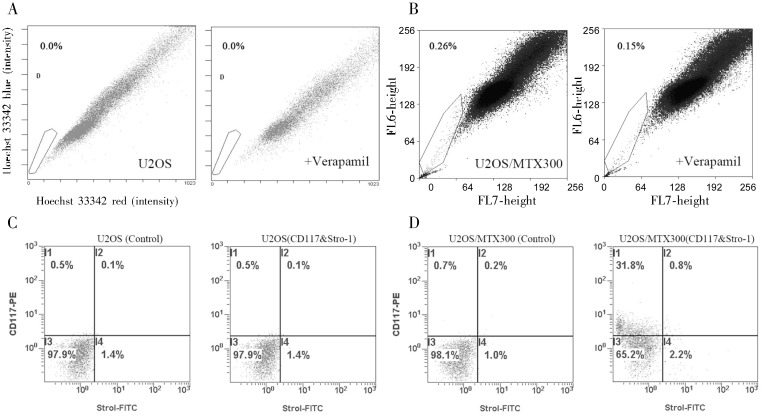
CSCs are commonly recognized to overexpress the ATP-binding cassette sub-family G member 2 (ABCG2). This property correlates with the ability to expel dyes like Hoechst 33342, making flow Cytometry a convenient method to identify this population of cells, which are deemed the side population (SP). We used flow Cytometry analysis to evaluate the SP in MTX-resistant and parental U2OS cells. Verapamil, a specific inhibitor of ABCG2 activity, was used as a negative control. Our results revealed that the SP cell fraction in U2OS/MTX300 cells was 0.11%, but no SP cells were detected from U2OS cells (Figure 3A), which was consistent with the studies of Murase et al.[11] and Tirino et al.[12]
Figure 3. MTX-resistant stable cells gain the CSC phenotype.
A and B, side population (SP) analysis with Hoechst 33342 staining was performed by flow Cytometry in U2OS/MTX300 and U2OS cells. The percentage of SP cells was 0 for U2OS and 0.11% for U2OS/MTX300. Verapamil, a specific inhibitor of ABCG2 activity, was used as a negative control. C and D, putative osteosarcoma CSC surface markers CD117 and Stro-1 were analyzed by flow Cytometry. The percentage of CD117+Stro-1+ cells was 0 for U2OS and 0.6% for U2OS/MTX300.
To determine surface markers of putative osteosarcoma stem cells, U2OS/MTX300 cells were analyzed by flow Cytometry for expression of CD117 and Stro-1, known markers of CSCs. The percentage of CD117+Stro-1+ U2OS/MTX300 cells was 0.6%, whereas no CD117+Stro-1+ U2OS cells were detected (Figure 3B).
MTX-resistant stable cells have high tumorigenic potential
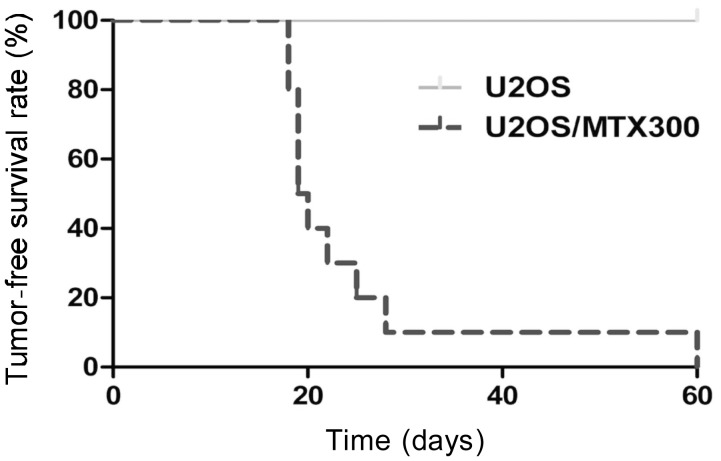
To compare the tumorigenic potential of U2OS/MTX300 cells and U2OS cells, nude mice were inoculated subcutaneously with 5 × 106 to 1 × 105 U2OS/MTX300 and U2OS cells. Tumor formation was observed in all mice inoculated with 5 × 106 U2OS/MTX300 cells, whereas no tumor was found in mice after inoculation with U2OS cells for 60 days (Figure 4). In addition, to evaluate the ability of initiating metastases, U2OS/MTX300 and U2OS cells (5 × 106) were injected into the tail vein of nude mice. However, no evidence of metastases existed in either group (data not shown).
Figure 4. MTX-resistant stable cells have high tumorigenic potential.
Survival curves of nude mice implanted with 5 × 106 U20S/MTX300 or U20S cells show that U2OS/MTX300 cells were highly tumorigenic.
Discussion
Recently, the cancer stem cell hypothesis has attracted a great deal of attention, with comprehensive evidence indicating such cells exist in human cancers of various types[4]. However, isolating and purifying CSCs are sometimes challenging because of their scarcity and continuous differentiation. At present, identifying and enriching CSCs are mainly based on surface markers or SP sorting. The problem is that exact surface antigens are usually unknown, as it seems somewhat impractical to screen large numbers of the entire known markers. Furthermore, the reported cytotoxicity of Hoechst 33342 has hindered its further application[13]–[15]. Therefore, functional enrichment based on the CSC property of chemoresistance[16] can be a feasible approach to identify CSCs. Chemotherapy enrichment of CSCs has important clinical significance because tumor relapse is not rare, even if prominent tumor shrinkage has obtained after chemotherapy. Nevertheless, there have been few studies of chemotherapy enrichment of CSCs in osteosarcoma to date[5].
In the present study, we report that the conventional chemotherapy drug MTX facilitates enrichment of CSCs in osteosarcoma, as indicated in MTX-resistant U2OS/MTX300 cells. This finding is consistent with the reports of Yu et al.[17] and Li et al.[18], who have successfully taken advantage of chemotherapy to enrich self-renewing breast cancer cells. Indeed, U2OS/MTX300 cells had higher clonogenic efficiency, were enriched in the SP phenotype, and expressed markers associated with CSCs, such as CD117 and Stro-1, which have been identified as putative osteosarcoma stem cell markers[19].
The capacity to form sarcospheres in a serum-free culture system is considered fundamental property of CSCs[20]. U2OS/MTX300 cells formed sarcospheres at a higher frequency than U2OS cells, and this ability to form sarcospheres was not impaired during 3 passages. This evidence suggests that U2OS/MTX300 cells possess enhanced self-renewal potential, which is significant as self-renewal is an indispensable functional feature for CSCs. Moreover, high tumorigenic potential is a hallmark of CSCs[4],[5],[20]. Compared to parental U2OS cells, U2OS/MTX300 cells had higher tumorigenic potential in vivo. However, U2OS/MTX300 and U2OS cells showed no distinct difference in inducing lung metastasis. The reason may be that both U2OS/MTX300 and U20S cells have only meager intrinsic ability for metastasis, or that the number of cells injected is too small and the course of the experiment needs to be prolonged.
In summary, our study supports a role for chemotherapy as a feasible approach to enrich osteosarcoma stem cells, providing a new way to further understand the biology of osteosarcoma stem cells.
Acknowledgments
We thank members of our lab for their kind help. This work was supported by grants from National Natural Science Foundation of China (No. 30872967 and No. 81072193 to Jin Wang) and from Natural Science Foundation of Guangdong Province (No. 9151008901000096 to Jin Wang).
References
- 1.Biermann JS, Adkins D, Benjamin R, et al. Bone cancer [J] J Natl Compr Canc Netw. 2007;5(4):420–437. doi: 10.6004/jnccn.2007.0037. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma [J] Curr Opin Pediatr. 2010;22(1):61–66. doi: 10.1097/MOP.0b013e328334581f. [DOI] [PubMed] [Google Scholar]
- 3.Gorlick R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary [J] Clin Cancer Res. 2003;9(15):5442–5453. [PubMed] [Google Scholar]
- 4.Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic [J] Mol Ther. 2009;17(2):219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell [J] J Orthop Surg Res. 2010;5:78. doi: 10.1186/1749-799X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe N, Gorlick R. High-dose methotrexate in osteosarcoma: let the questions surcease—time for final acceptance [J] J Clin Oncol. 2008;26(27):4365–4366. doi: 10.1200/JCO.2007.14.7793. [DOI] [PubMed] [Google Scholar]
- 7.Anderson P. Chemotherapy for osteosarcoma with high-dose methotrexate is effective and outpatient therapy is now possible [J] Nat Clin Pract Oncol. 2007;4(11):624–625. doi: 10.1038/ncponc0953. [DOI] [PubMed] [Google Scholar]
- 8.Yin JQ, Shen JN, Su WW, et al. Bufalin induces apoptosis in human osteosarcoma u-2os and u-2os methotrexate300- resistant cell lines [J] Acta Pharmacol Sin. 2007;28(5):712–720. doi: 10.1111/j.1745-7254.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 9.Zou CY, Wang J, Shen JN, et al. Establishment and characteristics of two syngeneic human osteosarcoma cell lines from primary tumor and skip metastases [J] Acta Pharmacol Sin. 2008;29(3):325–332. doi: 10.1111/j.1745-7254.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Zhong Z, Huang Y, et al. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells [J] J Biol Chem. 2010;285(7):4931–4940. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murase M, Kano M, Tsukahara T, et al. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas [J] Br J Cancer. 2009;101(8):1425–1432. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tirino V, Desiderio V, d'Aquino R, et al. Detection and characterization of CD133+ cancer stem cells in human solid tumours [J] PLoS One. 2008;3(10):e3459. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiezorek C. Cell cycle dependence of hoechst 33342 dye cytotoxicity on sorted living cells [J] Histochemistry. 1984;81(5):493–495. doi: 10.1007/BF00489756. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Dwarakanath BS, Mathew TL. DNA ligand hoechst- 33342 enhances uv induced cytotoxicity in human glioma cell lines [J] J Photochem Photobiol B. 2004;77(1–3):45–54. doi: 10.1016/j.jphotobiol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Chen AY, Yu C, Bodley A, et al. A new mammalian DNA topoisomerase I poison hoechst 33342: cytotoxicity and drug resistance in human cell cultures [J] Cancer Res. 1993;53(6):1332–1337. [PubMed] [Google Scholar]
- 16.Ishii H, Iwatsuki M, Ieta K, et al. Cancer stem cells and chemoradiation resistance [J] Cancer Sci. 2008;99(10):1871–1877. doi: 10.1111/j.1349-7006.2008.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Yao H, Zhu P, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells [J] Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Li HZ, Yi TB, Wu ZY. Suspension culture combined with chemotherapeutic agents for sorting of breast cancer stem cells [J] BMC Cancer. 2008;8:135. doi: 10.1186/1471-2407-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adhikari AS, Agarwal N, Wood BM, et al. CD117 and stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance [J] Cancer Res. 2010;70(11):4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clevers H. The cancer stem cell: premises, promises and challenges [J] Nat Med. 2011;17(3):313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]