Abstract
PARP is an important protein in DNA repair pathways especially the base excision repair (BER). BER is involved in DNA repair of single strand breaks (SSBs). If BER is impaired, inhibiting poly(ADP-ribose) polymerase (PARP), SSBs accumulate and become double stand breaks (DSBs). The cells with increasing number of DSBs become more dependent on other repair pathways, mainly the homologous recombination (HR) and the nonhomologous end joining. Patients with defective HR, like BRCA-deficient cell lines, are even more susceptible to impairment of the BER pathway. Inhibitors of PARP preferentially kill cancer cells in BRCA-mutation cancer cell lines over normal cells. Also, PARP inhibitors increase cytotoxicity by inhibiting repair in the presence of chemotherapies that induces SSBs. These two principles have been tested clinically. Over the last few years, excitement over this class of agents has escalated due to reported activity as single agent in BRCA1- or BRCA2-associated ovarian or breast cancers, and in combination with chemotherapy in triple negative breast cancer. This review covers the current results of clinical trials testing those two principles. It also evaluates future directions for the field of PARP inhibitor development.
Keywords: PARP, BRCA, PARP inhibitors
Poly(ADP-ribose) polymerase 1 (PARP1) is an important protein in the base excision repair (BER) pathway for DNA single strand breaks (SSBs). This makes PARP1 an interesting target for cancer therapy. Currently there are eight PARP inhibitors in clinical development testing two concepts. One applies the synthetic lethality principal and tests single agent PARP inhibitor in patients with deficient homologous recombination (HR) like BRCA-mutation tumors. The other concept is to compromise the cells ability to repair DNA damage caused by certain chemotherapies. The clinical data are just slowly maturing and hints of activity are seen in the BRCA-mutation cancers and triple negative breast cancer (TNBC). These early hints of activities have led to anticipatory excitement for this class of agents.
PARP
PARP
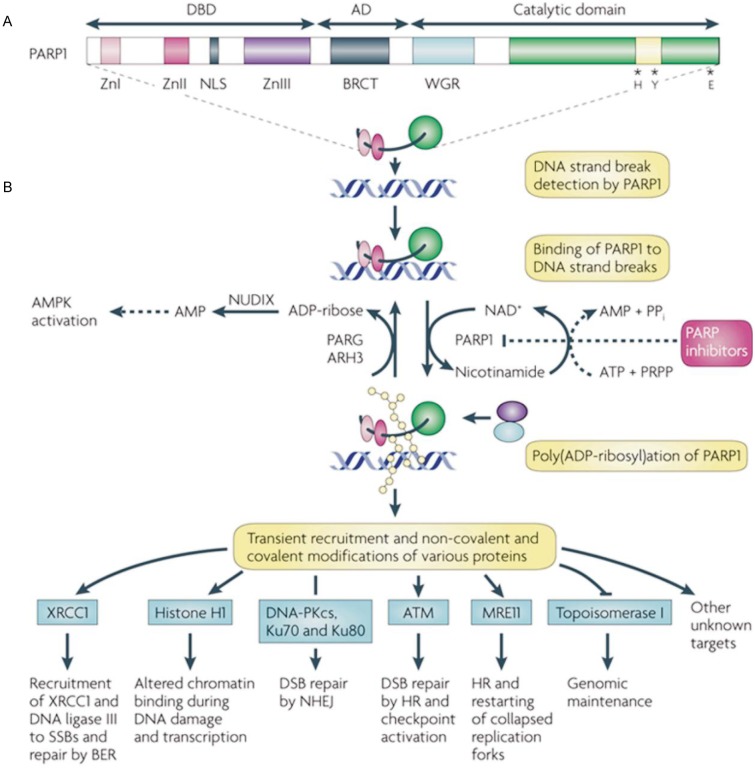
PARP is a family of proteins loosely based on structural similarity and function[1]. PARP proteins are composed of two ribose moieties and two phosphates per unit polymer. PARP1 and PARP2 are enzymes involved in a DNA repair pathway for SSBs called BER. The best-known PARP is PARP1 (Figure 1)[2]. This enzyme was first reported in 1963[6]. In 1980 Durkacz et al. [7] proposed that modulating PARP1 might augment the effect of alkylator chemotherapy. PARP1 detects and binds to sites of single strand DNA damage via the DNA-binding domain. It then synthesizes poly(ADP) ribose (PAR) and transfers it to acceptor proteins. PAR recruits other repair proteins to the damaged DNA site. In the case of extreme DNA damage, as with ischemia, PARP1 hyperactivation induces depletion of NAD+ and ATP, resulting in cell death by necrosis or apoptosis. PAR is involved in double strand breaks (DSBs) repair as well[8]. PAR recruits ATM, MRE11, and topoisomerase 1, which are involved in DSBs repair[9],[10].
Figure 1. Structural and functional characteristics of PARP1.
A, poly(ADP-ribose) polymerase 1 (PARP1) is shown with its DNA-binding (DBD), automodification (AD) and catalytic domains. The PARP signature sequence (yellow box within the catalytic domain) comprises the sequence most conserved among PARPs. Crucial residues for nicotinamide adenine dinucleotide (NAD+) binding (histidine; H and tyrosine; Y) and for polymerase activity (glutamic acid; E) are indicated. B, consequences of PARP1 activation by DNA damage. Although not shown to simplify the scheme, PARP1 is active in a homodimeric form[3],[4]. PARP1 detects DNA damage through its DBD. This activates PARP1 to synthesize poly(ADP) ribose (pADPr; yellow beads) on acceptor proteins, including histones and PARP1. Owing to the dense negative charge of pADPr, PARP1 loses affinity for DNA, allowing the recruitment of repair proteins by pADPr to the damaged DNA (blue and purple circles). Poly(ADP-ribose) glycohydrolase (PARG) and possibly ADP-ribose hydrolase 3 (ARH3) hydrolyse pADPr into ADP-ribose molecules and free pADPr. ADP-ribose is further metabolized by the pyrophosphohydrolase NUDIX enzymes into AMP, raising AMP:ATP ratios, which in turn activate the metabolic sensor AMP-activated protein kinase (AMPK). NAD+ is replenished by the enzymatic conversion of nicotinamide into NAD+ at the expense of phosphoribosylpyrophosphate (PRPP) and ATP. Examples of proteins non-covalently (pADPr-binding proteins) or covalently poly (ADP-ribosyl)ated are shown with the functional consequences of modification[5]. It is important to note that many potential protein acceptors of pADPr remain to be identified owing to the difficulty of purifying pADPr-binding proteins in vivo. PARP inhibitors prevent the synthesis of pADPr and hinder subsequent downstream repair processes, lengthening the lifetime of DNA lesions. ATM, ataxia telangiectasia-mutated; BER, base excision repair; BRCT, BRCA1 carboxy-terminal repeat motif; DNA-PKcs, DNA-protein kinase catalytic subunit; DSB, double-strand break; HR, homologous recombination; NHEJ, non-homologous end joining; NLS, nuclear localisation signal; PP,, inorganic pyrophosphate; SSB, single-strand break; Zn, zinc finger. Reprinted by permission from Macmillan Publishers Ltd: Nat Rev Cancer, 2010,10(4):293–301, copyright (2010).
Synthetic lethality
One of the rationales for using PARP inhibitors in oncology is synthetic lethality. Synthetic lethality is when two conditions that independently would not cause cell death applied in combination are lethal. In breast cancer susceptibility proteins 1 (BRCA 1) or BRCA2-mutation cell in which HR pathway is defective, the use of PARP inhibitors to hamper the BER causes cell damage and death[11],[12]. Other deficiencies in the HR pathway can also lead to cell death in the presence of PAPR inhibitors. Phosphatase and tensin homolog (PTEN) is a prevalent tumor suppressor gene involved in expression of RAD51 and important in the function of the HR. Another example of synthetic lethality is that PTEN-deficient cells are sensitive to PARP inhibitors in vitro and in vivo. Clinical trials are underway evaluating activity of PARP inhibitors in patients with decreased PTEN expression[13]. Disruption of the Fanconi Anemia (FA) pathway within HR makes cells more susceptible to synthetic lethality when exposed to agents that inhibit PARP[14]. This is currently being tested with veliparib, a PARP inhibitor.
There are two great advantages of exploiting synthetic lethality. The first is that only the tumor tissue will be targeted and thereby decreasing toxicity to the patient. Most people with BRCA mutations are heterozygous for the abnormality. Tumor, on the other hand, can be homozygous after a second hit occurs. Since only the tumor contains the homozygous pattern, resulting in defective HR, whereas the normal tissue carries the heterozygous and has intact HR, exposure to a PARP inhibitor would provide synthetic lethality selective for the tumor. Inhibiting PARP1 alone may be sufficient to cause tumor cell death and avoid the toxic effects of chemotherapy and radiation as shown in olaparib studies discuss below.
Inhibitors
All current PARP inhibitors in development are thought to inhibit both PARP1 and PARP2. Though nicotinamide's activity in inhibiting PARP was first noted in 1971[15], the exploration of this class of agent has recently become extensive[16],[17]. The first generation of inhibitors included nicotinamide analogues. In the 1980s, 3-aminobenzamide was reported to inhibit PARP but not considered as a selective agent, as it was not potent compared with newer inhibitors[3]. The second generation are more potent than 3-aminobenzamide. The third generation PARP inhibitors are the ones in current development and have greater potency and specificity for PARP than do inhibitors of prior generation (Table 1). These advantages allow for fewer off-target effects and greater efficacy.
Table 1. PARP inhibitors in clinical development.
| PARP inhibitor | Route of administration | Clinical development | Histology | Company |
| AG014699 | Intravenous | Phase II | Melanoma, breast cancer | Pfizer |
| Veliparib (ABT 888) | Oral | Phase II | Melanoma, breast cancer, glioblastoma, ovarian cancer | Abbott |
| Olaparib (AZ 2281, KU59436) | Oral | Phase II | Breast cancer, ovarian cancer, melanoma | AstraZeneca |
| Iniparib (BSI 201)/BSI 401 | Intravenous/oral | Phase III | Breast cancer, non–small cell lung cancer | Sanofi-Aventis/BiPar Sciences |
| MK4827 | Oral | Phase I | Ovarian cancer | Merck |
| CEP 9722 | Oral | Phase I | Cephalon | |
| BMN-673 | Oral | Phase I | Biomarin | |
| E7016 | Oral | Phase I | Eisai |
PARP inhibitors as single agents
PARP inhibitors killed BRCA2-deficient cells at doses that were nontoxic to normal cells in vitro and in xenograft models [11],[18]. BRCA2-deficient cells were 90 times more sensitive to PARP inhibition than to wild-type cells[19]. PARP inhibition was 3 times more potent than cisplatin cytotoxicity in BRCA-deficient cells. Ku0058684, a PARP inhibitor, inhibited tumor formation in mice injected with BRCA2-deficient but not normal cells[18]. p53 mutation does not interfere with the effect of PARP inhibitors[11],[20]. The potential of selectively targeting tumor cells without affecting the normal cells seemed possible in BRCA-associated tumors using single agent PARP inhibitor. This has been tested in various PARP inhibitors and reported with olaparib as discussed below.
PARP inhibitors in combination with cytotoxic therapy
Preclinically, PARP inhibitors have enhanced the effects of various chemotherapies. DNA methylating agents cause SSB that required BER. PARP1 elicited resistance to the effect of methylating agents, which is negated by the presence of a PARP inhibitor[21]. The accumulation of SSBs leads to DSBs and potentially overwhelms the HR pathway, resulting in cell death. Mismatch repair (MMR)–deficient cells do not respond well to the DNA methylating agent temozolomide (TMZ). In wild-type cells, MMR would either correct errors in replication or cause replication arrest or cell death; however, in MMR-deficient cells, abnormal DNA is not corrected and the cells survive[22]. 3-aminobenzamide augmented the efficacy of TMZ regardless of MMR status[23],[24]. AG14361, a PARP inhibitor and a sister agent to AG 14699, enhanced the effect of TMZ in MMR-deficient cells more than in MMR-proficient cells[25]. Veliparib in combination with TMZ significantly slowed tumor progression in an orthotopic rat glioma model over single agent TMZ[26]. When AG14361 was added to topoisomerase inhibitors, the lethal concentration (LC50) was significantly decreased and the resistance to topoisomerase inhibition was overcome in BER competent cells[27]. Resistance to camptothecins can develop due to overexpression of XRCC1, which is a protein involved in the repair of DNA SSBs formed by exposure to ionizing radiation and alkylating agents. PARP inhibition overcomes this resistance mechanism by interfering with XRCC1 recruitment to the DNA break site. In a mouse xenograft, veliparib enhanced the activity of cisplatin and carboplatin in a BRCA-mutated breast cell line[26]. Nicotinamide in combination with cisplatin increased the survival of a cisplatin-resistant ovarian cancer xenograft model[28]. CEP-6800 in a xenograft of non–small cell lung cancer showed enhancement of the cytotoxic effect of cisplatin [29]. In addition, cyclophosphamide is potentiated by veliparib[21],[26]. Preclinically, the evidence suggests tumors damaged by alkylating or methylating agents may be more sensitive to the chemotherapy in the presence of PARP inhibition.
PARP inhibitors in combination with ionizing radiation
PARP inhibitors potentiate ionizing radiation via inhibition of BER. DSBs accumulate in cells with defective PARP function after exposure to radiation. Radiation resistant cells treated with radiotherapy (XRT) after exposure to AG14361 experienced a 73% increase in tumor growth inhibition compared to treatment with XRT alone[30]. PARP inhibitors may also enhance XRT by impairment of NF-κB[8],[31]. In a mouse colon cancer xenograft, the addition of veliparib to irradiation extended survival from 23 to 36 days; notably, one mouse in this study experienced a complete response (CR)[26]. Given the preclinical evidence, PARP inhibitors are being tested with XRT and data should be available in the next few years.
Clinical Developments of PARP Inhibitors
AG014699 (PF01367338), Pfizer
AG014699, the phosphate salt of AG14361, was the first PARP inhibitor reported to have clinical activity. A phase I trial reported AG014699 in combination with temozolomide (TMZ) was tolerable. The PARP inhibitory dose (PID), defined as >50% decrease in PARP activity 24 h after dosing as evaluated in peripheral blood mononuclear cells (PBMCs), was determined to be 12 mg/m2. Dose-limiting toxicity (DLT) was not reached[32]. In a phase II trial, 40 patients with chemotherapy-naive advanced melanoma were treated with AG014699 at 12 mg/m2 plus TMZ at 200 mg/m2. Myelosuppression was greater than expected, with 5 cases (12.5%) of grade 4 thrombocytopenia, 6 cases (15.0%) of neutropenia, and 1 case (2.5%) of death from febrile neutropenia. Fatigue and nausea also occurred. There were 4 partial responses (PR) and 4 prolonged stable diseases (SD)[33]. This agent is given intravenously and potentially frequent administration limits its acceptance by patients. The other limiting factors are the enhancement of myelosuppression seen with TMZ. Despite those limitations, AG014699 are being further tested in combination with other agents, including carboplatin, carboplatin and paclitaxel, cisplatin and premetrexate, and epirubicin and cyclophosphamide[34].
Olaparib (AZD 2281, KU-0059436), AstraZeneca
Olaparib is an oral PARP inhibitor that has shown activity in ovarian and breast tumors with known BRCA mutations. A phase I study of single agent olaparib was conducted and included 60 patients, 22 of whom were positive for BRCA mutations[35]. In the expansion cohort, only patients with BRCA mutations were enrolled and treated at a continuous dosing schedule. A total of 50 ovarian cancer patients with BRCA mutations were enrolled. Twenty patients had CR or PR by response evaluation criteria in solid tumors (RECIST) and 3 patients had been SD for longer than 4 months, resulting in a clinical benefit rate of 46% (23/50). The median duration of response was 28 months. The most common drug-related toxicities were fatigue and mild gastrointestinal (GI) symptoms. A post ad hoc analysis showed a statistically significant difference in response among platinum-sensitive, -resistant, and -refractory populations (61%, 42%, and 15%, respectively), though no differences were noted in the duration of response or time to progression between the three platinum response groups[36]. These findings suggest that resistance to platinum decreases sensitivity to this PARP inhibitor.
In a phase II international trial in which 33 women with confirmed BRCA1 or BRCA2 mutation with recurrent ovarian cancer (ICEBERG 2) were enrolled, olaparib was given daily in a 28 day cycle at 400 mg twice per day, and a sequential cohort of 24 patients received 100 mg olaparib twice per day. The response rates by RECIST for the 400 mg and 100 mg doses were 33% and 12.5%, respectively. Two patients in the 400 mg cohort had CR. Toxicity was mild with only grade 3 nausea in 7% of patients and leucopenia in 5% of patients. This study implied that olaparib may have off-target effects since 100 mg inhibited PARP but was not as effective as a higher dose[37].
Kaye et al.[38] presented a randomized phase II trial including 97 patients with BRCA mutation–positive, platinum-resistant ovarian cancer treated with olaparib (200 mg or 400 mg, twice per day) or pegylated liposomal doxorubicin (PLD). The progression-free survival (PFS) was 6.5, 8.8, and 7.1 months for the 200 mg of olaparib, 400 mg of olaparib, and PDL arms, respectively. The primary objective of improving PFS was not reached due partly to a better PFS than expected in the PLD arm. Eight of 32 patients in the 200 mg olaparib arm, 10 of 31 in the 400 mg olaparib arm, and 6 of 33 in the PLD arm achieved PR. No difference in overall survival (OS) was evident at this time. Twice as many grade 3 or more severe toxicities were observed in the PLD arm. Though not achieving its primary objective, the study still shows consistent response and decreased toxicity with the use of single agent olaparib in the patients with BRCA-mutation ovarian cancer[38].
Gelmon et al.[39] reported response of high grade serous ovarian cancer (HGSOC) to olaparib at the 2010 ASCO Annual Meeting. Patients with HGSOC, regardless of BRCA status, were given olaparib at 400 mg twice per day continuously. Multiple biopsies were taken. Fifty-five patients prior to enrollment agreed to have BRCA testing as part of the study. PRs were seen in 14 (26.4%) of 53 patients. BRCA testing, which was undertaken after treatment, revealed that 7 patients with unknown BRCA status prior to enrollment had BRCA mutations with 43% (3/7) responses seen, whereas the remaining 46 patients negative for BRCA mutation had a response rate of 23.9%. The toxicity profiles were mild. Grade 3 toxicities were reported for fatigue, anemia, and diarrhea in more than one patient. This study showed PARP inhibitors has single agent activity outside of BRCA-mutation tumors.
Olaparib also showed activity in BRCA mutation– positive breast cancer. In a phase II trial of single agent olaparib, 54 patients with BRCA mutation–positive breast cancer were randomized to receive either 100 mg or 400 mg of olaparib twice per day. The overall response rate (ORR) was 22% and 41% and PFS was 3.8 and 5.7 months in the 100 mg and 400 mg cohorts, respectively. A median duration of response of ∼140 days was seen in both groups. Grade 3 or higher toxicities, including severe nausea, vomiting, and fatigue, were more prevalent in the higher dose cohort. This study again confirmed the activity seen in the phase I trial of olaparib as a single agent in the treatment of tumors with BRCA mutation[40]. Olaparib in combination with paclitaxel was also investigated in a phase I/II trial in TNBC. Nineteen patients, most of whom had received prior taxane therapy, were treated with daily 200 mg of olaparib given orally in combination with paclitaxel 90 mg/m2 intravenous drip weekly for 3 out of 4 weeks. Thirty-seven percent of patients had confirmed PRs. Given the prior taxane exposure, the use of olaparib may overcome resistance to taxane. Neutropenia despite growth factor support was the primary adverse event[41]. This study provided further evidence of PARP inhibitor activity in BRCA associated tumor.
Though olaparib has been well tolerated as single agent, one of the concerns regarding the addition of PARP inhibitors to chemotherapy is the potential of enhancing toxicity. The addition of olaparib to gemcitabine and cisplatin was not tolerable due to excessive myelosuppression. In this phase I trial, olaparib was given on days 1–4, cisplatin on day 3, and gemcitabine on days 3 and 10 every 3 weeks. DLTs were seen in 5 of 6 patients experiencing grade 3 or 4 thrombocytopenia. Despite de-escalation to dose level –1 (cisplatin of 50 mg/m2 and gemcitabine of 400 mg/m2), significant myelosuppression was still observed. Olaparib was further dose-reduced to being administrated only on day 1 and on this schedule, 2 of 6 patients still experienced grade 3 or 4 thrombocytopenia. One pancreatic cancer patient and 1 NSCLC patient experienced PR. The maximum tolerated dose (MTD) was 100 mg of olaparib on day 1 twice per day, cisplatin 60 mg/m2 on day 1 and gemcitabine of 500 mg/m2 on days 1 and 8 on a 21-day cycle[42]. Olaparib is also being combined with DNA damaging agents, such as topotecan, doxorubicin, carboplatin, carboplatin and paclitaxel, irinotecan, dacarbazine, and gemcitabine and cisplatin as well as with antiangiogenesis agents and as a single agent. The enhancement of myelosuppression in presence of PARP inhibitor and myelosuppressive chemotherapy is seen with other PARP inhibitors. A question that needs to be answered is whether the benefit of adding the PARP inhibitor to reduced dose chemotherapeutic agents outweighs that of the standard dose of the chemotherapeutic agents. This question will need to be addressed in future randomized trial.
Iniparib (BSI 201, NSC-746045; IND-71677), Sanofi-Aventis
Iniparib, also known as BSI 201, is a prodrug with a 4-min half-life. Data on its active metabolite are unknown at this time though a nitroso metabolite might be one of the active metabolites. Iniparib is given intravenously twice a week. It is the first PARP inhibitor to show survival advantage over patients with TNBC and has entered phase III trial.
The first phase I single agent trial included 23 patients with solid tumors. The patients were escalated through 7 dose levels up to 8 mg/kg without reaching a maximal tolerated dose. The 2.8 mg/kg dose caused PARP inhibition in PBMCs by more than 50% with the first dose of administration of BSI 201. Subsequent dosing increased the amount of PARP inhibition to more than 80%. SD was seen in 6 of 23 patients for at least 2 months (up to over 9 months in one patient). The adverse events were mostly GI symptoms. DLT was not observed[43].
In the combination phase I trial, patients with solid tumors were assigned to 1 of 4 combinations of iniparib, topotecan, gemcitabine, TMZ, or carboplatin with taxol. Assignment was based on physician preference. All regimens were reported to be well tolerated. There were no serious adverse events (SAEs) attributed to the drug. One ovarian cancer patient reached CR, lasting at least 6 months. Five patients with breast cancer, uterine cancer, renal cancer, or sarcoma reached PR[44]. The lack of enhancement of myelosuppression in combination with myelosuppressive chemotherapy led to suspicion that BSI 201 may not be a PARP inhibitor.
In a phase II study in TNBC, patients were randomized to gemcitabine with carboplatin group or gemcitabine with carboplatin and iniparib group. One hundred and sixteen patients were treated. Clinical benefit rate of 55.7% and 33.9% (P = 0.015), ORR of 52.5% and 32.5% (P = 0.023), median PFS of 5.9 months and 3.6 months (P = 0.012), and OS of 12.3 months and 7.7 months (P = 0.014) were observed in the iniparib combination arm and chemotherapy arm groups, respectively. No increase in adverse events was seen in the triple combination arm[45]. Though the regimen of gemcitabine and carboplatin was not a standard regimen used in treatment of TNBC, it has been gaining popularity due to this trial. A phase III trial evaluating this combination as first to third line treatment for metastatic TNBC has completed accrual. The Food and Drug Administration (FDA) recently approved an Expanded Access Protocol for iniparib in metastatic TNBC. Recently, Sanofi issued a press release announcing that adding iniparib to gemcitabine and carboplatin failed to provide any benefit for OS and PFS in the phase III trial. The phase II trial had led to excitement over the use of PARP inhibitors in TNBC. The details of the findings in the phase III trial will help guide future directions in the treatment of TNBC.
Veliparib (ABT888), Abbott Laboratories
In preclinical studies, veliparib was shown to be a potent inhibitor of PARP and was found to potentiate the effects of TMZ, platinum agents, cyclophosphamide, and radiation in syngeneic and xenograft tumor models. It was also reported to have good bioavailability and the capability to cross the blood-brain barrier[26].
The first phase 0 study performed under the FDA's new exploratory investigational new drug was conducted by the National Cancer Institute with veliparib. Veliparib was chosen because of its wide therapeutic index. A validated pharmacodynamic assay was developed for assessing PARP inhibition by measuring PAR. The pharmacokinetics and pharmacodynamics were evaluated over a short time period after a single dose of nontoxic veliparib (10, 25, and 50 mg, which were each tested in 3 patients). The target concentration based on PARP inhibition concentration in animals was exceeded even in the patients receiving the 10 mg dose. Statistically significant decreases in PAR were defined as 55% reduction in PBMCs and 95% in tumors. Significant reduction in PAR was seen at the 25 and 50 mg dose 3–6 h after dosing for both tumor and PBMC, respectively. Three additional subjects at the 50 mg dose underwent tumor biopsy around 24 h after the administration of veliparib. Even at 24 h after dosing, 49% reduction below baseline PAR level was reported[46].
There are multiple ongoing phase I and II trials with veliparib as single agent and in various chemotherapy combinations. A phase I trial of veliparib in combination with metronomic cyclophosphamide in patients with refractory solid tumors and lymphomas included 18 patients. PBMC PAR level reductions of >50% were seen in 16 of 18 patients. Two patients with tumor biopsy exhibited >95% reduction in PAR in the tumor. Two patients with BRCA2-mutation ovarian cancer and a single breast cancer patient achieved PR. A phase II randomized trial evaluating the role of veliparib in combination with oral cyclophosphamide in ovarian cancer patients with BRCA mutation or high-grade serous ovarian cancer, TNBC, or lymphoma have been initiated[47].
Veliparib in combination with TMZ was evaluated in metastatic breast cancer. Forty-one patients were treated with 40 mg oral veliparib, twice per day, on days 1–7 and TMZ at a dose of 150 mg/m2 on days 1–5 every 28 days. The initial schedule was revised due to higher than expected grade 4 thrombocytopenia. Veliparib was reduced to 30 mg on days 1–7. Fifteen patients had TNBC. Of 24 evaluable patients, 1 reached CR and 2 reached PR[48].
Like olaparib, veliparib enhances the myelosuppression of chemotherapeutic agents. When combined with topotecan, doses of veliparib and topotecan were significantly reduced due to myelosuppression. The original schedule was 1.2 mg/m2 topotecan at days 1–5 and 10 mg veliparib twice per day on days 1–7. The final schedule found to be tolerable was 0.6 mg/m2 topotecan and 10 mg veliparib twice per day on days 1–5. Six out of 10 patients at the higher dose levels had increases in tumor Υ H2AX levels with the addition of veliparib. There was also a correlation of γH2AX up-regulation with PARP inhibition[49].
MK4827, Merck
MK4827 is an oral inhibitor of PARP1 and PARP2. It is currently being tested in phase I trial as a single agent in advanced solid tumors, ovarian tumors, and prostate tumors, and as combination therapy with carboplatin, with or without paclitaxel, and carboplatin with liposomal doxorubicin in patients with advanced solid tumors. In a phase I trial of single agent MK4827 enriched with patients having BRCA1 or BRCA2 mutations, 6 patients, including 5 with BRCA mutation, achieved PR. The study excluded patients with prior PARP inhibitor exposure. PARP inhibition was shown in PBMC of patients treated with doses higher than 110 mg. The MTD was 300 mg once per day with thrombocytopenia being the DLT[50].
Resistance to PARP inhibitor
Resistance to PARP inhibitors has been reported. BRCA deficiency may be reversed by changes in the mutational reading frame, resulting instead in production of wild-type BRCA protein. These changes in the mutational reading frame of BRCA may potentially occur through a second mutation, compensatory mutations, or crossovers[51]. This may explain why not all BRCA-mutation tumors respond to PARP inhibitors. It may become necessary to check for restoration of the HR in patients with BRCA mutation–associated tumors. These patients should not be treated with single agent PARP inhibitor. Another mechanism hypothesized includes up-regulation of the p-glycoprotein efflux pump reducing intracellular PARP inhibitor concentrations[52]. In additional, resistance has been shown in tumors with increased tumor expression of PARP.
Future Directions
PARP inhibitors are an exciting new class of agents that have shown efficacy, especially for BRCA-related and high-grade serous ovarian cancer, and BRCA- mutation breast cancer and TNBC. There are many PARP inhibitors in development. They vary in administration route, toxicity profile, efficacy, and resistance mechanism. Currently, it is not clear if PARP inhibitors behave similarly in the clinic as few are studied in like conditions. To date, PARP inhibitors have shown activity as single agents as well as in combination with chemotherapy in the histologies above.
Tumors with certain DNA repair defects that lead to defective HR, based on synthetic lethality, are very sensitive to PARP inhibition. This has been shown Preclinically in BRCA deficient cells and now seen clinically with single agent studies using olaparib or MK4827 in BRCA mutation–associated breast and ovarian cancers. Studies are underway evaluating patients with other HR defects like PTEN and FA pathway defects. This will widen the therapeutic population in which PARP inhibitors may be of use. The search for biomarkers to identify tumors that are more likely to respond to PARP inhibitors and screening for resistance to PARP inhibitors are of great interest and ongoing. One possibility to screen for resistance is to measure PAR in PBMCs ex vivo or by genome analysis, which might select for patients with increased susceptibility to PARP inhibitor treatment. Studies are also underway to detect other genetic defects in the HR pathway to identify populations which may be more susceptible to PARP inhibitors.
Combination of PARP inhibitor with DNA damaging chemotherapeutic agents, especially those that cause SSBs, is currently in broad clinical testing. Various PARP inhibitors (Table 1) are being combined with chemotherapies including TMZ, topotecan, irinotecan, and carboplatin. Radiation is another area of interest since it is also depends on the BER for repair. In combination with chemotherapy, olaparib and veliparib enhance the myelosuppression of their chemotherapy partner, as observed with cisplatin/gemcitabine and topotecan, respectively. It is not clear if PARP inhibitors potentiate other toxicities, i.e., neuropathy or nephropathy, when in use with platinum and taxanes. This will become more evident as more clinical trials using these agents in combination with PARP inhibitors mature. The increase in myelosuppression had led to dose reduction of the chemotherapy partner(s). Randomized studies of reduced dose chemotherapy in combination with PARP inhibitor to standard dose chemotherapy will have to be tested to define the role of PARP inhibitor in this setting.
As this class of agents move into the (neo)adjuvant and prevention setting, development of secondary malignancy is a concern. PARP plays a role in monitoring DNA mutations, which occur daily. Long-term inhibition of PARP leading to continual inability to repair a normal process may lead to malignancy similar to a BRCA mutation. The late and prolonged effects of being treated with PARP inhibitors will become more evident in the setting of (neo)adjuvant and prevention trials. The complexity is knowing when there is an increase in the secondary malignancy and the number of patients put at risk to answer this question.
Overall, this is an exciting new class of agents with great potential for development. Maturation of current studies over the next few years will lead to a better understanding of the PARP inhibitors and define its role in the therapy of cancer.
References
- 1.Zaremba T, Curtin NJ. PARP inhibitor development for systemic cancer targeting [J] Anticancer Agents Med Chem. 2007;7(5):515–523. doi: 10.2174/187152007781668715. [DOI] [PubMed] [Google Scholar]
- 2.Rouleau M, Patel A, Hendzel MJ, et al. PARP inhibition: PARP1 and beyond [J] Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer PI, Buki KG, Hakam A, Kun E. Macromolecular association of ADP-ribosyltransferase and its correlation with enzymic activity [J] Biochem J. 1990;270:17–26. doi: 10.1042/bj2700017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza-Alvarez H, Alvarez-Gonzalez R. Poly (ADP-ribose) polymerase is a catalytic dimer and the automodification reaction is intermolecular [J] J Biol Chem. 1993;268:22575–22580. [PubMed] [Google Scholar]
- 5.Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases [J] Front Biosci. 2008;13:3046–3082. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- 6.Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme [J] Biochem Biophys Res Commun. 1963;11:39–43. doi: 10.1016/0006-291x(63)90024-x. [DOI] [PubMed] [Google Scholar]
- 7.Durkacz BW, Omidiji O, Gray DA, et al. (ADP-ribose)n participates in DNA excision repair [J] Nature. 1980;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Wu W, Rosidi B, et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways [J] Nucleic Acids Res. 2006;34(21):6170–6182. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschhorn R. In vivo reversion to normal of inherited mutations in humans [J] J Med Genet. 2003;40(10):721–728. doi: 10.1136/jmg.40.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haince JF, McDonald D, Rodrigue A, et al. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites [J] J Biol Chem. 2008;283(2):1197–1208. doi: 10.1074/jbc.M706734200. [DOI] [PubMed] [Google Scholar]
- 11.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumors with inhibitors of poly (ADP-ribose) polymerase [J] Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 12.Bryant HE, Petermann E, Schultz N, et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination [J] EMBO J. 2009;28(17):2601–2615. doi: 10.1038/emboj.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors [J] EMBO Mol Med. 2009;1(6–7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly (ADP-ribose) polymerase inhibition [J] Cancer Res. 2006;66(16):8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 15.Clark JB, Ferris GM, Pinder S. Inhibition of nuclear NAD nucleosidase and poly ADP-ribose polymerase activity from rat liver by nicotinamide and 5′-methyl nicotinamide [J] Biochim Biophys Acta. 1971;238(1):82–85. doi: 10.1016/0005-2787(71)90012-8. [DOI] [PubMed] [Google Scholar]
- 16.Zaremba T, Thomas H, Cole M, et al. Doxorubicin-induced suppression of poly (ADP-ribose) polymerase-1 (PARP-1) activity and expression and its implication for PARP inhibitors in clinical trials [J] Cancer Chemother Pharmacol. 2010;66(4):807–812. doi: 10.1007/s00280-010-1359-0. [DOI] [PubMed] [Google Scholar]
- 17.Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside [J] Ann Oncol. 2010;22(2):268–279. doi: 10.1093/annonc/mdq322. [DOI] [PubMed] [Google Scholar]
- 18.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy [J] Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 19.Evers B, Drost R, Schut E, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin [J] Clin Cancer Res. 2008;14(12):3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Gámez JA, Martín-Oliva D, Aguilar-Quesada R, et al. PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis [J] Biochem J. 2005;386(Pt 1):119–125. doi: 10.1042/BJ20040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tentori L, Graziani G. Chemopotentiation by PARP inhibitors in cancer therapy [J] Pharmacol Res. 2005;52(1):25–33. doi: 10.1016/j.phrs.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Jiricny J. The multifaceted mismatch-repair system [J] Nat Rev Mol Cell Biol. 2006;7(5):335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 23.Wedge SR, Porteus JK, May BL, et al. Potentiation of temozolomide and BCNU cytotoxicity by O(6)-benzylguanine: a comparative study in vitro [J] Br J Cancer. 1996;73(4):482–490. doi: 10.1038/bjc.1996.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Shi Y, Guan R, et al. Potentiation of temozolomide cytotoxicity by poly (ADP)ribose polymerase inhibitor ABT-888 requires a conversion of single-stranded DNA damages to double-stranded DNA breaks [J] Mol Cancer Res. 2008;6(10):1621–1629. doi: 10.1158/1541-7786.MCR-08-0240. [DOI] [PubMed] [Google Scholar]
- 25.Curtin NJ, Wang LZ, Yiakouvaki A, et al. Novel poly (ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells [J] Clin Cancer Res. 2004;10(3):881–889. doi: 10.1158/1078-0432.ccr-1144-3. [DOI] [PubMed] [Google Scholar]
- 26.Donawho CK, Luo Y, Penning TD, et al. ABT-888, an orally active poly (ADP–ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models [J] Clin Cancer Res. 2007;13(9):2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 27.Smith LM, Willmore E, Austin CA, et al. The novel poly(ADP-Ribose) polymerase inhibitor, AG14361, sensitizes cells to topoisomerase I poisons by increasing the persistence of DNA strand breaks [J] Clin Cancer Res. 2005;11(23):8449–8457. doi: 10.1158/1078-0432.CCR-05-1224. [DOI] [PubMed] [Google Scholar]
- 28.Bürkle A, Chen G, Küpper JH, et al. Increased poly (ADP-ribosyl)ation in intact cells by cisplatin treatment [J] Carcinogenesis. 1993;14(4):559–561. doi: 10.1093/carcin/14.4.559. [DOI] [PubMed] [Google Scholar]
- 29.Miknyoczki SJ, Jones-Bolin S, Pritchard S, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly (ADP-ribose) polymerase inhibitor [J] Mol Cancer Ther. 2003;2(4):371–382. [PubMed] [Google Scholar]
- 30.Calabrese CR, Almassy R, Barton S, et al. Anticancer chemosensitization and radiosensitization by the novel poly (ADP-ribose) polymerase-1 inhibitor AG14361 [J] J Natl Cancer Inst. 2004;96(1):56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 31.Boulton S, Kyle S, Durkacz BW. Interactive effects of inhibitors of poly (ADP-ribose) polymerase and DNA-dependent protein kinase on cellular responses to DNA damage [J] Carcinogenesis. 1999;20(2):199–203. doi: 10.1093/carcin/20.2.199. [DOI] [PubMed] [Google Scholar]
- 32.Plummer R, Jones C, Middleton M, et al. Phase I study of the poly (ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors [J] Clin Cancer Res. 2008;14(23):7917–7923. doi: 10.1158/1078-0432.CCR-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer R, Lorigan P, Evans J, et al. ASCO. Alexandria VA: American Society of Clinical Oncology; 2006. First and final report of a phase II study of the poly (ADP-ribose) polymerase (PARP) inhibitor, AG014699, in combination with temozolomide (TMZ) in patients with metastatic malignant melanoma (MM) [C] p. 8013. [Google Scholar]
- 34.2010. http://ClinicalTrials.gov. A study of poly (ADP-Ribose) polymerase inhibitor PF-01367338 in combination with several chemotherapeutic regimens.
- 35.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers [J] N Engl J Med. 2009;361(2):123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 36.Fong PC, Yap TA, Boss DS, et al. Poly (ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval [J] J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 37.Audeh MW, Carmichael J, Penson RT, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial [J] Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 38.Kaye S, Kaufman B, Lubinski J, et al. ESMO. Glascow, UK: Oxford University Press; 2010. Phase II study of the oral PARP inhibitor olaparib (AZD2281) versus liposomal doxorubicin in ovarian cancer patients with BRCA1 and/or BRCA2 mutations [C] p. 9710. [Google Scholar]
- 39.Gelman K, Hirte HW, Robidoux A, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2010. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple negative breast cancer [C] p. 3002. [Google Scholar]
- 40.Tutt A, Robson M, Garber JE, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial [J] Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 41.Rajan A, Kelly RJ, Gutierrez M, et al. TAT. Amsterdam, Netherlands: NDDO Education Foundation; 2010. A Phase I combination study of olaparib (AZD 2208; KU-0059436) and cisplatin plus gemcitabine in adults with solid tumors [C] p. 1. [Google Scholar]
- 42.Dent R, Lindeman GJ, Clemons M, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2010. Safety and efficacy of the oral PARP inhibitor olaparib (AZD2281) in combination with paclitaxel for the first- or second-line treatment of patients with metastatic triple-negative breast cancer: results from the safety cohort of a phase I/II multicenter trial [C] p. 1018. [Google Scholar]
- 43.Kopetz S, Mita MM, Mok I, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2008. First in human phase I study of BSI-201, a small molecule inhibitor of poly ADP-ribose polymerase (PARP) in subjects with advanced solid tumors [C] p. 3577. [Google Scholar]
- 44.Mahany J, Lewis N, Heath EI, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2008. A phase IB study evaluating BSI-201 in combination with chemotherapy in subjects with advanced solid tumors [C] p. 3579. [Google Scholar]
- 45.O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer [J] N Engl J Med. 2011;364(3):205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 46.Kummar S, Kinders R, Gutierrez ME, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies [J] J Clin Oncol. 2009;27(16):2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kummar S, Chen AP, Ji JJ, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2010. A phase I study of ABT-888 (A) in combination with metronomic cyclophosphamide (C) in adults with refractory solid tumors and lymphomas [C] p. 2605. [Google Scholar]
- 48.Isakoff J, Overmoyer B, Tung NM, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2010. Phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancerS [C] p. 1019. [Google Scholar]
- 49.Ji JJ, Kummar S, Chen AP, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2010. Pharmacodynamic response in phase I combination study of ABT-888 and topotecan in adults with refractory solid tumors and lymphomas [C] p. 2010. [Google Scholar]
- 50.Sandhu SK, Wenham RM, Wilding G, et al. ASCO. Alexandria, VA: American Society of Clinical Oncology; 2010. First-in-human trial of a poly(ADP-ribose) polymerase (PARP) inhibitor MK-4827 in advanced cancer patients (pts) with antitumor activity in BRCA-deficient and sporadic ovarian cancers [C] p. 2001. [Google Scholar]
- 51.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers [J] Nature. 2008;451(7182):1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum durgs [J] PROC Natl Acad Sci USA. 2008;105(44):17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]