Abstract
Oxaliplatin-based chemotherapy is used for treating gastric cancer. Autophagy has been extensively implicated in cancer cells; however, its function is not fully understood. Our study aimed to determine if oxaliplatin induce autophagy in gastric cancer MGC803 cells and to assess the effect of autophagy on apoptosis induced by oxaliplatin. MGC803 cells were cultured with oxaliplatin. Cell proliferation was measured using MTT assay, and apoptosis was determined by flow Cytometry. Protein expression was detected by Western blot. Autophagy was observed using fluorescent microscopy. Our results showed that the rate of apoptosis was 9.73% and 16.36% when MGC803 cells were treated with 5 and 20 µg/mL oxaliplatin for 24 h, respectively. In addition, Caspase activation and poly ADP-ribose polymerase (PARP) cleavage were detected. Furthermore, when MGC803 cells were treated with oxaliplatin for 24 h, an accumulation of punctate LC3 and an increase of LC3-II protein were also detected, indicating the activation of autophagy. Phosphorylation of Akt and mTOR were inhibited by oxaliplatin. Compared to oxaliplatin alone, the combination of autophagy inhibitor chlorochine and oxaliplatin significantly enhanced the inhibition of cell proliferation and the induction of cell apoptosis. In conclusion, oxaliplatin-induced protective autophagy partially prevents apoptosis in gastric cancer MGC803 cells. The combination of autophagy inhibitor and oxaliplatin may be a new therapeutic option for gastric cancer.
Keywords: Autophagy, oxaliplatin, gastric neoplasm, apoptosis
Autophagy, a major protein degradation pathway in eukaryotic cells, eliminates damaged cellular machineries, aged organelles, and unwanted macromolecules and recycles cellular components for reuse. In the process of autophagy, partial or whole cytoplasm or organelles are wrapped into bilayer membrane vesicles to form autophagosomes, which subsequently change into monolayer membrane vesicles and then fuse with lysozymes to complete content degradation. Additionally, harsh microenvironments beyond cell tolerance can also trigger autophagy to cause cell suicide, and up-regulated autophagy can lead to autophagic programmed cell death, also called programmed cell death type II. Therefore, the role of autophagy in cell homeostasis is indispensible[1].
Recent studies show that autophagy plays key roles in cancer treatment and is associated with cell apoptosis[2]. Furthermore, numerous chemotherapeutic drugs, including topotecan, cyclophosphamide, temozolomide, and gemcitabine, have been found to induce cellular autophagy[3],[4]. Gastric cancer is common in China, and most patients are diagnosed at advanced stage. In general, the median survival is less than 1 year, even when patients are treated with aggressive combination chemotherapy. In contrast to the conventional cisplatin-based ECF regimen, the EOX regimen with oxaliplatin, a third-generation platinum drug, can improve survival rate substantially. Hence, oxaliplatin has become an effective agent for treating gastric cancer[5],[6]. However, whether oxaliplatin can induce autophagy in gastric cancer cells has not been reported. PI3K/AKT and the downstream mammalian target of rapamycin (mTOR) play important roles in regulating cell proliferation, cell cycle, nutri tion-related signaling transduction, protein synthesis and turnover, and other processes. Moreover, mTOR is a key regulator for autophagy initiation[7]. However, it remains unknown whether the PI3K/AKT/mTOR pathway is involved in regulating cell apoptosis and autophagy in gastric cancer.
Whether chemotherapy-induced autophagy promotes or inhibits tumor cell death is unclear. A recent study indicated that autophagy was likely involved in chemo-resistance of cancer cells, as evidenced by the much higher autophagic activity found in cisplatin-resistant cancer cells than in drug-sensitive cancer cells[8]. Studies showed that 5-fluorouracil (5-FU) led to autophagic response in colon cancer cells, and inhibition of autophagy enhanced the efficacy of 5-FU[9],[10]. Additionally, cisplatin has also been reported to induce protective autophagy in esophageal cancer cells, which enabled them to escape from cisplatin-induced apoptosis[11]. Chloroquine (CQ), an autophagic inhibitor, can inhibit autophagy by blocking lysozyme-induced autophagosome degradation. CQ has been reported to enhance the efficacy of chemotherapeutic drugs[12]. Thus, CQ may play an important role in clarifying autophagy in gastric cancer.
Here, we determined that oxaliplatin induced autophagy in gastric cancer MGC803 cells, explored the role of the PI3K/AKT/mTOR pathway in the apoptosis and autophagy, and further clarified the role of oxaliplatin-induced autophagy in the apoptosis of gastric cancer cells using the combination of CQ and oxaliplatin.
Materials and Methods
Reagents
RPMI-1640 medium was purchased from Gibco. Fetal bovine serum (FBS) was from Tianjin Institute of Hematology. RNase A was purchased from AMRESCO. Propidium iodide (PI), methyl thiazolyl tetrazolium (MTT), and CQ were from Sigma. Hoechst33342 was from Invitrogen. Actin and Akt antibody were from Santa Cruz. Microtubule-associated protein light chain 3 (LC3), poly ADP-ribose polymerase (PARP), phospho-Akt (Ser-473), phospho-mTOR, and mTOR antibodies were from Cell Signaling.
Cell culture
Gastric cancer MGC803 cells were cultured in RPMI-1640 medium containing 10% heat-inactivated FBS and 12 U/mL gentamicin and maintained at 37°C in an incubator with saturated humidity and 50 mL/L CO2.
MTT assay
MGC803 cells seeded in 96-well plates with a final volume of 200 µL medium were treated with oxaliplatin and CQ alone or in combination in triplicate wells. After a 20-hour incubation, 25 µL MTT solution (5 mg/mL) was added to each well and the cells were then incubated for another 4 h before the supernatant was aspirated. The cells were lysed in 200 µL DMSO and mixed thoroughly. The absorbance (A) values were measured at 570 nm. The cell survival rate was calculated as follow: cell proliferation rate (%) = (average A value in treated group - average A value in blank group) / (average A value in control group - average A value in blank group) × 100%.
Detection of apoptosis
MGC803 cells were seeded in 6-well plates and then incubated with oxaliplatin and CQ alone or in combination for 24 h. Each sample was collected and fixed with 70% ethanol for 4 h. The samples were labeled with 10 µL PI (20 µg/µL) for 30 min in dark and were subsequently analyzed with FACS flow Cytometry. The WinMDI software was used for data analysis.
Western blotting
Each sample was collected and lysed in 200 µL RIPA buffer [0.1% SDS, 1% Triton-100, 150 mmol/L NaCl, 1 mmol/L EDTA (pH 8.0), 10 mmol/L Tris-HCI (pH 7.5)] supplemented with protease inhibitors (100 µg/mL PMSF, 2 µg/mL Aprotitin) at 4°C for 40 min. Cell lysates were centrifuged at 15 000 r/min for 20 min and aliquots of the supernatants were used to measure protein concentration by the Lowry method. Proteins were mixed with 3x sample buffer and boiled for 5 min. Proteins (50 µg/lane) were resolved by 12% SDS-polyacrylamide gel electrophoresis for 3 h and then transferred onto nitrocellulose membranes (at a voltage of 2 mV/cm2 for 40 min). The membranes were blocked with 5% skim milk for 2 h and then were cut to proper sizes for overnight antibody staining at 4°C. The next day, membranes were washed with Tris-buffered saline Tween 20 (TBST) buffer [10 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, and 0.1% Tween 20] buffer 4 times and stained with horseradish peroxidase-conjugated secondary antibody at room temperature for 30 min. The immunoreactive proteins were visualized and analyzed with the ECL method in the GIS gel image analysis system.
Fluorescence microscopy
To monitor the distribution of the green fluorescent protein-fused LC3 (GFP-LC3) in MGC803 cells, a stably transfected cell line was established by transfecting GFP-LC3 vector (kindly provided by Høyer-Hansen M, Danish Cancer Society) into MGC803 cells using Lipofectamine 2000 followed by selection with 200 µg/mL G418. The GFP-LC3 stable cells were then treated with oxaliplatin at desired concentrations for 24 h. The distribution of GFP-LC3 was observed under the microscope after Hoechst33342 nuclear staining.
Statistical analysis
All results were from 3 independent experiments and data are shown as mean ± standard deviation (SD). SPSS13.0 statistical software was used for statistical analysis. The t test was used for intergroup comparison and a P value of < 0.05 was considered significant.
Results
Oxaliplatin induces apoptosis in MGC803 cells
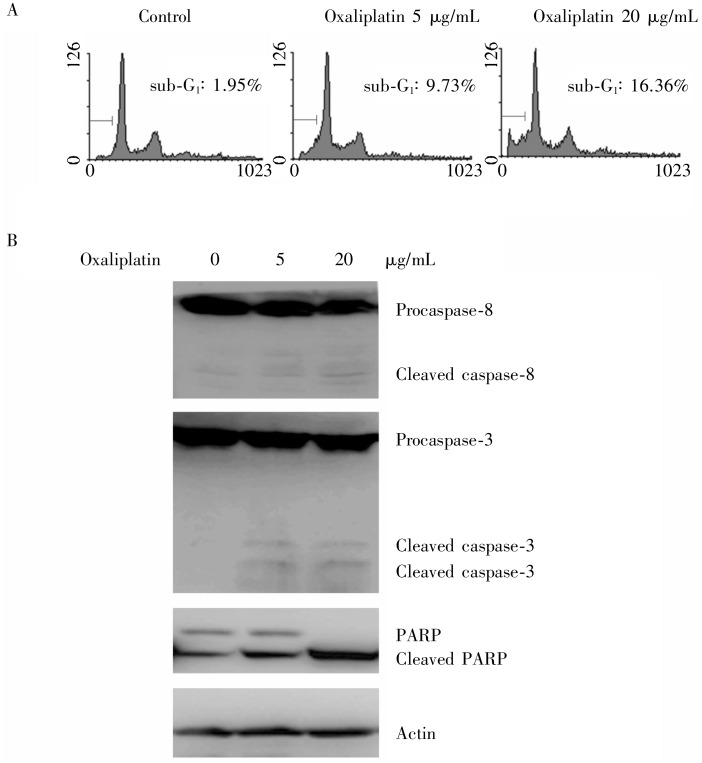
The MGC803 cells treated with oxaliplatin at 5 µg/mL and 20 µg/mL for 24 h had apoptosis rates of 9.73% and 16.36%, respectively (Figure 1A). When treated with 5 µg/mL oxaliplatin for 24 h, levels of procaspase-3 and procaspase-8 decreased, caspase-3, caspase-8, and PARP were cleaved in MGC803 cells. When treated with 20 µg/mL oxaliplatin, MGC803 cells showed markedly enhanced cleavage of caspase-3, caspase-8, and PARP (Figure 1B). Taken together, these results suggest that oxaliplatin induces apoptosis in MGC803 cells.
Figure 1. Oxaliplatin induces apoptosis in gastric cancer MGC803 cells.
MGC803 cells were exposed to 5 and 20 µg/mL oxaliplatin for 24 h. A, cell apoptosis was quantified with flow Cytometry. The rate of cell apoptosis is enhanced at increasing concentrations of oxaliplatin. B, the cleavage of caspase-8, caspase-3, and poly ADP-ribose polymerase (PARP) protein were detected by Western blotting, indicating cell apoptosis.
Oxaliplatin induces autophagy in MGC803 cells
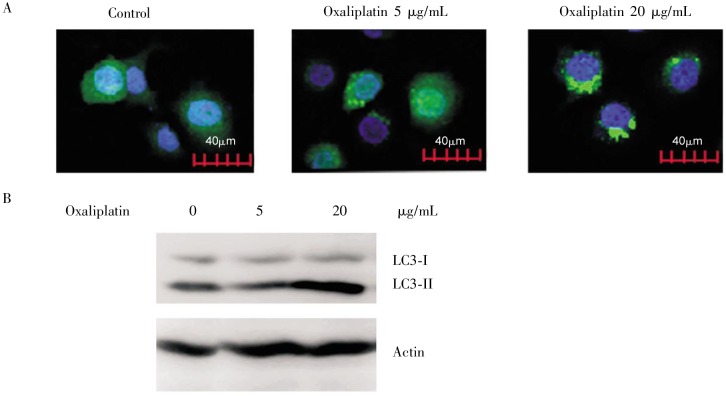
LC3 was accumulated in the stably transfected GFP-LC3 cells treated with 5 µg/mL oxaliplatin for 24 h, and the accumulation became more apparent when cells were treated with 20 µg/mL oxaliplatin (Figure 2A). Similarly, Western blot results also showed that protein expression of LC3-II was largely increased in the 20 µg/mL oxaliplatin-treated cells, whereas remained unchanged in the 5 µg/mL oxaliplatin-treated cells (Figure 2B). In summary, our results suggest that oxaliplatin induces autophagy in MGC803 cells.
Figure 2. Oxaliplatin induces autophagy in gastric cancer MGC803 cells.
MGC803 cells stably expressing green fluorescent protein-fused LC3 (GFP-LC3) were exposed to 5 and 20 µg/mL oxaliplatin for 24 h. A, dotted accumulation of LC3 was observed by fluorescence microscopy, suggesting the accumulation of autophagosomes. B, the increase of LC3-II protein was detected by Western blotting, indicating cell autophagy.
Oxaliplatin inhibits the PI3K/Akt/mTOR pathway
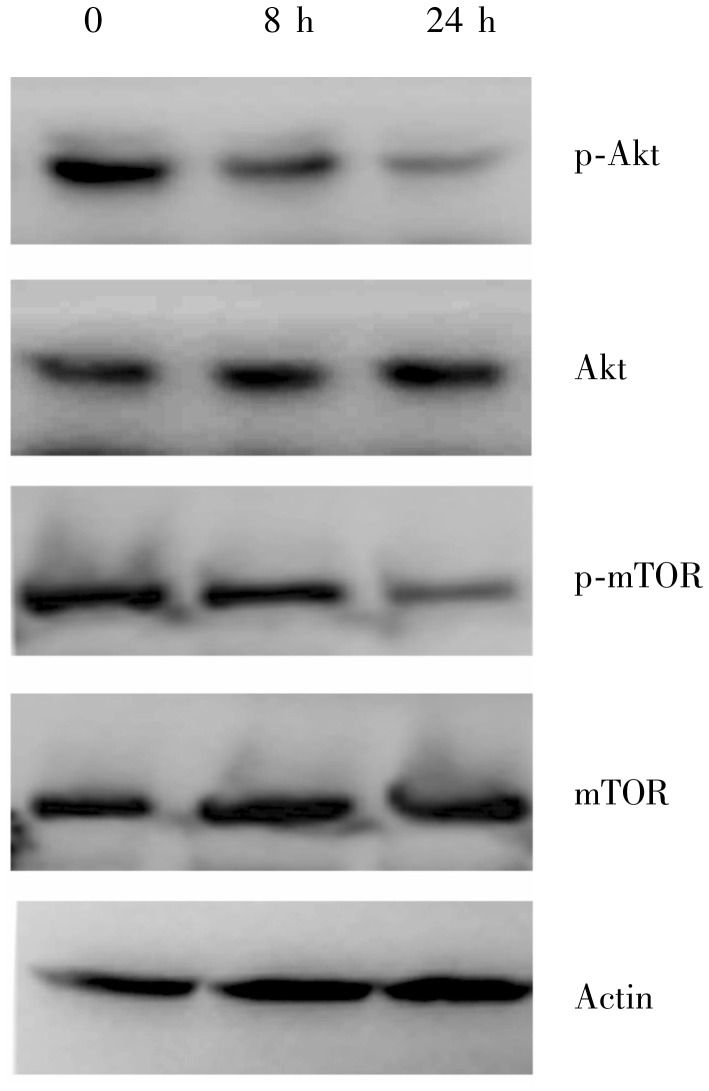
Compared to the control, both phosphorylated Akt and phosphorylated mTOR were slightly decreased in the MGC803 cells treated with 20 µg/mL oxaliplatin for 8 h (Figure 3). When oxaliplatin treatment was extended to 24 h, the phosphorylation of Akt and mTOR were further inhibited. The results suggest that oxaliplatin may induce apoptosis and autophagy by inhibiting the PI3K/Akt/mTOR pathway in MGC803 cells.
Figure 3. Oxaliplatin inhibits the phosphorylation of Akt and mammalian target of rapamycin (mTOR) in gastric cancer MGC803 cells.
MGC803 cells were exposed to 20 µg/mL oxaliplatin for 8 h and 24 h. Down-regulation of p-Akt and p-mTOR proteins was detected by Western blotting, suggesting that oxaliplatin inhibits the activation of Akt and mTOR.
Oxaliplatin-induced protective autophagy inhibits apoptosis in MGC803 cells
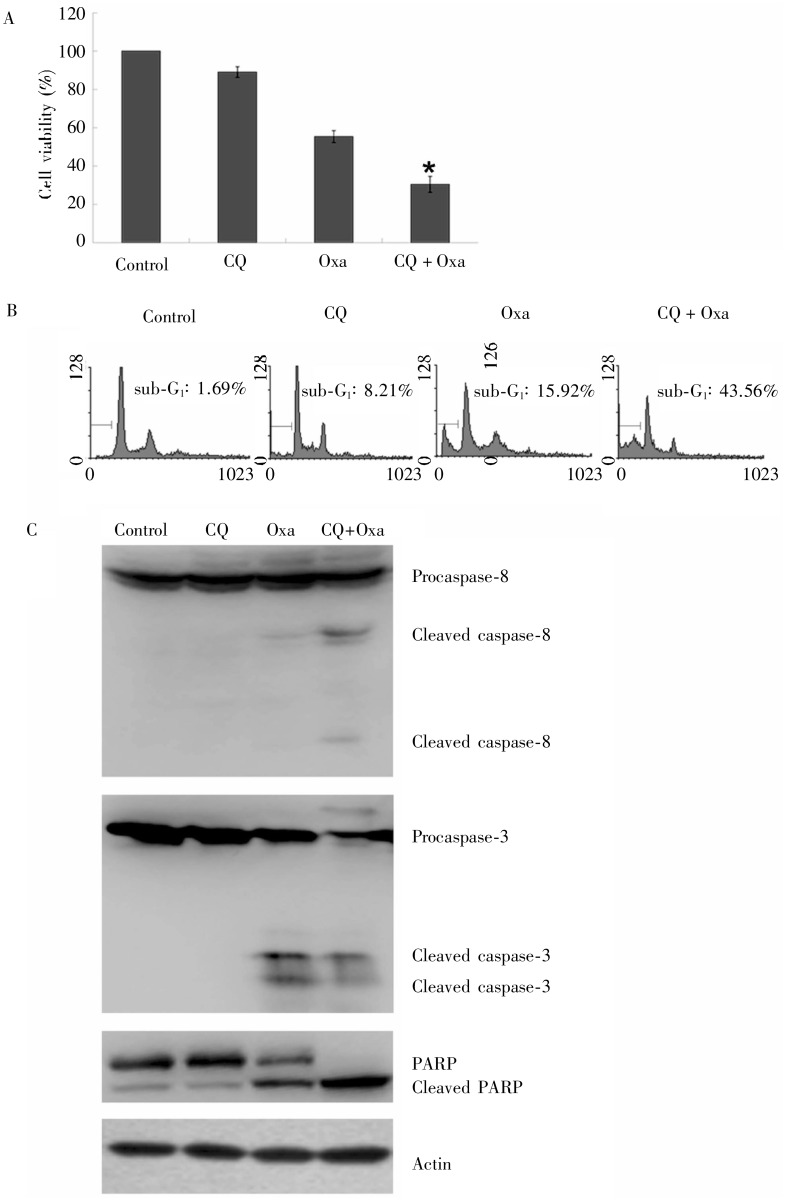
Compared with oxaliplatin alone, 20 µg/mL oxaliplatin and 20 µmol/L CQ significantly inhibited the proliferation of MGC803 cells at 24 h [(69.53 ± 4.17)% vs. (44.59 ± 3.09)%, P < 0.05] (Figure 4A), increased the apoptosis (43.56% vs. 15.92%, P < 0.05) (Figure 4B), and increased the cleavage of caspase-3, caspase-8, and PARP (Figure 4C). The results suggest that inhibiting protective autophagy enhances oxaliplatin-induced apoptosis in MGC803 cells.
Figure 4. The effect of oxaliplatin and chloroquine on survival and apoptosis in gastric cancer MGC803 cells.
MGC803 cells were exposed to 20 µg/mL oxaliplatin and 20 µmol/L chloroquine for 24 h. A, cell survival was determined by MTT assay. *Compared to oxaliplatin alone, the combination of oxaliplatin and chloroquine significantly inhibited cell survival (P < 0.05). B, cell apoptosis was quantified with flow Cytometry. Compared to oxaliplatin alone, the combination of oxaliplatin and chloroquine significantly enhanced cell apoptosis. C, the cleavage of caspase-8, caspase-3, and PARP protein was detected by Western blotting, indicating the increase of cell apoptosis. CQ, chloroquire; Oxa, oxaliplatin.
Discussion
The significance of oxaliplatin, a third-generation platinum drug, in treating gastric cancer has been increasingly recognized. Previously, we showed that oxaliplatin induced apoptosis in gastric cancer cells[13]. Oxaliplatin inhibited MGC803 cell proliferation by 50% at (23.44 ± 3.15) µg/mL (IC50) after 24 h. Therefore, we treated MGC803 cells with 5 and 20 µg/mL oxaliplatin. Here, we report that apoptosis, Caspase activation, and PARP cleavage were robustly enhanced in the oxaliplatin-treated MGC803 cells in a dose-dependent manner, suggesting that oxaliplatin induces apoptosis in MGC803 cells.
Recent studies showed that some chemotherapeutic drugs induce protective autophagy in cancer cells, thus allowing cancer cells to escape drug-induced apoptosis [14]. However, it remains unknown whether oxaliplatin can induce apoptosis and autophagy in gastric cancer cells simultaneously. In cancer cells, the autophagosome marker LC3 is processed to produce cytoplasmic LC3-I, which then undergoes ubiquitin-like modification and covalent attachment to phosphatidylethanolamine on autophagosome membranes to form LC3-II. Autophagic initiation can be determined by examining LC3-II levels or by visualizing LC3-expressing autophagosome punctate in cells based on the principle that the amount of LC3-II is proportional to the number of autophagosomes. Our study showed that oxaliplatin treatment enhanced autophagosome punctate and LC3-II protein expression, suggesting that autophagy was induced in MGC803 cells. Thus, oxaliplatin induces both cell apoptosis and autophagy in MGC803 cells.
The PI3K/Akt pathway plays a key role in cancer cell proliferation and apoptosis inhibition[15]. Akt activation leads to activation of downstream mTOR. Inhibiting mTOR, a critical factor for autophagy regulation, can induce autophagy[16]. We found that oxaliplatin slightly inhibited the phosphorylation of both Akt and mTOR in MGC803 cells after treatment for 8 h and significantly blocked their activation when the treatment time was extended to 24 h. These results suggest that oxaliplatin-induced apoptosis and autophagy may be due to inhibition of the PI3K/Akt/mTOR pathway in MGC803 cells.
Autophagy can either promote cell survival or cell death. Recent studies indicate that treatment with chemotherapeutic drugs can lead to protective autophagy that blocks apoptosis in cancer cells. 5-FU induced protective autophagy in colon cancer cells, and autophagy inhibitors enhanced cancer cell sensitivity to 5-FU[17]. Claerhout et al.[18] reported that autophagy inhibition enhanced cisplatin-induced apoptosis in skin cancer. CQ, a lysosome targeting drug, can block the final step of autophagy by altering the lysosomal pH, thereby affecting the lysosomal protein degradation[19]. Our results showed that CQ combined with oxaliplatin significantly inhibited proliferation and increased apoptosis in MGC803 cells, as evidenced by cleavage of caspase-3, caspase-8, and PARP. This result indicates that oxaliplatin-induced protective autophagy partially inhibits apoptosis of gastric cancer cells.
In summary, oxaliplatin can induce both apoptosis and protective autophagy in MGC803 cells. The autophagy inhibitor CQ can enhance oxaliplatin's inhibitory effect on cell proliferation and promote oxaliplatin-induced apoptosis in gastric cancer cells. We hypothesize that CQ could serve as a novel chemotherapeutic sensitizer, which may lead to a new treatment approach for gastric cancer in clinics. Nevertheless, further study of CQ is essential.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 30770993); Specialized Research Fund for the Doctoral Program of Higher Education (No. 20102104120008); Fund for Scientific Research of the First Hospital of China Medical University (No. fsfh1003); Scientific Research Foundation for Doctors in Liaoning Province (No. 20101146).
References
- 1.Mizushima N. Autophagy: process and function [J] Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 2.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy [J] Curt Opin Cell Biol. 2004;16(6):663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Li DD, Wang LL, Deng R, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells [J] Oncogene. 2009;28(6):886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 4.Oehadian A, Koide N, Hassan F, et al. Differential expression of autophagy in Hodgkin lymphoma cells treated with various anti-cancer drugs [J] Acta Med Indones. 2007;39(4):153–156. [PubMed] [Google Scholar]
- 5.Sumpter K, Harper-Wynne C, Cunningham D, et al. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF [J] Br J Cancer. 2005;92(11):1976–1983. doi: 10.1038/sj.bjc.6602572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer [J] N Engl J Med. 2008;358(1):36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 7.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma [J] J Clin Invest. 2007;117(2):326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren JH, He WS, Nong L, et al. Acquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagy [J] Cancer Biother Radiopharm. 2010;25(1):75–80. doi: 10.1089/cbr.2009.0701. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Hou N, Faried A, et al. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model [J] Eur J Cancer. 2010;46(10):1900–1909. doi: 10.1016/j.ejca.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Hou N, Faried A, et al. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells [J] Ann Surg Oncol. 2009;16(3):761–771. doi: 10.1245/s10434-008-0260-0. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, Yang Y, Liu Q, et al. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells [J] Med Oncol. 2011;28(1):105–111. doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- 12.Brech A, Ahlquist T, Lothe R A, et al. Autophagy in tumor suppression and promotion [J] Mol Oncol. 2009;3(4):366–375. doi: 10.1016/j.molonc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Qu X, Zhang Y, et al. Oxaliplatin enhances TRAIL-induced apoptosis in gastric cancer cells by CBL-regulated death receptor redistribution in lipid rafts [J] FEBS Lett. 2009;583(5):943–948. doi: 10.1016/j.febslet.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki K, Tsuno NH, Sunami E, et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells [J] BMC Cancer. 2010;10:370. doi: 10.1186/1471-2407-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT—a major therapeutic target [J] Biochim Biophys Acta. 2004;1697(1–2):3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Fu L, Kim YA, Wang X, et al. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy [J] Cancer Res. 2009;69(23):8967–8976. doi: 10.1158/0008-5472.CAN-09-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bijnsdorp IV, Peters GJ, Temmink OH, et al. Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells [J] Int J Cancer. 2010;126(10):2457–2468. doi: 10.1002/ijc.24943. [DOI] [PubMed] [Google Scholar]
- 18.Claerhout S, Verschooten L, Van Kelst S, et al. Concomitant inhibition of AKT and autophagy is required for efficient cisplatin-induced apoptosis of metastatic skin carcinoma [J] Int J Cancer. 2010;127(12):2790–2803. doi: 10.1002/ijc.25300. [DOI] [PubMed] [Google Scholar]
- 19.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies [J] Eur J Pharmacol. 2009;625(1–3):220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]