Abstract
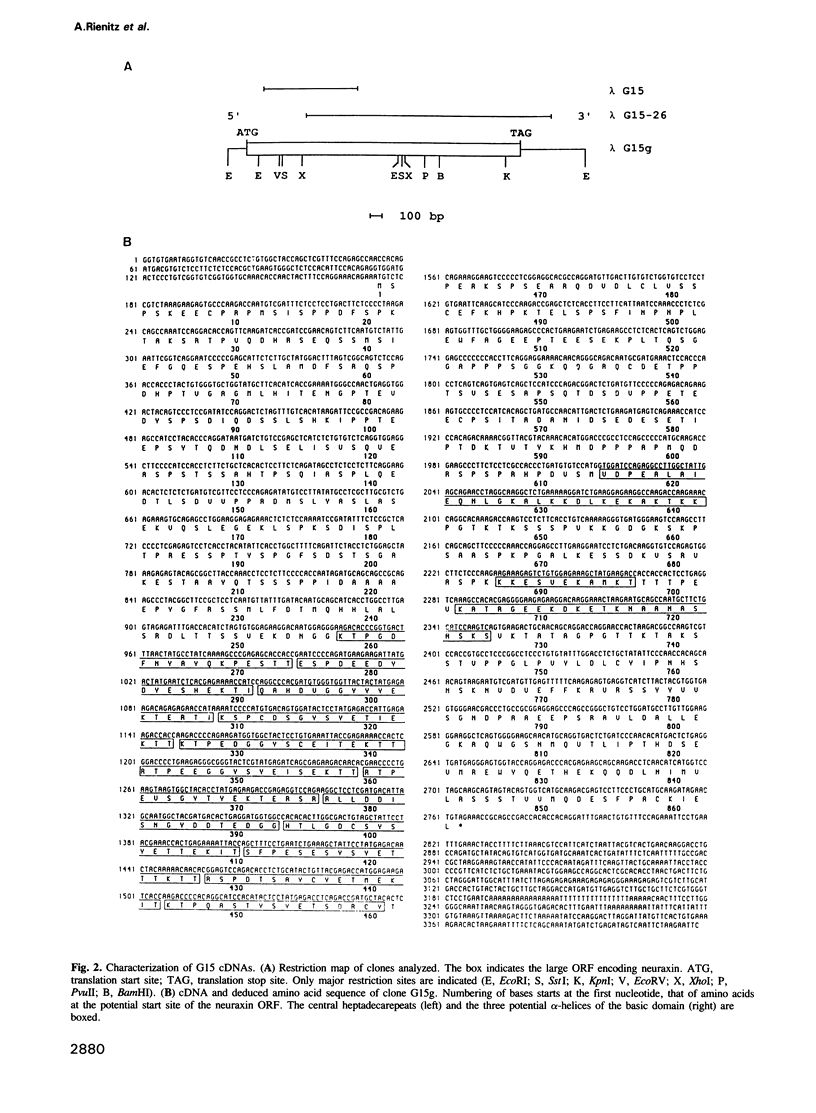
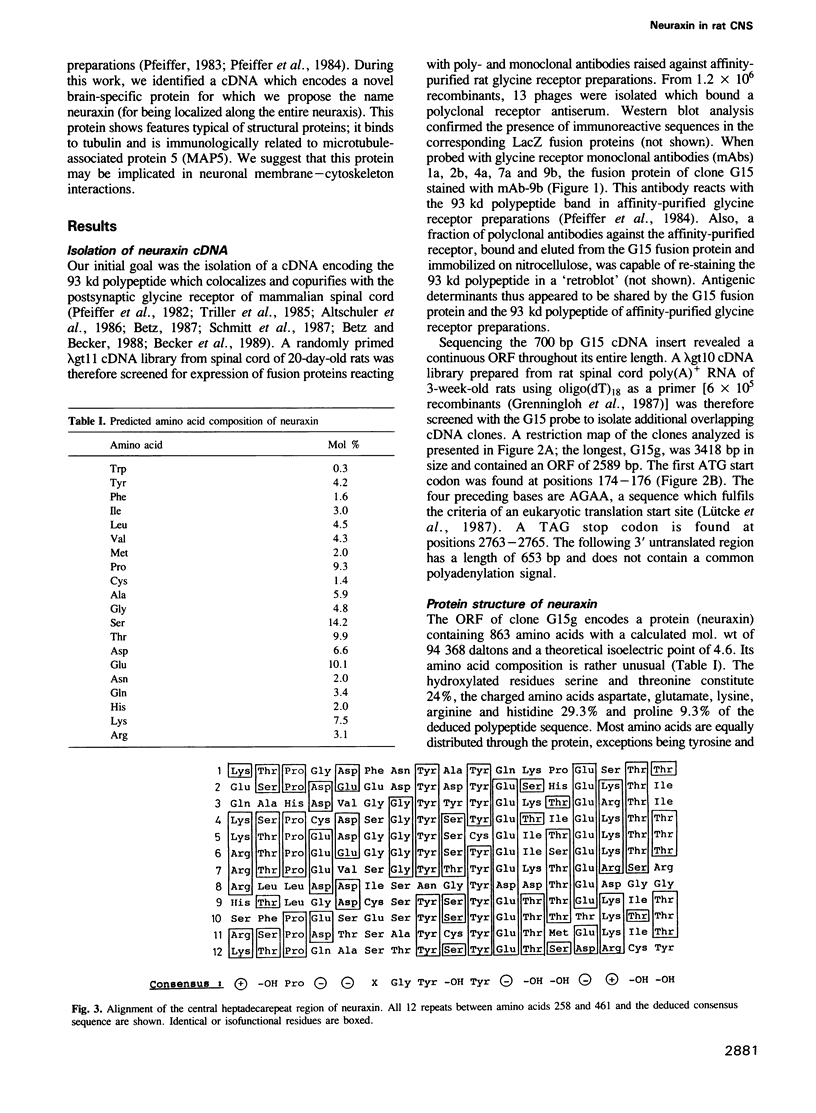
During screening of a rat spinal cord lambda gt11 cDNA library with poly- and monoclonal antibodies against the postsynaptic glycine receptor a cDNA was isolated which covers an open reading frame encoding a protein of calculated mol. wt 94 kd. Sequence analysis identified a novel type of neuron-specific protein (named neuraxin) which is characterized by an unusual amino acid composition, 12 central heptadecarepeats and putative protein and/or membrane interaction sites. The gene encoding neuraxin appears to be unique in the haploid rat genome and conserved in higher vertebrates. Northern blot and in situ hybridization revealed neuraxin mRNA to be expressed throughout the rodent central nervous system (CNS). In spinal cord, neuraxin transcripts were abundant in motoneurons which also expressed glycine receptor subunit mRNA. A bacterial fusion protein containing approximately 90% of the neuraxin sequence was found to specifically bind tubulin. Polyclonal neuraxin antibodies cross-reacted with microtubule-associated protein 5 (MAP5), and a monoclonal antibody against MAP5 recognized the neuraxin fusion construct. Based on these data we suggest that neuraxin is related to MAP5 and may be implicated in neuronal membrane-microtubule interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuler R. A., Betz H., Parakkal M. H., Reeks K. A., Wenthold R. J. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986 Mar 26;369(1-2):316–320. doi: 10.1016/0006-8993(86)90542-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988 Dec 1;7(12):3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Sensitive immunoassay shows selective association of peripheral and integral membrane proteins of the inhibitory glycine receptor complex. J Neurochem. 1989 Jul;53(1):124–131. doi: 10.1111/j.1471-4159.1989.tb07303.x. [DOI] [PubMed] [Google Scholar]
- Birkenmeier C. S., Bodine D. M., Repasky E. A., Helfman D. M., Hughes S. H., Barker J. E. Remarkable homology among the internal repeats of erythroid and nonerythroid spectrin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5671–5675. doi: 10.1073/pnas.82.17.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Burden S. J., DePalma R. L., Gottesman G. S. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983 Dec;35(3 Pt 2):687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Sobel A., Rousselet A., Devaux P. F., Changeux J. P. Consequences of alkaline treatment for the ultrastructure of the acetylcholine-receptor-rich membranes from Torpedo marmorata electric organ. J Cell Biol. 1981 Aug;90(2):418–426. doi: 10.1083/jcb.90.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frail D. E., Mudd J., Shah V., Carr C., Cohen J. B., Merlie J. P. cDNAs for the postsynaptic 43-kDa protein of Torpedo electric organ encode two proteins with different carboxyl termini. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6302–6306. doi: 10.1073/pnas.84.17.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. C., Matus A. Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol. 1988 Mar;106(3):779–783. doi: 10.1083/jcb.106.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. The alpha-subunit of tubulin is preferentially associated with brain presynaptic membrnae. FEBS Lett. 1979 Mar 1;99(1):86–90. doi: 10.1016/0014-5793(79)80255-0. [DOI] [PubMed] [Google Scholar]
- Green N. M., Wrigley N. G., Russell W. C., Martin S. R., McLachlan A. D. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983;2(8):1357–1365. doi: 10.1002/j.1460-2075.1983.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Scarmato P., Harrison S. C., Monroe J. J., Chow E. P., Mattaliano R. J., Ramachandran K. L., Smart J. E., Ahn A. H., Brosius J. Clathrin light chains LCA and LCB are similar, polymorphic, and share repeated heptad motifs. Science. 1987 Apr 17;236(4799):320–324. doi: 10.1126/science.3563513. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins: their potential role in determining neuronal morphology. Annu Rev Neurosci. 1988;11:29–44. doi: 10.1146/annurev.ne.11.030188.000333. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Microtubule-associated proteins. Annu Rev Cell Biol. 1986;2:421–457. doi: 10.1146/annurev.cb.02.110186.002225. [DOI] [PubMed] [Google Scholar]
- Peng I., Binder L. I., Black M. M. Biochemical and immunological analyses of cytoskeletal domains of neurons. J Cell Biol. 1986 Jan;102(1):252–262. doi: 10.1083/jcb.102.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
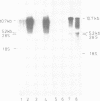
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Pfeiffer F., Simler R., Grenningloh G., Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer B., Cohen R., Matus A. MAP5: a novel brain microtubule-associated protein under strong developmental regulation. J Neurocytol. 1986 Dec;15(6):763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
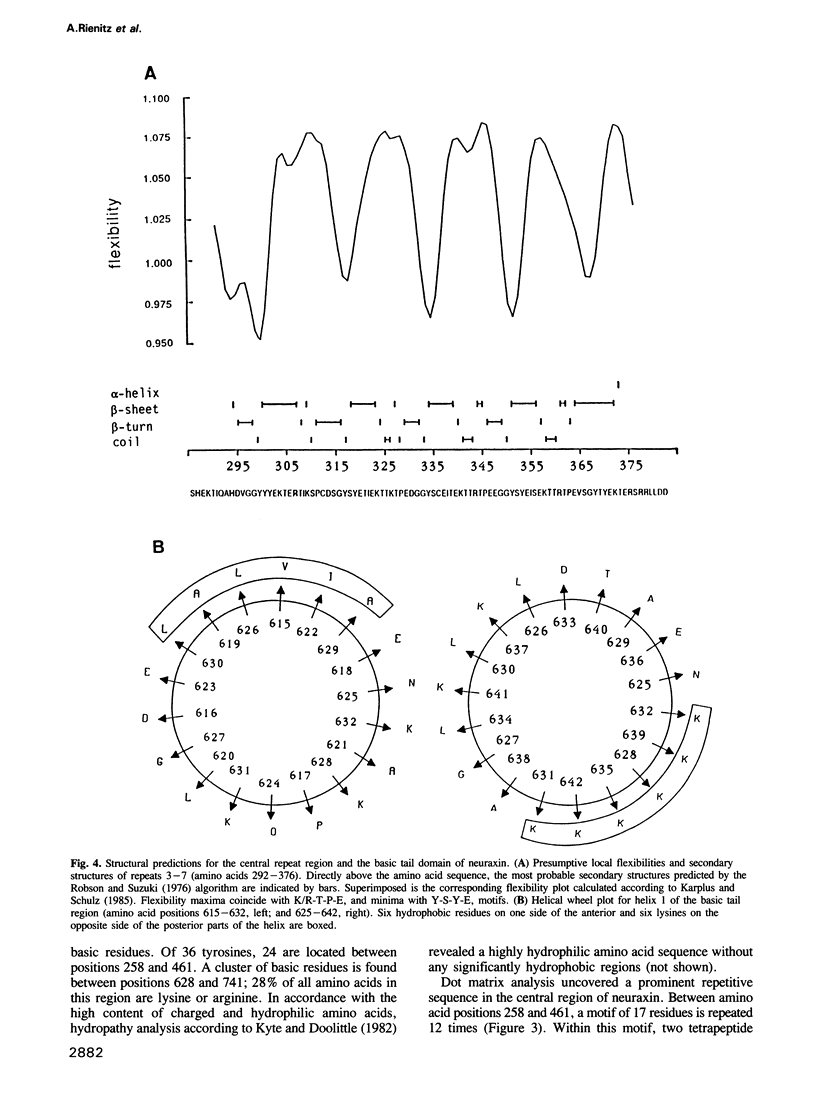
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P., Hermans-Borgmeyer I., Betz H., Gundelfinger E. D. Neuronal acetylcholine receptors in Drosophila: the ARD protein is a component of a high-affinity alpha-bungarotoxin binding complex. EMBO J. 1988 Sep;7(9):2889–2894. doi: 10.1002/j.1460-2075.1988.tb03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt B., Knaus P., Becker C. M., Betz H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987 Feb 10;26(3):805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y., Elmer L., Davis J., Bennett V., Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988 May 12;333(6169):177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Pfeiffer F., Betz H., Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J Cell Biol. 1985 Aug;101(2):683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten G. F., Kopin I. J., Axelrod J. Effects of colchicine and vinblastine on axonal transport and transmitter release in sympathetic nerves. Ann N Y Acad Sci. 1975 Jun 30;253:528–534. doi: 10.1111/j.1749-6632.1975.tb19226.x. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D., Hermans-Borgmeyer I., Gundelfinger E. D., Betz H. Identification of gene products expressed in the developing chick visual system: characterization of a middle-molecular-weight neurofilament cDNA. Genes Dev. 1987 Sep;1(7):699–708. doi: 10.1101/gad.1.7.699. [DOI] [PubMed] [Google Scholar]