Abstract
The hypothalamic-pituitary-adrenal (HPA) axis modulates individuals’ physiological responses to social stress, which is an inevitable aspect of the daily lives of group-living animals. Previous nonhuman primate studies have reported that sex, age, rank and reproductive condition influence cortisol levels under stressful conditions. In this study we investigated cortisol responses to stress among 70 multiparous, free-ranging female rhesus macaques (Macaca mulatta) on the island of Cayo Santiago, PR. Plasma cortisol samples were collected in two consecutive years under similar conditions. Twenty-two females were sampled both years, and most of those females were lactating in only one of the years. Individual differences in cortisol levels were stable across years, even though reproductive condition changed for most individuals. No relationship was found between age or social rank and cortisol levels. Of the females that changed reproductive conditions, cortisol levels were higher when they were lactating than when they were cycling, and the amount of change in cortisol from cycling to lactating was greatest for low-ranking individuals. Heightened reactivity to stress during lactation may be the result of concerns about infant safety, and such concerns may be higher among low-ranking mothers than among higher ranking mothers. Psychosocial stress and hyperactivation of the HPA axis during lactation can suppress immune function and increase vulnerability to infectious diseases, thus explaining why adult females in the free-ranging rhesus macaque population on Cayo Santiago have a higher probability of mortality during the birth season than during the mating season.
Keywords: reproductive condition, cortisol, rank, rhesus macaques
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis plays an important role in modulating an organism's responses to perturbations of the environment. Social stress is an inevitable aspect of the daily lives of group-living animals, particularly for animals such as many nonhuman primates that live in complex societies. Understanding interindividual variation in HPA axis function in group-living primates can enhance our understanding of how individuals and their behavior are affected by their social environment and the extent to which their adjustments to environmental challenges are adaptive or maladaptive.
In nonhuman primates, the activity of the HPA axis is typically assessed by measuring concentrations of cortisol in blood or feces under baseline conditions or in response to stress. A large number of studies have demonstrated that there are significant differences in basal or stress cortisol levels among individuals living in the same group and that these individual differences are stable over time, at least in the laboratory [e.g. Higley et al., 1992a; Bercovitch and Clarke, 1995; Cavigelli et al., 2003; Crockford et al., 2008; see below]. Sex, dominance rank, age, and female reproductive condition are variables that have been shown to affect variation in cortisol levels in nonhuman primates. Although males and females generally have similar cortisol levels under basal conditions, there is some evidence that females exhibit greater cortisol responses to stress [e.g., Meyer and Bowman, 1972; Scallet et al., 1981; but see Higley et al., 1992b, for a lack of significant sex differences] and pharmacological challenges [ACTH challenge: Bowman and Wolf, 1969; CRH challenge: Sanchez et al., in press] than males.
The relation between dominance rank and cortisol level is complex, as some primate studies have reported higher cortisol levels in low-ranking individuals, whereas others have reported higher cortisol levels in high-ranking individuals [see Abbott et al., 2003 for review]. In cercopithecine monkeys such as rhesus macaques and baboons, dominance ranks are stable for females but can be very unstable for males [Melnick and Pearl, 1987]. In these species, studies have generally reported higher cortisol levels in low-ranking than in high-ranking females [e.g. Gust et al., 1993; Maestripieri et al., 2008], whereas the relation between cortisol and rank in males is more variable and different in periods of rank stability and instability [Sapolsky, 1992; Bercovitch and Clarke, 1995].
Reports on the effects of age on cortisol in nonhuman primates are mixed. Gust et al. [2000] reported that the basal cortisol levels of rhesus macaques 15 years and older were higher at the evening nadir but lower at the morning zenith than the cortisol levels of younger females, suggesting that aging flattens circadian rhythm in circulating cortisol. The authors found no age-related differences in cortisol response to a social stressor. Goncharova and Lapin [2002] reported evidence for adrenal cortex hyperreactivity in aging monkeys; furthermore, it took old monkeys longer to return to basal cortisol levels following ACTH administration.
The relation between female reproductive condition and cortisol levels has been poorly investigated in nonhuman primates. Some studies of rodents and humans have shown that lactation is accompanied by reduced HPA axis reactivity to stress, suggesting that breastfeeding has anxiolytic properties, possibly mediated by oxytocin [Neumann et al., 2000]. Maestripieri et al. [2008], however, reported that among a population of free-ranging rhesus macaques, lactating females had higher cortisol levels in response to stress than did cycling females, suggesting that in this primate species, motherhood may be associated with heightened emotional reactivity and anxiety, possibly because of concerns about infant safety [see also Maestripieri, 1993a; 1993b]. The higher cortisol responses to stress exhibited by lactating females were unlikely to be the result of higher basal cortisol levels because basal cortisol levels return to pre-pregnancy baseline levels within one week following parturition [Taylor et al., 1994; McLean and Smith, 1999; Mastorakos and Ilias, 2003], and no lactating females in the study gave birth during the week that preceded plasma collection.
Studies in which HPA axis function is repeatedly assessed over long periods of time are rare in free-ranging nonhuman primates. The only relevant studies have been conducted in rhesus monkeys reared and/or housed under artificial laboratory conditions. For example, among rhesus monkeys separated from their mothers after birth and housed in pairs or small groups in cages, individual differences in cortisol levels in response to mild stress were relatively stable from infancy through the juvenile years [Suomi, 1983; Higley et al., 1992b; Davenport et al., 2003; see also Erwin et al., 2004]. Temporal consistency was also evident among a population of singly-housed, adult male rhesus macaques when tested under both basal and restraint conditions [Capitanio et al., 1998; 2004]. Given that relevant data from free-ranging monkeys are lacking, we do not yet know whether the stability in individual differences in cortisol levels observed in the laboratory also would occur in individuals living in a complex social and ecological environment.
In this study we investigated cortisol responses to stress (capture and individual housing) among multiparous female rhesus macaques in the free-ranging population on the island of Cayo Santiago, PR. Data were collected in two consecutive years under very similar conditions. Some of our subjects were sampled both years, whereas others were sampled in one year but not in the other. Of the subjects sampled both years, some of them were sampled in different reproductive conditions, i.e., they were cycling one year and lactating the other year.
We tested the following hypotheses: 1) individual differences in cortisol responses to stress would be stable across the two years, despite changes in reproductive condition for some females; 2) younger individuals would have the lowest cortisol levels but middle- and old-aged individuals may not differ in their cortisol; 3) cortisol responses to stress would be lowest in high-ranking females and highest in low-ranking females; and 4) females that were sampled twice and in different reproductive conditions would have higher cortisol levels when lactating than when cycling. With the latter hypothesis, we aimed to replicate, using a within-subjects analysis, our previous finding obtained with a between-subjects comparison that lactation is associated with increased cortisol responses to stress in female rhesus macaques.
Methods
Subjects
This study was conducted with the free-ranging population of rhesus macaques on Cayo Santiago, a 15.2 ha island located 1 km off the southeastern coast of Puerto Rico. This colony was established in 1938, with free-ranging monkeys captured in India [Rawlins and Kessler, 1986]. Since then, no new individuals have been introduced into the population, except through births. To maintain a stable population size, a fraction of the yearlings and two-year olds are culled each year. During the study period, the population included approximately 850 animals distributed among 6 naturally formed social groups. Monkeys on Cayo Santiago forage on vegetation and are provisioned with rainwater and commercial monkey chow. Rhesus macaques are seasonal breeders. In the Cayo Santiago population, there is a 6-month mating season beginning in March, followed by a 6-month birth season beginning in September. Some rhesus females give birth every year while others do so every other year. Colony records are updated with daily censuses of all animals. The Cayo Santiago database includes information on each animal's date of birth and death, maternal relatedness and genealogy, as well as a history of each individual's group membership, reproduction and health.
The data presented here were collected as part of a larger study of stress and reproduction in multiparous females. Study subjects included all adult females in the population who, when the study began, were older than 15 years of age (n= 55) and a control group of younger females (n=15) aged between 6 and 15 years. Average age in the sample was 16.60±0.50 years. Each subject was multiparous and belonged to one of 6 social groups (F=23 R=19; S=15; V=5; HH=4; KK=4). Subjects, with the exception of 12 individuals for which behavioral data were not available, were classified as being high-, middle-, or low-ranking depending on whether their rank fell within the top, middle, or bottom third of the dominance hierarchy within their group. Dominance hierarchies were established on the basis of data collected by trained observers on aggressive and submissive interactions. Blood samples were collected and body mass was recorded from 52 females between January and March 2007, and from 40 females between January and February 2008. Twenty-two of the females were caught and sampled both years. During the 2007 trapping season, 25 females were lactating and had a live infant (infant age range: 8–134 days; mean±S.E.=72.8±7.5 days), 4 of them had given birth to an infant within 6 months but the infant had died for unknown reasons, 2 of them were pregnant, and 21 of them were cycling. During the 2008 trapping season, 26 of the females were lactating and had a live infant (infant age range: 1-134 days; mean±S.E.=86.3±6.3 days), 1 of them had given birth to an infant within 6 months but the infant had died for unknown reasons, and 13 were cycling. Of the 22 females caught both years, 6 were lactating in 2007 and cycling in 2008; 6 were cycling in 2007 and lactating in 2008; 2 were pregnant in 2007 and cycling in 2008; 6 were lactating both years; and 2 were cycling both years. All females were inspected by a veterinarian at the time of sample collection and found to be in general good health. Therefore, there was no hint that the cycling females in this study did not conceive as a result of health problems, or that the lactating females were in poor health. For females who had not given birth within the 6 months prior to their being trapped, pregnancy was determined at the time of sampling through a physical inspection (palpation of the abdomen) by the veterinarian and reconfirmed retrospectively at the end of the birth season.
Procedure
Monkeys were captured in a feeding corral, approximately 100 m2, which was provisioned daily with monkey chow. Trapping generally occurred between 8:30 and 12:00. Subjects were netted or captured by hand in the corral, transferred to a holding cage (0.62×0.42×0.62 m), and moved to a small field laboratory. The adult females and their infants were then placed into a standard squeeze cage for overnight housing. The morning after capture, the monkeys were anesthetized with ketamine (approximately 10mg/kg via IM injection), a blood sample was collected, and the females were weighed. Mother-infant pairs were separated only for the period of time when mothers were anesthetized. Blood samples were collected between 7:15 and 10:40 (average time of day: 8:18±5.0 min) and, on average, 66.5±5.5 min after the door of the laboratory was first opened (range: 7-213 min) and 22.1±2.7 min after ketamine administration (range 0–127 min). Samples collected the morning after capture presumably reflect the cumulative stressful effects of capture and overnight housing [see Laudenslager et al., 1999; Maestripieri et al., 2008]. All blood samples were collected from the femoral vein into heparin-treated Vacutainer tubes. Samples were refrigerated for 20 minutes and were then centrifuged for 20 minutes. Following centrifugation, the plasma was aliquotted into microcentrifuge tubes, and tubes were stored at −80°C until shipped to the Biomarker Assay Core Lab of the Yerkes National Primate Research Center, where they were assayed for cortisol by radioimmunoassay using a commercially available kit (Diagnostic Systems Laboratories, Webster, TX). The samples collected in 2007 were assayed in April 2007 [see Maestripieri et al. 2008, for some of these data], while the samples collected in 2008 were assayed in July 2008. The assay used has an intra-assay coefficient of variation of 4.90%. Measures of inter-assay variation, assessed by adding low and high concentration standards to each assay, were 15.6% (low) and 13.5% (high).
Analyses
Raw cortisol values were used in the analyses. One female was excluded from analyses because her cortisol level was more than 2.5 standard deviations below the group average. We used general linear mixed models (GLMM) to assess the effects of 3 categorical variables and 3 continuous variables on cortisol levels. Dominance rank, social group and reproductive condition were fixed factors in the models, and female age, female mass and infant age were continuous factors. Because blood samples and masses were collected from some females both in 2007 and 2008, we included individual identity and year of sample collection as random factors in the models. We used restricted maximum likelihood methods for model estimation and Sattherwaite's F tests to gauge fixed effects. We chose the best model using Akaike's information criterion (AIC), starting with all main effects and interactions between each main effect and the random factors “identity” and “year.” None of the interactions with identity or year were included in the final model.
Other statistical tests included Student's t-tests for paired and unpaired samples, Pearson's correlations, analysis of variance (ANOVA), and chi-square tests. All tests were two-tailed, unless otherwise noted. Probabilities <0.05 were considered statistically significant. All analyses were conducted in SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
Research Ethics
The research protocol followed was assessed and approved by the Institutional Animal Care and Use Committees of the University of Chicago and the University of Puerto Rico and adhered to the ASP principles for the ethical treatment of nonhuman primates.
Results
Our final model included only female reproductive state as a predictor of cortisol levels (GLMM: F1,58.11=7.86, p=.007). Female age, female mass, infant age, dominance rank, and social group were not included in the final model because they were not significant predictors of cortisol. The model indicated that cortisol levels were significantly higher for lactating females than for cycling females (t=-2.80, df=19, p=.007).
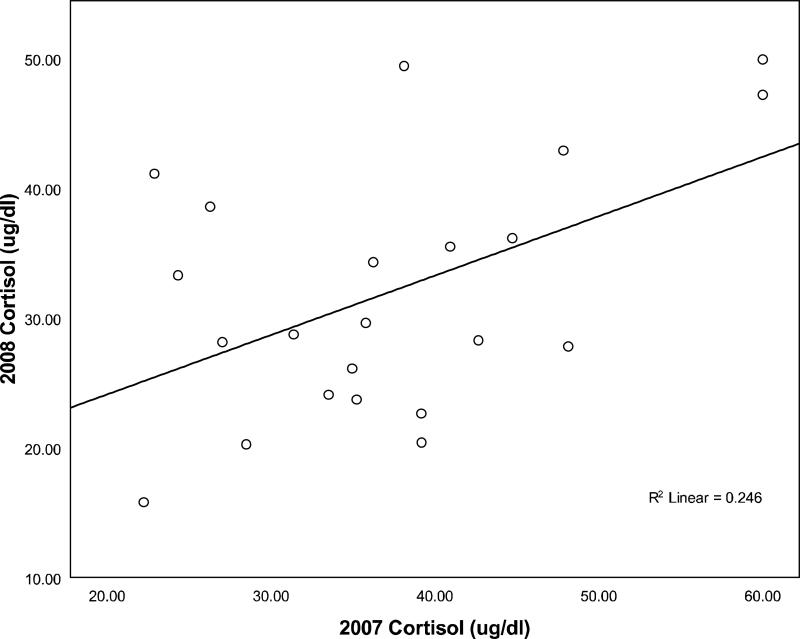
There was a significant positive correlation between 2007 and 2008 cortisol levels for the 22 females that were trapped both years (n=22, r=.50, p=.02), suggesting that individual differences in cortisol levels were stable across the two years (see Fig. 1). To confirm that the correlation was not driven by the 10 females who were either cycling both years or lactating both years, we examined 2007 and 2008 cortisol values for females who were cycling one year and lactating in the other. Six of these females were cycling in 2007 and lactating in 2008, and 6 were lactating in 2007 and cycling in 2008. Again, we found a positive correlation between 2007 and 2008 cortisol values (n=12, r=.52, p=.04; one-tailed).
Figure 1.
Correlation between 2008 cortisol levels and 2007 cortisol levels.
Plasma cortisol levels measured in 2007 were significantly higher than those measured in 2008 (2007=36.09±1.36 ug/dl; 2008=30.33±1.56 ug/dl; t=2.79; df=89; p=0.01). Since females in 2007 were, on average, older than females in 2008 (2007=18.32±0.34; 2008=15.83±0.84; t=3.00; df=91; p= 0.003), the difference in cortisol between years may have been due to the possibility that cortisol is higher in older than in younger females. When only the 22 females that were trapped both years were considered, however, cortisol levels were still significantly higher in 2007 (37.23±2.25 ug/dl) than in 2008 (31.97±2.07 ug/dl; t=2.42; df=21; p= 0.03), despite the fact that these females were obviously younger in 2007 than in 2008. Therefore, the difference between years was not due to age. We examined whether the difference in cortisol between years may have been due to a difference in the relative proportion of cycling versus lactating females in the two years since we previously reported that in the 2007 females, cortisol was significantly higher in lactating than in cycling females [Maestripieri et al., 2008]. However, the proportion of lactating females was significantly higher in 2008 (cycling: 13; lactating= 26) than in 2007 (cycling=22; lactating= 25; chi square= 6.27; df=1; p=0.01). Therefore, higher cortisol levels in 2007 were not due to a higher proportion of lactating females in the sample.
Since body mass can influence cortisol production, we examined whether differences in the 2007 and 2008 cortisol averages could have been related to differences in body mass. The body masses of lactating and non-lactating females were equivalent in both 2007 and 2008 (2007: lactating: 9.32±.30 kg; cycling: 9.10±.41 kg; t=.43, df=44, p=.67; 2008: lactating: 8.24±.29 kg; cycling: 8.82±.57 kg; t=-1.01, df=38, p=.32), but females were heavier in 2007 than in 2008 (2007: 9.15±.25 kg; 2008: 8.43±.27; t=1.96, df=88, p=.05). Body masses also were higher in 2007 than 2008 for the 22 females that were trapped both years (2007: 8.62±.34 kg; 2008: 7.97±.28 kg; t=3.90, df=21, p=.001). There was no correlation, however, between body mass and cortisol in 2007 or 2008 (2007: n=51, r=-.07, p=.63; 2008: n=40, r=-.16, p=.33).
We concluded from these analyses that the difference in cortisol between years may have been due to the fact that the hormonal assays for the samples collected in the two years were run separately, at different times and using different kits. To be able to conduct within-subjects comparisons across years, we calculated z-scores for the 2007 samples and z-scores for the 2008 samples.
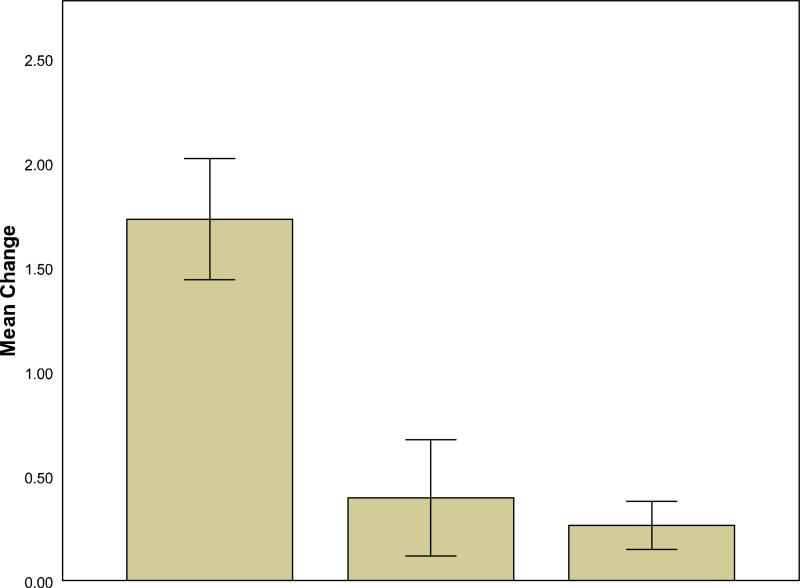
Among the females sampled both years, 12 females were lactating one year and cycling the other. Within-subjects comparisons using z-scores revealed that cortisol was significantly higher when females were lactating than when they were cycling (lactating: 0.44±.29; cycling: -.26±.32; t=2.98, df=11, p=.01). For the females that either were cycling both years (n=2) or lactating both years (n=6), there were no differences in 2007 and 2008 cortisol values (2007: .39±.35; 2008: .47±.36; t=-.19, df=7, p=.86). The increase in cortisol from the cycling condition to the lactating condition was not related to female age (n=12, r=-.36, p=.25) but varied significantly in relation to dominance rank: low-ranking females had greater increases in cortisol from the cycling condition to the lactating condition than did middle- or high-ranking females (F2,9=6.99, p=.02) (Fig. 2).
Figure 2.
Mean (±S.E.M.) change in cortisol levels from the cycling condition to the lactating condition. The z-score values are used here because, as described in the text, the 2007 and 2008 mean cortisol values differed significantly, even though there were no obvious differences in the 2007 and 2008 samples. Thus, z-scores were necessary to compare values collected in separate years.
Discussion
The results of this study demonstrate that the cortisol responses to stress of multiparous female rhesus macaques are stable across two consecutive years. Stable individual differences in cortisol levels have previously been reported in rhesus monkeys living in controlled and homogeneous laboratory conditions [e.g. Higley et al., 1992b; Capitanio et al., 1998]. Remarkably, our study shows that such stability also can be observed among rhesus females belonging to a large free-ranging population living in a heterogeneous natural environment.
Neither variation in age between 6 and 27 years nor social rank were significantly associated with variation in cortisol response to stress. Differences in reproductive condition, however, were associated with differences in females’ cortisol levels. In a previous study, we showed that cortisol responses to capture and individual housing are higher in lactating females than in cycling females [Maestripieri et al., 2008]. In the present study, we replicated this finding using within-subjects analyses and showed that the same individual females have higher cortisol responses to stress when lactating than when cycling. Given that lactation is energetically costly, higher levels of cortisol among mothers may be an artifact of increased glucose production during lactation [Gittleman and Thompson, 1988; Bell and Bauman, 1997]. However, we found no relationships between reproductive condition and body mass or between cortisol and body mass; therefore, it is unlikely that the metabolic costs associated with lactation accounted for the observed differences in cortisol levels. The higher cortisol responses to stress exhibited by lactating females may have been the result of concerns about infant safety. Consistent with this explanation, we found that the increase in cortisol responses to stress occurring during lactation was higher in low-ranking than in middle- and high-ranking females. Low-ranking mothers may perceive their infants to be at risk from other group members to a greater extent than middle- and high-ranking females and experience greater constraints in their ability to provide protection for offspring [Maestripieri, 1995]. Analysis of longitudinal mortality records has demonstrated that infants born to low-ranking females have a lower probability of surviving their first year than infants born to high-ranking females [Drickamer, 1974]. This implies that motherhood may be particularly challenging for low-ranking females.
In a previous study, we demonstrated that adult females in the free-ranging population of rhesus macaques on Cayo Santiago are more likely to die during the birth season than during the mating season, suggesting that the processes of pregnancy, parturition, and lactation have significant survival costs for females [Hoffman et al., 2008]. Infectious diseases are a significant cause of adult mortality in the Cayo Santiago population [Kessler et al., 2006]. Sustained hyperactivation of the HPA axis during lactation can impair immune function and therefore contribute to increased vulnerability to infection and increased risk of mortality. Thus, in addition to the energetic demands of pregnancy and lactation, the psychosocial stress associated with motherhood can be a significant cost of reproduction for rhesus macaque females. Future work should investigate whether females who reproduce every year are more likely to exhibit signs of chronic stress than females that reproduce every other year, and whether frequent reproduction, especially among low-ranking females, is associated with reduced health and longevity.
Acknowledgments
We thank Richelle Fulks and Misael Rivera for assistance with data collection, and Melissa Gerald and the staff of the Caribbean Primate Research Center for logistical support and assistance with animal capture and handling. We also thank James Higham for statistical advice. This study was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The protocol for this study was approved by the Institutional Animal Care and Use Committee, Medical Sciences Department, University of Puerto Rico. This research was supported by NIH grant R21-AG029862 to D.M. This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources (NCRR) to the Caribbean Primate Research Center of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Abbott DH, Keverne DB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr., Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 1997;2:265–278. doi: 10.1023/a:1026336505343. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Clarke S. Dominance rank, cortisol concentrations, and reproductive maturation in male rhesus macaques. Physiol Behav. 1995;58:215–221. doi: 10.1016/0031-9384(95)00055-n. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Wolf R C. Plasma 17-hydroxycorticosteroid response to ACTH in M. mulatta: dose, age, weight, and sex. Proc Soc Exp Biol Med. 1969;130:61–64. doi: 10.3181/00379727-130-33488. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta). Psychoneuroendocrino. 2004;29:1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): evidence for temporal and situational consistency. Am J Primatol. 1998;44:29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: ring-tailed lemurs (Lemur catta). Horm Behav. 2003;43:166–179. doi: 10.1016/s0018-506x(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Whitten PL, Seyfarth RL, Cheney DL. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm Behav. 2008;53:254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Novak MA, Meyer JS, Tiefenbacher S, Higley JD, Lindell SG, Champoux M, Shannon C, Suomi SJ. Continuity and change in emotional reactivity in rhesus monkeys throughout the prepubertal period. Motiv Emotion. 2003;27:57–76. [Google Scholar]
- Drickamer LC. A ten-year study of reproductive data for free-ranging Macaca mulatta. Folia Primatol. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Erwin JM, Tigno XT, Gerzanich G, Hansen BC. Age-related changes in fasting plasma cortisol in rhesus monkeys: implications of individual differences for pathological consequences. J Gerontol A-Biol. 2004;59A:424–432. doi: 10.1093/gerona/59.5.b424. [DOI] [PubMed] [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. Am Zool. 1988;28:863–875. [Google Scholar]
- Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing Dev. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Horm Behav. 1993;27:318–331. doi: 10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- Gust DA, Wilson ME, Stocker T, Conrad S, Plotsky PM, Gordon TP. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocr Metab. 2000;85:2556–2563. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Linnoila M, Vickers JH. Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psych. 1992a;49:436–441. doi: 10.1001/archpsyc.1992.01820060016002. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiat. 1992b;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behav Ecol Sociobiol. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler MJ, Berard JD, Rawlins RG, Bercovitch FB, Gerald MS, Laudenslager ML, Gonzalez JM. Tetanus antibody titers and duration of immunity to clinical tetanus infections in free-ranging rhesus monkeys (Macaca mulatta). Am J Primatol. 2006;68:725–731. doi: 10.1002/ajp.20262. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Rasmussen KL, Berman CM, Lilly AA, Shelton SE, Kalin NH, Suomi SJ. A preliminary description of responses of free-ranging monkeys to brief capture experience: behavior, endocrine, immune, and health relationships. Brain Behav Immun. 1999;13:124–137. doi: 10.1006/brbi.1998.0548. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993a;95:19–31. [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). II. Emotional bases of individual differences in mothering style. Ethology. 1993b;95:32–42. [Google Scholar]
- Maestripieri D. Assessment of danger to themselves and their infants by rhesus macaque (Macaca mulatta) mothers. J Comp Psychol. 1995;109:416–420. doi: 10.1037/0735-7036.109.4.416. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Fulks R, Gerald MS. Plasma cortisol responses to stress in lactating and nonlactating female rhesus macaques. Horm Behav. 2008;53:170–176. doi: 10.1016/j.yhbeh.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic–pituitary–adrenal axes during pregnancy and postpartum. Ann. N.Y. Acad. Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol Metab. 1999;10:174–178. doi: 10.1016/s1043-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- Melnick DJ, Pearl MC. Cercopithecines in multimale groups: Genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 121–134. [Google Scholar]
- Meyer JS, Bowman RE. Rearing experience, stress, and adrenocorticosteroids in the rhesus monkey. Physiol Behav. 1972;8:339–343. doi: 10.1016/0031-9384(72)90382-4. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques. History, behavior, and biology. SUNY Press; Albany, NY: 1986. [Google Scholar]
- Sanchez MM, McCormack K, Grand AP, Fulks R, Graff AE, Maestripieri D. Effects of sex and early maternal abuse on ACTH and cortisol responses to the CRH challenge during the first 3 years of life in group-living rhesus monkeys. Dev Psychopathol. doi: 10.1017/S0954579409990253. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinolo. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Suomi SJ, Bowman RE. Sex differences in adrenocortical response to controlled agonistic encounters in rhesus monkeys. Physiol Behav. 1981;26:385–390. doi: 10.1016/0031-9384(81)90163-3. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Social development in rhesus monkeys: considerations of individual differences. In: Oliverio A, Zappella M, editors. The Behavior of Human Infants. Plenum; New York: 1983. pp. 71–92. [Google Scholar]
- Taylor A, Littlewood J, Adams D, Dore' C, Glover V. Serum cortisol levels are related to moods of elation and dysphoria in new mothers. Psychiatr Res. 1994;54:241–247. doi: 10.1016/0165-1781(94)90018-3. [DOI] [PubMed] [Google Scholar]