Abstract
Cytomegalovirus (CMV) induces the expansion of a unique subset of human NK cells expressing high levels of the activating CD94-NKG2C receptor that persist after control of the infection. We investigated whether this subset is indeed CMV-specific or is also responsive to acute infection with Epstein-Barr virus (EBV). Here we describe a longitudinal study of CMV-seronegative and -seropositive students who were acutely infected with EBV. The NKG2Chi NK subset was not expanded by EBV infection. However, EBV infection caused a decrease in the absolute number of immature CD56brightCD16− NK cells in the blood, and in CMV-seropositive individuals, induced an increased frequency of mature CD56dimNKG2A+CD57+ NK cells in the blood that persisted into latency. These results provide further evidence that NKG2C+ NK cells are CMV-specific, and suggest that EBV infection alters the repertoire of NK cells in the blood.
Keywords: Human, Natural Killer cells, viral infection, cytomegalovirus, memory
Introduction
The human herpesvirus family, including cytomegalovirus (CMV) and Epstein-Barr virus (EBV), are ubiquitous human pathogens that infect a majority of the world’s population. These viruses have co-evolved with their human host, usually causing asymptomatic primary infection after which the virus goes latent and persists for the lifetime of the individual (1). There are certain populations, however, who are at risk for life-threatening consequences of these viruses, for example individuals who are immunocompromised due to hematopoietic stem cell (HSCT) or solid organ transplantation, cancer treatment, or HIV infection. In addition, pregnant women who contract CMV can pass the infection to the fetus, often resulting in birth defects (2).
Natural Killer (NK) cells play a significant role in the immune response against viral infection. Their importance is underscored by rare individuals who are selectively deficient in NK cells, and are highly susceptible to herpesviruses, including CMV, EBV, and varicella zoster virus (3). Recently, a unique population of NK cells expressing the CD94-NKG2C receptor at high levels was found in CMV-seropositive, but not -seronegative individuals (4, 5). These NKG2Chi cells also express CD57, which marks a population of mature NK cells with distinct phenotype and function (6). An increased frequency of NKG2C+ NK cells was also found in CMV-seropositive patients (but not CMV-seronegative patients) acutely infected with chikungunya (7) and hantavirus (8), and chronically infected with HIV-1 (9) and hepatitis B and C (10), indicating that CMV infection is essential for the generation of these NKG2ChiCD57+ NK cells. In addition, these NK cells were expanded after CMV-reactivation in patients undergoing HSCT or solid organ transplantation and persist for over one year after the acute CMV infection (5, 11, 12). These findings are in line with those from mouse models, in which Ly49H+ NK cells specifically respond to CMV infection (13–15), suggesting that NKG2ChiCD57+ NK cells might similarly be involved in controlling human CMV infection.
NKG2C belongs to the NKG2 family of C-type lectin-like receptors expressed by NK cells and some T cells (16). Members of this family form heterodimers with CD94, and transmit inhibitory or activating signals, depending on the receptor. NKG2C is an activating member of the family, associating with the ITAM-containing adaptor protein DAP12, whereas NKG2A is an inhibitory receptor, possessing two ITIMs in its cytoplasmic tail (17). Both the CD94-NKG2A and CD94-NKG2C receptors recognize the non-classical HLA-E molecule, although NKG2A binds with higher affinity than NKG2C. HLA-E presents leader peptides from classical MHC class I molecules, and recognition by NKG2A transmits an inhibitory signal, protecting healthy cells from attack by NK cells. HLA-E expressed by healthy cells does not trigger activation of CD94-NKG2C+ NK cells, suggesting that alterations in the peptide repertoire of HLA-E during CMV infection might cause the specific expansion of CD94-NKG2C+ NK cells.
Epstein-Barr virus is another prevalent herpesvirus, typically causing asymptomatic and persistent infection (18). If EBV is not acquired at a young age, it is often contracted when young adults enter college and manifests as infectious mononucleosis (IM) (18, 19). There is growing evidence that NK cells play a role during EBV infection. During acute EBV infection, NK cell numbers are significantly increased (19–21). NK cells exhibit greater cytotoxicity against an EBV-transformed cell line during acute EBV infection (20), and limit EBV viral load, IM symptoms, and tumor formation in a humanized mouse model (21). CD56bright NK cells may control EBV infection and limit transformation of B cells in tonsils and secondary lymphoid tissues (22, 23).
A recent study reported an increased frequency of NKG2C+ NK cells in pediatric patients who were both CMV- and EBV-seropositive, compared to those who were only CMV-seropositive (24), suggesting that EBV infection might modulate this NK cell population. While this study compared groups of children based on CMV and EBV serological status, it did not address the effect of acute EBV infection and latency on this compartment. In this study, we performed a longitudinal analysis of a cohort of EBV-naïve university students, who experienced acute IM to determine whether CD94-NKG2C+ NK cells are affected by, and whether a unique subset of peripheral blood NK cells responds preferentially to acute EBV infection.
Materials and Methods
Peripheral blood
Cryopreserved PBMC were available from a longitudinal study performed at the University of Minnesota (19, 25). Pre-infection, acute, and latent EBV infection samples were available for 15 subjects (8 CMV-seronegative and 7 CMV-seropositive). In addition, blood samples from 3 CMV- and EBV-seronegative individuals (healthy controls) were analyzed. All participants gave informed consent, and the University of Minnesota Institutional Review Board approved all protocols used.
NK cell phenotype and function
To assess function, 5×106 PBMC were recovered overnight in a 37° C incubator in RP10 medium [RPMI-1640 (Corning) supplemented with 10% heat-inactivated FBS (Hyclone), L-glutamine, penicillin, streptomycin (Corning), and 200 U/ml recombinant human IL-2 (NCI Biological Repository)]. NK cell degranulation was induced by co-culture at a 1:1 ratio with the MHC class I-deficient EBV-transformed B lymphoblastoid cell line, 721.221 with FITC-conjugated mouse anti-human CD107a (BioLegend), and Golgistop (BD Biosciences) in 96-well flat-bottom plates in a 37° C incubator with 5% CO2. After 4 hours, cells were harvested and stained with fluorochrome-conjugated antibodies against CD3, CD56, CD16, CD57, NKG2C, NKG2A, NKG2D, KIR2DL2/DS2/3 (DX27), KIR3DL1 (DX9), KIR3DL2 (DX31), and KIR2DL1/DS1 (EB6) (5). Cells were analyzed on a BD LSRII flow cytometer (BD Biosciences) using FlowJo software (Tree Star, Inc.).
Statistical analysis
Statistical analysis was performed using either one-way or two-way ANOVA with a Tukey’s posttest with Prism software (GraphPad Software). P values of <0.05 were considered significant.
Results and Discussion
EBV infection does not induce the expansion of NKG2C+ NK cells
We analyzed the phenotype of NK cells for 15 individuals who were EBV-naïve and experienced IM during college. Subject information is detailed in Table 1. Of the 7 CMV+ individuals, 3 had an expanded NKG2ChiCD57+ subset in the CD56dimCD16+ NK cell population before EBV infection, while none of the CMV− individuals exhibited this phenotype (Fig. 1A). Individual 5186 does not express NKG2C, and may have homozygous deletion of this gene, a trait found in approximately 4% of the general population (26). The frequency of NKG2ChiCD57+ NK cells was remarkably stable (Fig. 1B). Acute EBV infection did not induce the expansion of the NKG2ChiCD57+ NK cell subset in either CMV+ or CMV− individuals nor did this population change during EBV latency, up to 939 days post-IM. In 2 of the 3 CMV+ individuals with the NKG2ChiCD57+ subset the frequency of these cells decreased during acute infection, but returned to pre-infection levels in latency. EBV viremia during acute infection shows no correlation with the frequency of NKG2ChiCD57+ NK cells in either CMV− or CMV+ individuals (data not shown). The frequency of NKG2CdimCD57− cells in the CD56bright immature NK cell population, which we hypothesize are the progenitors of the NKG2Chi NK cells that expand and acquire CD57 during CMV infection, was stable during EBV infection and into latency (Fig. 1C). We analyzed NKG2C expression in a small cohort of 3 rare individuals who are CMV− and EBV-seronegative (“healthy controls”) and found, as expected, very stable expression even over a 2.5-year period (Supplemental Fig. 1). Taken together, these data demonstrate that expansion of the NKG2ChiCD57+ NK cell subset is not a general response to herpesvirus infections, but is CMV-specific.
Table I.
Subject information
| Subject ID |
CMV status† |
NKG2Chi CD57+ |
*Pre- infection |
**Latent infection |
|---|---|---|---|---|
| 5001 | Pos | No | −361 | 516 |
| 5036 | Pos | No | −698 | 341 |
| 5065 | Pos | Yes | −552 | 179 |
| 5139 | Pos | Yes | −284 | 534 |
| 5186 | Pos | Null | −132 | 919 |
| 5243 | Pos | No | −202 | 118 |
| 5524 | Pos | Yes | −188 | 939 |
| 5068 | Neg | No | −480 | 333 |
| 5178 | Neg | No | −304 | 662 |
| 5214 | Neg | No | −197 | 782 |
| 5219 | Neg | No | −402 | 681 |
| 5406 | Neg | No | −498 | 384 |
| 5428 | Neg | No | −108 | 597 |
| 5477 | Neg | No | −185 | 886 |
| 5483 | Neg | No | −100 | 328 |
| 5099 | Neg | No | No EBV control | |
| 5128 | Neg | No | No EBV control | |
| 5144 | Neg | No | No EBV control | |
Pos = positive; Neg = negative; Subject 5186 does not express surface NKG2C
Numbers show days before and
days after onset of acute EBV infection
Figure 1. Acute EBV infection does not expand the NKG2C+ NK cell compartment.
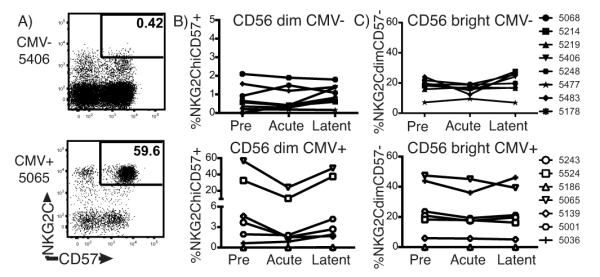
PBMC from CMV− (n=8) and CMV+ (n=7) individuals who contracted EBV were analyzed by flow cytometry for NKG2C and CD57 expression. A) Representative plots with gates showing the NKG2ChiCD57+ subset in the CD3−CD56dimCD16+ NK cell population from CMV− (top panel) and CMV+ (bottom panel) individuals. Frequency per individual before and during acute and latent EBV infection of: B) CD3−CD56dimCD16+ NK cells that are NKG2ChiCD57+, and C) CD3−CD56brightCD16− NK cells that are NKG2CdimCD57−, graphed by CMV serological status (CMV− top; CMV+ bottom).
EBV infection induces a CD56dimNKG2A+CD57+ population in CMV+ individuals
As reported (19), we observed that the frequency of CD8+ T cells increases during EBV infection. Although we did not detect an increase in the frequency of total CD3−CD56+ NK cells, we found a significant decrease in the blood in the absolute number of the immature CD56brightCD16− NK cell population (Fig. 2A), reflected as a decrease in the frequency of CD56bright immature NK cells (Fig 2B). While it is unclear from our studies of PBMC whether this shift is due to maturation or trafficking, Munz and colleagues have reported that the CD56bright NK cell subset is important for controlling infection in the tonsils (22, 23), suggesting that these cells may be recruited from peripheral blood even into latency. Another study reported an increased frequency of the CD56bright subset in acute IM subjects compared to healthy controls (20). However, our data are based on a prospective cohort for which pre-infection, acute IM and latent infection samples were analyzed, and this may account for the discrepancy. In EBV- and CMV-seronegative healthy controls, the frequency of both the CD56bright and CD56dim subsets are remarkably stable over the course of at least 2.5 years, suggesting that EBV infection is modulating this population shift (Fig 2C). We also analyzed expression of NKG2A, the inhibitory counterpart to NKG2C, and CD57 during EBV infection (Fig. 3A). Expression of NKG2A on the immature CD56bright NK cells was stable during infection and into latency, regardless of CMV status (Fig. 3B). In contrast, the frequency of total NKG2A+ NKG2C− NK cells in the mature CD56dim population was markedly increased during acute IM, and remained high in latency, in CMV+ but not CMV− individuals (Fig. 3C). In latency, the population of NKG2A+ cells co-expressing CD57 is significantly higher in CMV+ compared to CMV-individuals, and compared to pre- and acute infection timepoints (Fig. 3D). In healthy controls, however, the frequencies of NKG2A+ and NKG2A+CD57+ NK cells in the CD56dim population were remarkably stable (Fig. 3E). Taken together, these data indicate that CMV and EBV co-infection induces an increased frequency of a distinct CD56dimNKG2A+CD57+ NK cell population in the blood that persists into latency.
Figure 2. The CD56brightCD16− immature NK cell population decreases in EBV infection.
Graphs show A) the numbers and B,C) percentages of CD3−CD56brightCD16− (left panels) and CD3−CD56dimCD16+ (right panels) NK cells from PBMC. A,B) CMV− (closed circles ●; n=8) and CMV+ (open circles ○; n=7) individuals before (pre), during (acute) and after (latent) EBV infection. C) Healthy controls (CMV− and EBV−; n=3) at four timepoints from 2007-2010. Significance was determined by one-way ANOVA, *p<0.05, **p<0.009, ***p<0.001, ****p=0.0001.
Figure 3. EBV infection induces a CD56dimNKG2A+CD57+ population in CMV+ individuals.

PBMC from CMV− (n=8) and CMV+ (n=7) individuals before (pre), during (acute), and after (latent) EBV infection and from healthy controls (n=3) were analyzed by flow cytometry for NKG2A and CD57 expression on CD3−CD56+ NK cells. A) Representative plots showing NKG2A and CD57 expression by CD56bright (left panels) and CD56dim (right panels) NK cells. Frequency of NKG2A expression by: B) CD3−CD56brightCD16−, and C) CD3−CD56dimCD16+ NK cells, and D) frequency of NKG2A+CD57+ NK cells in the CD3−CD56dimCD16+ population from CMV− (closed circles ●) and CMV+ (open circles ○) individuals. E) Frequency of NKG2A and CD57 expression by CD3−CD56dimCD16+ NK cells from healthy controls. Significance was determined by two-way ANOVA, *p< 0.05, **p<0.007.
EBV infection does not induce expansion of a specific KIR subset
We examined the cell surface expression of the killer-cell immunoglobulin-like receptors (KIR) -2DL2/3/DS2, -3DL1, -3DL2, and -2DL1/DS1 to determine whether there is EBV-specific modulation of these receptors. Expression of these KIRs fluctuates during acute IM, regardless of CMV status (Fig. 4A and B), likely a reflection of the changing environment in acute infection. In EBV-infected, CMV− individuals, return to baseline KIR expression occurs within a year of infection (latent samples ranged from 333-886 days post-IM) (Fig. 4A). For three of the CMV+ individuals, there is a trend towards a decreased frequency of KIR+ cells in latency, compared to pre-infection frequencies (Fig. 4B), indicating that up to 939 days post infection, the KIR repertoire is still altered. These data are in line with those of Malmberg and colleagues, suggesting that CMV infection alters the KIR repertoire, and that this phenotype is stable for up to 4 years (27). While they found that these alterations are not seen in CMV− individuals, even those who are EBV-seropositive, our data would suggest that co-infection further alters KIR expression. KIR expression on NK cells from healthy controls was stable (Supplemental fig. 2). Nevertheless, there was no expansion of a unique KIR subset during EBV infection, suggesting that these KIRs are not specifically involved in the response to EBV infection.
Figure 4. EBV infection does not induce expansion of a specific KIR subset.

PBMC from A) CMV− (n=6) and B) CMV+ (n=5) individuals before (pre), during (acu), and after (lat) EBV infection were analyzed for expression of the KIRs -2DL2/3/DS2, -3DL1, -3DL2, and -2DL1/DS1 by CD3−CD56dimCD16+ NK cells. Graphs show percentage of KIR+ cells for each individual at pre, acute, and latent infection timepoints.
The NKG2Chi NK cell subset does not show altered function after EBV infection
The persistence of NKG2ChiCD57+ cells in CMV+ individuals suggests that these may be a population of long-lived NK cells involved in controlling CMV. In support of this, Miller and colleagues have shown that after HSCT, NKG2Chi NK cells expand in response to CMV-reactivation (11). It is unknown whether NKG2Chi or NKG2A+CD57+ NK cells respond with more potent function after infection with EBV. To test this, we co-cultured NK cells with the EBV-transformed cell line 721.221 and measured degranulation by CD107a expression (Fig. 5A). Due to the heterogeneity of the response from individual-to-individual, we calculated the ratio of CD107a expression in the NKG2ChiCD57+ or the NKG2A+CD57+ NK cell subsets to that of the total NK population. Before and after acute EBV infection, regardless of CMV status, a similar proportion of either NKG2ChiCD57+ (Fig. 5B) or NKG2A+CD57+ (Fig. 5C) NK cells degranulated in response to EBV-transformed targets. These data suggest that acute EBV infection does not modulate the function of these NK cell subsets.
Figure 5. NKG2ChiCD57+ and NKG2A+CD57+ NK cells exhibit similar function before and after EBV infection.

PBMC were co-cultured with the EBV-transformed cell line 721.221 or left unstimulated and stained with CD107a to assess degranulation by total, NKG2ChiCD57+, and NKG2A+CD57+ NK cells in the CD3−CD56dimCD16+ population. A) Representative plots showing CD107a expression by unstimulated (unstim) or stimulated (721.221) total (top panels), NKG2ChiCD57+ (bottom left panel), and NKG2A+CD57+ (bottom right panel) NK cells. Graphs show the ratio of CD107a expression by B) NKG2ChiCD57+ and C) NKG2A+CD57+ NK cells to that of total NK cells for each individual before (pre), during (acu), and after (lat) EBV infection. Ratios were determined by dividing the percentage of CD107a+ NKG2ChiCD57+ or NKG2A+CD57+ by the percentage of CD107a+ total NK cells.
In summary, these data provide strong evidence that the NKG2ChiCD57+ NK cell subset is specific for CMV infection, and is not a generalized response to herpesvirus infections. It is surprising that the frequency of NKG2A+CD57+ NK cells is higher only in CMV-seropositive individuals. Along with the decrease in KIR+ NK cell frequency, these data suggest that co-infection alters the repertoire, with an increased population of KIR− NK cells in the blood. The frequency of NKG2A+CD57+ NK cells in the KIR− subset is higher than in cells expressing one or more KIRs only in CMV+ individuals (Hendricks, unpublished observations), suggesting that NKG2A may be licensing these cells in the absence of inhibitory KIR expression, and CD57 is marking their prior expansion in response to EBV infection. These longitudinal studies clearly show that EBV infection can affect the NK cell repertoire and suggest that persistent infection with EBV may have long-term effects on the NK cell population in the individual.
Supplementary Material
Acknowledgements
The authors would like to thank nurses Julie Ed and Beth Mullen; Jennifer Knight for technical assistance; and the L.L.L. laboratory for comments and discussions.
This work was supported by National Institutes of Health grants AI068129 to L.L.L. DWH is supported by an American Lung Association Senior Research Training Fellowship, and SKD is supported by T32 AI007313.
Abbreviations
- KIR
Killer cell immunoglobulin-like receptors
- HSCT
Hematopoietic stem cell transplant
- IM
infectious mononucleosis
References
- 1.Smith C, Khanna R. Immune regulation of human herpesviruses and its implications for human transplantation. Am. J. Transplant. 2013;13(Suppl 3):9–23. doi: 10.1111/ajt.12005. [DOI] [PubMed] [Google Scholar]
- 2.Crough T, Khanna R. Immunobiology of Human Cytomegalovirus: from Bench to Bedside. Clin. Microbiol. Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 4.Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang S-M, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petitdemange C, Becquart P, Wauquier N, Béziat V, Debré P, Leroy EM, Vieillard V. Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity. PLoS Pathog. 2011;7:e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaëlsson J, Malmberg K-J, Klingström J, Ahlm C, Ljunggren H-G. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gumá M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, López-Botet M. Human Cytomegalovirus Infection Is Associated with Increased Proportions of NK Cells That Express the CD94/NKG2C Receptor in Aviremic HIV-1–Positive Patients. J. Infect. Dis. 2006;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- 10.Béziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debré P, Björkström NK, Malmberg K-J, Marcellin P, Vieillard V. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 11.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human Cytomegalovirus (CMV)-Induced Memory-like NKG2C+ NK Cells Are Transplantable and Expand In Vivo in Response to Recipient CMV Antigen. J. Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct Recognition of Cytomegalovirus by Activating and Inhibitory NK Cell Receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 14.Dokun AO, Kim S, Smith HRC, Kang H-SP, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 15.Smith HRC, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanier LL. NK Cell Recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 17.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odumade OA, Hogquist KA, Balfour HH. Progress and Problems in Understanding and Managing Primary Epstein-Barr Virus Infections. Clin. Microbiol. Rev. 2011;24:193–209. doi: 10.1128/CMR.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balfour HH, Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, Vezina HE, Thomas W, Hogquist KA. Behavioral, Virologic, and Immunologic Factors Associated With Acquisition and Severity of Primary Epstein–Barr Virus Infection in University Students. J. Infect. Dis. 2013;207:80–88. doi: 10.1093/infdis/jis646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams H, McAulay K, Macsween KF, Gallacher NJ, Higgins CD, Harrison N, Swerdlow AJ, Crawford DH. The immune response to primary EBV infection: a role for natural killer cells. Br. J. Haematol. 2005;129:266–274. doi: 10.1111/j.1365-2141.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 21.Chijioke O, Müller A, Feederle R, Barros MHM, Krieg C, Emmel V, Marcenaro E, Leung CS, Antsiferova O, Landtwing V, Bossart W, Moretta A, Hassan R, Boyman O, Niedobitek G, Delecluse H-J, Capaul R, Münz C. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5:1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA, Münz C. Tonsilar NK Cells Restrict B Cell Transformation by the Epstein-Barr Virus via IFN-γ. PLoS Pathog. 2008;4:e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lünemann A, Vanoaica LD, Azzi T, Nadal D, Münz C. A Distinct Subpopulation of Human NK Cells Restricts B Cell Transformation by EBV. J. Immunol. 2013;191:4989–4995. doi: 10.4049/jimmunol.1301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saghafian-Hedengren S, Sohlberg E, Theorell J, Carvalho-Queiroz C, Nagy N, Persson J-O, Nilsson C, Bryceson YT, Sverremark-Ekström E. Epstein-Barr Virus Co-infection in Children Boosts Cytomegalovirus-induced Differentiation of Natural Killer Cells. J. Virol. 2013 doi: 10.1128/JVI.02382-13. JVI.02382–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odumade OA, Knight JA, Schmeling DO, Masopust D, Balfour HH, Jr, Hogquist KA. Primary Epstein-Barr virus infection does not erode preexisting CD8+ T cell memory in humans. J. Exp. Med. 2012;209:471–478. doi: 10.1084/jem.20112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyashita R, Tsuchiya N, Hikami K, Kuroki K, Fukazawa T, Bijl M, Kallenberg CGM, Hashimoto H, Yabe T, Tokunaga K. Molecular genetic analyses of human NKG2C (KLRC2) gene deletion. Int. Immunol. 2004;16:163–168. doi: 10.1093/intimm/dxh013. [DOI] [PubMed] [Google Scholar]
- 27.Béziat V, Liu LL, Malmberg J-A, Ivarsson MA, Sohlberg E, Björklund AT, Retière C, Sverremark-Ekström E, Traherne J, Ljungman P, Schaffer M, Price DA, Trowsdale J, Michaëlsson J, Ljunggren H-G, Malmberg K-J. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



