Abstract
Curcumin is known to induce apoptosis of cancer cells by different mechanisms, but its effects on cancer stem-like cells have been less investigated. Here we report that curcumin promotes the survival of DCLK1-positive colon cancer stem-like cells (CSC), potentially confounding application of its anticancer properties. At optimal concentrations, curcumin greatly reduced expression levels of stem cell markers (DCLK1/CD44/ALDHA1/Lgr5/Nanog) in 3D spheroid cultures and tumor xenografts derived from colon cancer cells. However, curcumin unexpectedly induced proliferation and autophagic survival of a subset of DCLK1-positive CSCs. Spheroid cultures were disintegrated by curcumin in vitro but re-grew within 30–40 days of treatment, suggesting a survival benefit from autophagy, permitting long-term persistence of CRC. Notably, RNAi-mediated silencing of DCLK1 triggered apoptotic cell death of colon cancer cells in vitro and in vivo, and abolished CRC survival in response to curcumin; combination of DCLK1-siRNA and curcumin dramatically reversed CSC phenotype, contributing to attenuation of the growth of spheroid cultures and tumor xenografts. Taken together, our findings confirm a role of DCLK1 in colon cancer stem cells and highlight DCLK1 as a target to enhance antitumor properties of curcumin.
Keywords: DCLK1, CD44, autophagy, apoptosis, tumorigenesis
INTRODUCTION
Colorectal cancer (CRC) incidence remains very high in the US and western world (1), and even though several antibodies/molecules have been developed for treating CRCs, surgical removal and chemo/radiation therapy remains standard of care. Colon cancer stem cells (CSCs) are believed to be resistant to chemo/radiation therapy, and a major cause of relapse (2). Several CRC stem cell markers, with extracellular domains, have been identified, including CD44 (3), Lgr5 (4,5) and DCLK1 (6,7). We recently reported that immortalized embryonic epithelial cells, induced to overexpress progastrin (HEKmGAS), developed tumorigenic/metastatic potential (8). Transformed HEKmGAS CSCs co-expressed stem cell markers DCLK1/CD44, while non-tumorigenic (HEKC) cells did not (8). Normal stem cells (NSCs) from colonic-crypts, positive for DCLK1/Lgr5, also do not co-express CD44 (9). In here, we confirmed CRC cells (HCT-116, DLD-1, HT-29) co-express stem cell markers DCLK1/CD44, similar to tumorigenic HEKmGAS cells (8).
Curcumin (active ingredient in turmeric powder), isolated from Curcuma longa (10), is an important ingredient of Asian foods. Curcumin has potent anti-inflammatory, anti-bacterial and anti-cancer effects (10), but lacks solubility in aqueous solutions; bioavailable formulations have been developed (11) and are being examined in phaseI/II clinical trials (12, personal communications with Dr. Lawrence Helson). Curcumin is non-toxic at high doses (12g/day), and targets multiple oncogenic pathways (10,12–14). However, pro-apoptotic effects of curcumin are attenuated by autocrine growth factors (14). Thus, sub-populations of cancer cells may escape inhibitory effects of curcumin; this concept was extended to CSCs in the current studies.
Curcumin induces caspase-3 dependent and independent apoptosis, due to autophagy (13,15). Autophagy represents a double-edged sword which causes either cell death or survival of cells (16). Majority of the studies suggest that curcumin-induced autophagy is a pro-death signal (13,17,18). However, curcumin-induced autophagy also allows tumor initiating cells to survive and either differentiate (19), or become senescent/quiescent (20,21). Possible autophagic effects of curcumin on CSCs remain unknown. Inhibitory effects of curcumin have been reported on CSCs (22–25). However, response of CSCs, positive for DCLK1/CD44/Lgr5, to curcumin remains unknown. In here, we examined inhibitory effects of curcumin against colon cancer cells in vitro and in vivo, in relation to effects on apoptosis/autophagy/proliferation of DCLK1/CD44/Lgr5+ stem cells. Spheroidal re-growth assay was used to examine resistance of CSCs to curcumin. Our results suggest the novel possibility that DCLK1+ve cells survive curcumin induced autophagy.
Since a sub-population of DCLK1+ve cells survived inhibitory effects of curcumin, we examined inhibitory efficacy of DCLK1-siRNA±curcumin, against growth of HCT-116 cells in vitro and in vivo. Our studies demonstrate that DCLK1-siRNA induces apoptosis of colon cancer cells/tumors, in the absence of autophagy; combination of curcumin+DCLK1-siRNA induced massive apoptotic/autophagic cell death, resulting in almost complete loss of stem cell populations expressing DCLK1/CD44/Lgr5.
MATERIALS AND METHODS
Materials and methods used in the current studies are similar to that described previously (8). Reagents used are detailed in supplementary-methods(A). HCT-116, DLD-1 and HT-29 colon-cancer-cells from American Tissue Culture Collection (ATCC) (Manassas, VA) were maintained in DMEM medium as previously described (26). These cells were purchased in 1990’s from ATCC and were authenticated by BioSynthesis DNA Identity Center in 2012.
Treatment of cells
Sub-confluent cells in monolayer-cultures were treated with either DMSO or Non-Targeting siRNA (controls) or optimally effective concentrations of curcumin (25μM)±DCLK1-siRNA (100nM), based on preliminary studies with increasing concentrations of these agents. After 24–48h of treatment, cells were processed for measuring viability/proliferation/apoptosis/autophagy by published methods (8,17,18), as detailed in supplementary-methods(B). Briefly, Viability/proliferation of cells was measured by trypan blue exclusion test and MTT assay, respectively, as described (8). For assessing % cells undergoing apoptosis/autophagy/proliferation, control and treated cells/spheroids, dissociated enzymatically and cytospun onto slides, were fixed and processed for staining with antibodies against apoptotic-marker (activated-caspase-3), autophagic-marker (LC3A/B-I/II) and proliferation-marker (PCNA). Live cells were also stained with Acridine-Orange to visualize autophagic vesicles.
Colon-cancer-cells were grown as primary/secondary spheroids, as previously described by us (8). Primary-spheroids were treated on Day6 after seeding the wells (at which time well-formed primary-spheroids were present), with optimally effective concentrations of curcumin±DCLK1-siRNA, as described in legend of Figure 2A. For generating secondary-spheroids, wells containing primary-spheroids were enzymatically dissociated, and ~5000 cells re-plated in low-attachment plates as described in Supplementary-Methods(C).
Fig. 2.
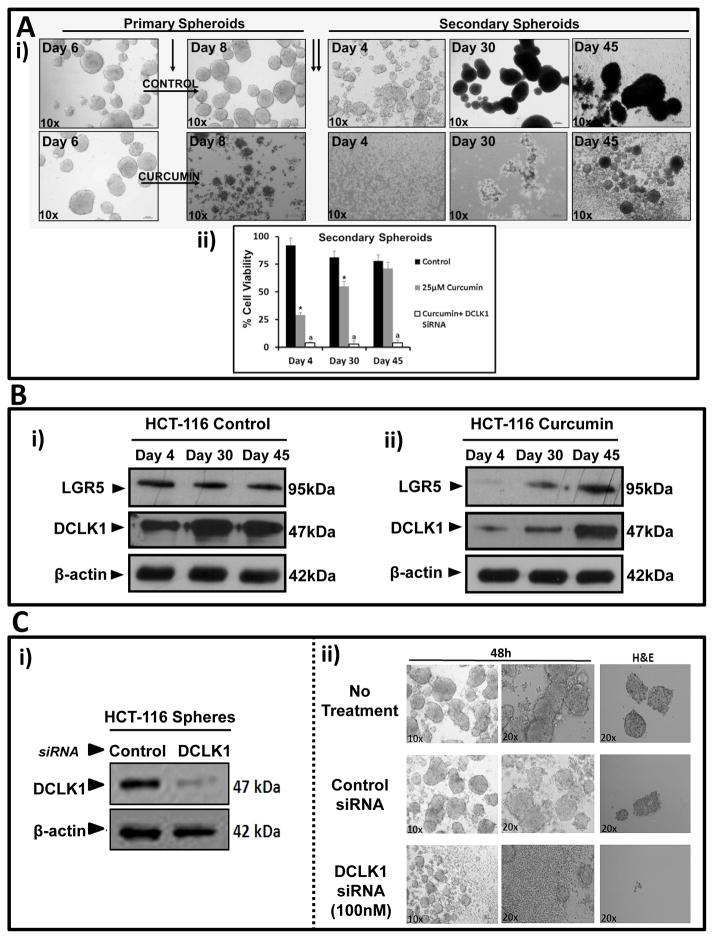
Figure 2A–B: Regrowth/relapse and re-expression of stem cell markers in curcumin treated HCT-116 spheroids.. Equal number of HCT-116 cells, seeded in 24 well plates, were treated on Day 6 with or without curcumin (25μM) (single arrow). (Ai) After 48h, primary spheroids/cells were harvested and plated as secondary spheroids (two arrows). Representative images of secondary spheroids/cells imaged until Day 45; (Aii) Cell viability (%) of secondary spheroids/cells; Data=Mean±SEM of eight wells/two experiments. *=P<0.05 vs control values; a=P<0.05 vs curcumin values. (Bi–ii) Representative WB data from secondary spheroids/cells in duplicate wells derived from control/curcumin treated samples. Fig 2C: Treatment of HCT-116 spheroids with DCLK1-siRNA. Representative WB data (i) and images (ii) of HCT-116 primary spheroids treated with control or DCLK1-siRNA (100nM) for 48h. Representative H&E staining of spheroid sections shown in the last panels.
For the relapse experiment (Fig 2), control/treated primary-spheroids, were dissociated and re-plated as secondary-spheroids, and imaged daily with white light microscopy. In some experiments, spheroids were processed for paraffin-embedding, followed by H&E/IHC/IF staining, as previously described (8). Control/treated spheroids were also processed for either western blotting (WB) or cell-viability, as described above.
Colon-cancer-cells were FACsorted and Analyzed for stem-cell-markers, as described (8) and detailed in Supplementary-methods(D). Briefly, sub-confluent cells were harvested and processed for labeling with fluorophore-tagged antibodies against DCLK1/Lgr5, FACsorted into distinct populations of positive/negative cells, cytospun and fixed on slides, and processed for IF staining with anti-CD44-antibody and 4′, 6-diamidino-2-phenylindole(DAPI). Images acquired with an epifluorescent microscope were analyzed using METAMORPH, v6.0 software (Molecular Devices).
Cells were grown as subcutaneous xenografts in athymic (SCID/nude) mice, essentially as described (8), and detailed in Supplementary-methods(E). Briefly, 5×106 cells were inoculated s.c. in both flanks of female athymic mice. One week post-injection, xenografts were visible on both sides. Mice, bearing tumors, were randomly divided into groups of 3each. Mice were injected every 2 days with either: 0.01%DMSO (Control), Curcumin(3mg/100μL in 0.01%DMSO=100μM), non-targeting siRNA (control) (100nM/100μL PBS), DCLK1-siRNA(0.5pmol/100μL in PBS=100nM), or Curcumin (100μM) and DCLK1-siRNA(100nM). Tumor volume was measured every other day. Mice were sacrificed 3 weeks after initiating treatment, and tumors removed and weighed and processed for WB/IF analysis.
Western blot (WB) analysis of cells growing either as 2D-cultures, 3D-spheroids or xenografts, was conducted as detailed in Supplementary-Methods(F). Briefly, cells/tumors were processed for WB analysis as previously described (8). Blots were cut into horizontal strips containing target or loading control proteins, and processed for detection of antigen-antibody complexes by chemiluminescese. Membrane-strips containing target/loading control proteins were simultaneously exposed to autoradiographic films. In cases where limited samples were analyzed for multiple proteins, loading-control β-actin was measured in a corresponding sample containing equivalent-protein. In a few cases, β-actin was stripped to measure target- protein with similar Mr. Relative band density on scanned autoradiograms was analyzed using Image J program (rsbweb.nih.gov/ij/download), and expressed as a ratio of β-actin in corresponding samples.
Transient transfection of cell/spheroids with double-stranded siRNA-oligonucleotide is detailed in supplementary-methods(G), and was conducted as previously described (8). Transfected cells in 2D were propagated in normal growth medium containing 10% FCS, and growth was examined after 48h in an MTT assay. Transfected spheroids were maintained in spheroid medium as described in Supplementary-Methods(C).
Statistical analysis of data
Data are presented as mean±SEM of values obtained from 4–6 samples from 2–3 experiments/mice. To test for significant differences between means, nonparametric Mann-Whitney test was employed using Statview 4.1 (Abacus Concepts, Inc., Berkeley, CA); P values were considered statistically significant if less than 0.05.
RESULTS
DCLK1+ve colon cancer stem cells (CSCs) co-express CD44
Monolayer cultures of colon cancer cell lines (HCT-116/DLD-1/HT-29) were analyzed by either immunofluorescence (IF) or FACSorting for expression levels of CSC markers, DCLK1/CD44/Lgr5, as previously described (8). On an average 2–3% of cells expressed stem cell markers (data not shown). We recently reported that transformed/tumorigenic embryonic epithelial cells co-expressed stem cell markers DCLK1/CD44, unlike isogenic non-tumorigenic cells (8). Human colon cancer cell lines were similarly positive for transformed phenotype (representative data from HCT-116 cells are presented in Supplementary Fig 1i). Majority of FACSorted DCLK1+ve cells (>80%) co-expressed CD44, while FACSorted Lgr5+ve cells did not (Supplementary Figs 1ii,iii). Surprisingly, a large number of CD44+ve cells co-sorted with Lgr5+ve cells (Supplementary Figs 1i,iii), suggesting that a sub-population of Lgr5+ve cells may be tightly adherent to CD44+ve cells. Lineage tracing studies in the future may allow us to determine if the adherent CD44+ve cells perhaps represent daughter progenitor cells, derived from Lgr5+ve cells. CD44+ve cells, which co-sorted with Lgr5+ve cells, did not co-express DCLK1 (data not shown), unlike co-expression of CD44 by a majority of DCLK1+vecells (described above). CSCs, positive for either DCLK1 or Lgr5, were mostly present along outer edges of spheroids, derived from colon cancer cells (Supplementary Fig 2A); CD44+ve cells, on the other hand, were distributed throughout the spheroids, providing further evidence that cells positive for only CD44 may perhaps represent daughter progenitor cells. Co-expression of CD44 and DCLK1 was evident in cells along outer edges of spheroids, while co-expression of Lgr5 and CD44 was less frequent (Supplementary Figs 2Bi,ii).
Curcumin attenuates growth of HCT-116 cells/spheroids, associated with loss of stem cell markers
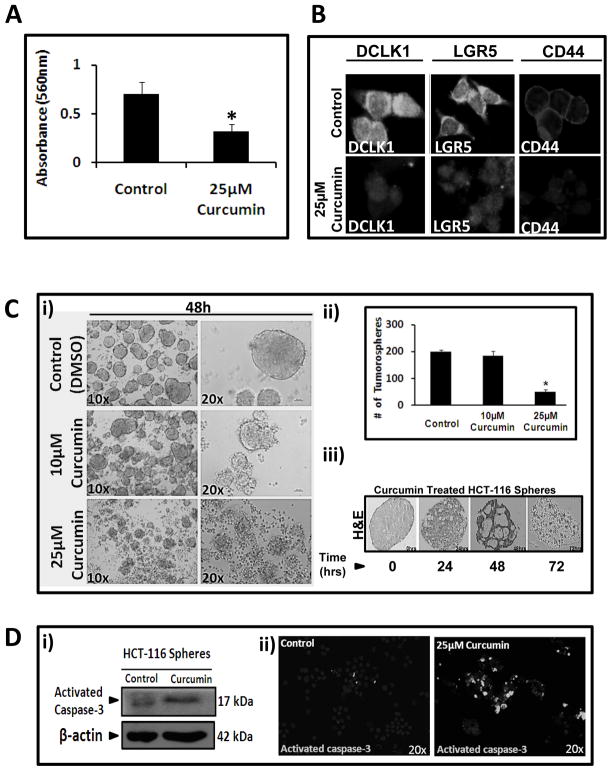
Curcumin (25μM) was optimally effective in reducing growth of HCT-116 cells in 2D cultures by >50% (Fig 1A), resulting in reduced expression of stem cell markers DCLK1/Lgr5/CD44 (Fig 1B). Low doses of curcumin (10μM) did not significantly reduce number of spheroidal growths/well, but had morphological effects (Figs 1Ci,ii). Curcumin (25μM), reduced total number of tumorospheres by >60%/well, associated with disintegration of spheroids (Figs 1Ci,ii), in a time-dependent manner (Fig 1Ciii), along with caspase-3 activation (Figs 1Di,ii).
Fig. 1.
Figure 1A–B: Curcumin inhibits growth of HCT-116 cells/spheroids, and reduces expression of stem cell markers.. (A) MTT assay results (absorbance at 560nm) with control/curcumin treated HCT-116 cells in culture. Data=Mean±SEM of eight wells/two experiments. (B) Representative images (one of three experiments) of 2D cells, treated with curcumin/vehicle for 48h, stained with indicated stem cell markers. Fig 1C–D: Curcumin disintegrates spheroids and induces apoptosis. (Ci) Equal number of HCT-116 spheroids/well, imaged at 48h after treatment, as shown. (Cii) Number of HCT-116 spheroids after 48h of treatment. Data=Mean±SEM of spheroids in 24 wells from one of two experiments. (Ciii) Representative H&E images of single HCT-116-spheroids sections after 0–72h of curcumin treatment. (D) WB (i) and staining (ii) data for activated caspase-3 from a representative of two experiments with spheroids after 48h of treatment. Images in B,Cii,iii are digitally enhanced. *=P<0.05 vs control values.
Regrowth (relapse) of curcumin-treated HCT-116 spheroids
HCT-116 primary spheroids growing in 24-well plates were treated on Day 6 with either control-vehicle or 25μM curcumin for 48h (Fig 2Ai). Primary spheroids on Day 8 were dissociated and replated as secondary spheroids. By Day 4, cells from control group started growing as secondary spheroids; curcumin treated cells did not (Fig 2Ai). For ~28 days, secondary spheroids did not form from curcumin treated samples, while control samples developed dense secondary spheroids. By Days 28–30, small spheroidal structures appeared in wells containing curcumin treated samples (Fig 2Ai). By Day 45, curcumin treated spheroidal cells had re-grown as secondary spheroids, suggesting that a subset of stem cells survived curcumin. Secondary cells/spheroids, were isolated as single cells at indicated days, and analyzed for cell viability (Fig 2Aii). Surprisingly, ~25% of curcumin treated cells were viable on Day 4 after re-plating (Fig 2Aii), increasing dramatically by Days 30–45, matching regrowth of treated-cells as secondary spheroids (Figs 2Ai,ii). Relative levels of Lgr5 remained stable in secondary spheroids from control wells, while relative levels of DCLK1 increased 2–3 fold by Days 30–45 (Figs 2Bi–ii). Curcumin treatment of primary spheroids resulted in almost complete attenuation of Lgr5; but low levels of DCLK1 remained (Fig 2Bii). These results suggest that a subset of DCLK1+ve cells is resistant to curcumin. At Day 30, after re-plating, relative levels of Lgr5/DCLK1 were increased in curcumin-treated spheroids; by Day 45 levels had increased 2–4 fold (Fig 2Bii). Since curcumin attenuated Lgr5 expression but not DCLK1, we used DCLK1-siRNA for targeting DCLK1+ve CSCs.
DCLK1-siRNA targets DCLK1 expression and induces disintegration of HCT-116 spheroids
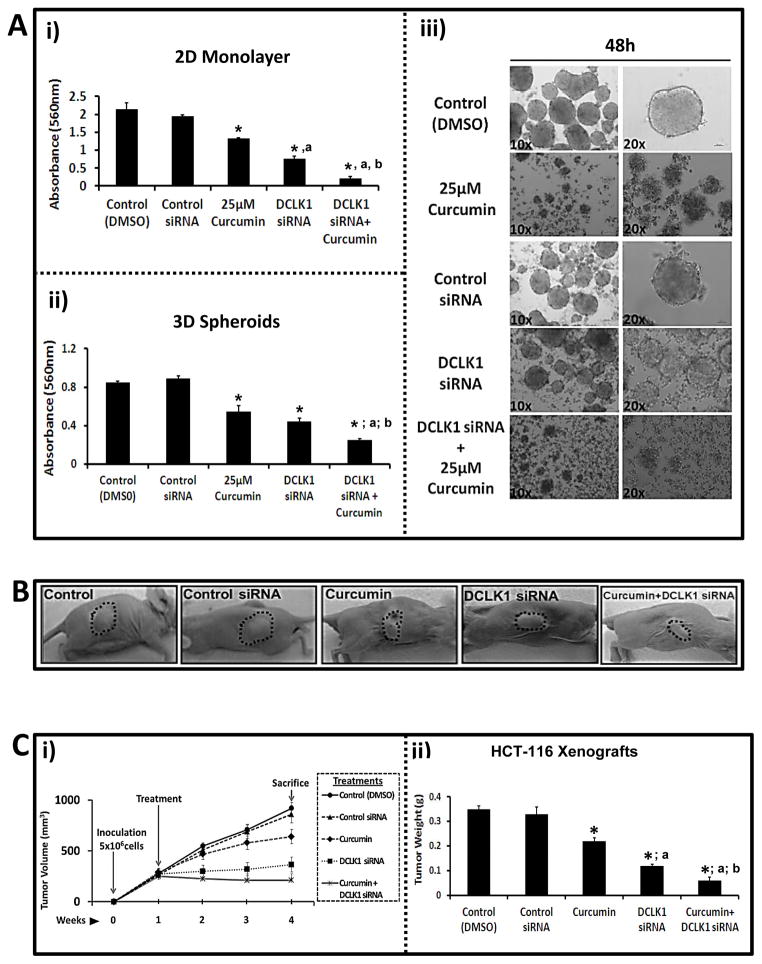
HCT-116 spheroids were treated with either control or DCLK1-siRNA. DCLK1-siRNA (100nM) attenuated DCLK1 expression in HCT-116 spheroids (Fig 2Ci), disintegrating spheroids within 48h (Fig 2Cii); lower concentrations were less effective (data not shown). Control-siRNA had no effects. Surprisingly DCLK1-siRNA was more effective than curcumin, while combination of DCLK1-siRNA+curcumin was significantly more effective than either agent alone, in both 2D (Fig 3Ai) and 3D (Figs 3Aii–iii).
Fig. 3.
Figure 3A–C: Inhibitory effects of curcumin (25μM) ± DCLK-siRNA (100nM) on growth of HCT-116 cells, growing either as 2D (Ai) / 3D (Aii,Aiii) in vitro, or as xenografts in vivo (B,C). Ai–Aiii: Cells/spheroids treated for 48h as shown. Controls treated with 0.01% DMSO or control-siRNA (100nM). Growth of cells/spheroids (absorbance at 560nM) was analyzed as described in Supplementary Methods-C. Data=Mean±SEM of 8–12 wells/two experiments for 2D cells (Ai) and 3D-spheroids (Aii). Representative images of spheroids are presented in Aiii. B–C: Athymic mice (three/group) inoculated bilaterally with 5X106 HCT-116 cells and treated with curcumin±DCLK1-siRNA (as described in Supplementary Methods-E). (B) Representative images of tumor bearing mice at Day 28. (Ci) Tumor volumes measured at indicated time points. (Cii) Tumors weights (g) at time of sacrifice. Data in Ci/ii=Mean±SEM of six tumors/three mice. *=P<0.05 vs control values; a=P<0.05 vs curcumin values; b=P<0.05 vs DCLK1-siRNA values.
Inhibitory effects of curcumin±DCLK1-siRNA against growth of HCT-116 xenografts in vivo
Mice inoculated subcutaneously with 5×106 HCT-116 cells had palpable tumors by Day 7, and were injected on ventral side of tumors with either control or DCLK1-siRNA±curcumin, every 2nd day for 3 weeks. Representative tumor bearing mice, from the five treatment groups, are shown at Day 28 in Figure 3B. Tumor size (volume) continued to enlarge in mice in the order of, control (DMSO)=control-siRNA>curcumin>DCLK1-siRNA (Fig 3Ci). However, in curcumin+DCLK1-siRNA group, the pre-formed tumors actually began to shrink in size (Fig 3Ci). After 3 weeks of treatment, mice were euthanized and tumor weights noted. Tumor weights in control vs treated mice followed a similar pattern described above for tumor size (Fig 3Cii). Surprisingly, DCLK1-siRNA was more effective than curcumin against growth of HCT-116 cells/tumors (Figs 3Ai–ii,B,C), suggesting a functional role of DCLK1 in proliferative/tumorigenic potential of CSCs. The latter possibility was confirmed in the relapse experiment with spheroids. Primary spheroids treated with curcumin+DCLK1-siRNA did not re-form secondary spheroids even after 60 days of re-plating (data not shown), and cell viability remained <5% after re-plating (Fig 2Aii).
Inhibitory efficacy of curcumin on relative expression of stem cell markers/transcription factors in HCT-116 cells
Autocrine progastrin exerts growth promoting effects on colon cancer cells by activating NFκBp65/β-catenin and up-regulating relative expression of DCLK1/Lgr5/CD44 (9,26–29). Control and curcumin-treated monolayer cultures (M), spheroids (S) and tumors (T) were harvested and analyzed by WB analysis for the above indicated proteins. Representative WB data are presented in Supplementary Figures 3Ai–ii; data from several blots are presented as a % change in ratio of relative levels of target proteins/β-actin in control vs curcumin-treated cells (Supplementary Fig 2B). Results confirmed that curcumin attenuates activation of β-catenin/NFκBp65 and reduces relative expression of stem cell markers. Surprisingly, even though curcumin reduced expression of indicated proteins by >40–90% (Supplementary Fig 3), inhibitory efficacy on growth of 2Dcells/spheroids/tumors was <50% (Figs 3A–C). Since curcumin induces autophagy (13,15–20), which can lead to either cell survival or cell death (16), we next examined autophagy/apoptosis in response to curcumin±DCLK1-siRNA.
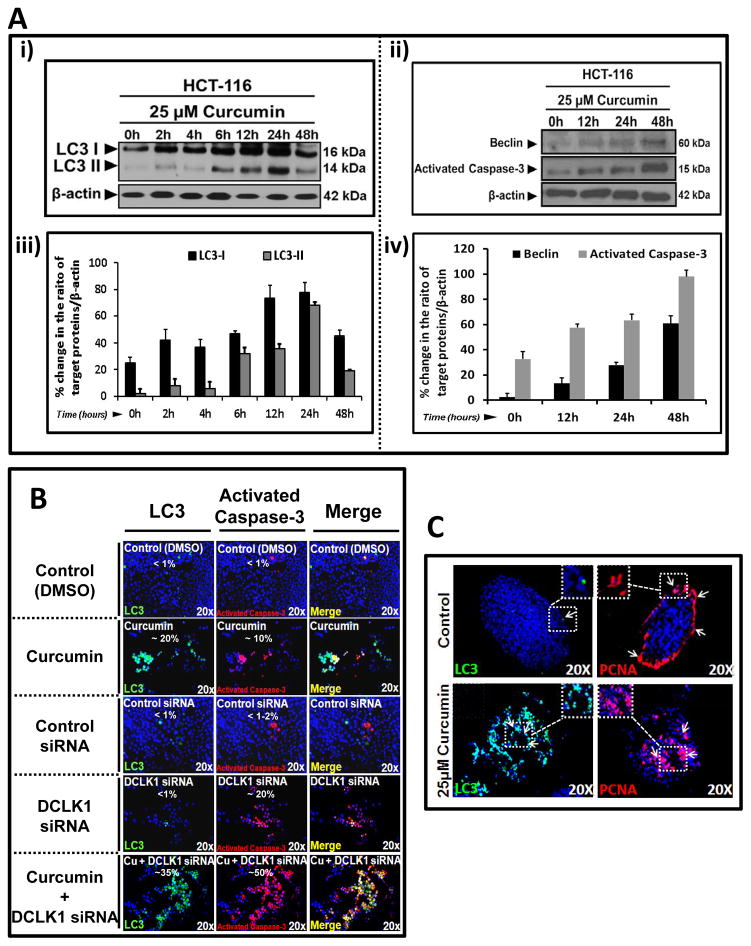
Curcumin induces autophagy and apoptosis of colon cancer cells while DCLK1-siRNA only induces apoptosis
Curcumin treated HCT-116 cells were analyzed for relative levels of autophagic markers (LC3A/BI-II, Beclin-1) and activated caspase-3. Representative WB data are shown in Figures 4Ai,ii. Data from several blots are presented as % change in the ratio of relative levels of target proteins/β-actin in control vs curcumin treated cells (Figs 4Aiii,iv). Curcumin increased relative levels of LC3-I between 12–24h, which was processed to generate LC3-II (Figs 4Ai–ii), confirming previous reports (20). Beclin-I (autophagy-latent gene, ATG6), required to initiate autophagosome formation (30), also increased in a time-dependent manner in response to curcumin (Figs 4Aii,iv). Acidic vesicular organelles (AVOs) stain orange/red with acridine orange, and specify autophagy (31). Formation of AVOs was confirmed in HCT-116 cells in response to curcumin in a dose- and time-dependent manner (Supplementary Figs 4Ai–iv). Increased expression/formation of LC3-I/II in response to curcumin was confirmed by IF staining of HCT-116 cells in culture (Fig 4B). A significant % of LC3+ve cells in curcumin treated samples co-expressed activated caspase-3 (Fig 4B, merged images), suggesting curcumin induced autophagy results in apoptotic death of many HCT-116 cells. DCLK1-siRNA significantly increased staining for activated-caspase-3, but not LC3 (Fig 4B). Combination of curcumin+DCLK1-siRNA significantly increased staining for LC3/activated caspase-3, suggesting combined regimen may synergistically induce autophagy/apoptosis, wherein majority of autophagic cells go through apoptosis (Fig 4B).
Fig. 4.
Figure 4A–C: Curcumin induces autophagy/apoptosis in HCT-116 cells/spheroids while DCLK1-siRNA induces apoptosis. Ai–ii: Representative WB data from one of three experiments with curcumin treated HCT-116 cells in 2D; LC3-I/II (Ai) and Beclin-1/activated caspase-3 (Aii) as shown. Aiii–iv: % change in WB data from all three experiments in Ai–ii. Ratio of target protein/β-actin at 0h assigned 0% value; ratios at increasing time points presented as % of 0h value. B: IF staining for LC3-I/II and activated caspase-3 from representative control/treated cells, cultured on coverslips, from one of two experiments. Yellow color in merged images=co-staining of LC3/activated caspase-3 (~% cells stained for indicated protein[s] shown in each panel). C: Representative IF staining for LC3 and PCNA in control/treated HCT-116 spheroidal sections, from one of two experiments. Arrows=staining for indicated proteins.
PCNA+ve cells are mainly present at outer edges of control HCT-116 spheroids (Fig 4C), similar to staining pattern of DCLK1/Lgr5 (Supplementary Figs 2A,B). Curcumin treated HCT-116-spheroids become positive for PCNA and LC3-I/II in an overlapping area of spheroids (Fig 4C), suggesting that curcumin induced autophagy is associated with both apoptosis (Fig 4B) and proliferation (Fig 4C).
To further examine cell death/cell survival role of autophagy, HCT-116 cells were treated with an inhibitor of autophagy (3-methylalanine, 3-MA) (Supplementary Fig 4B). Inhibitory effects of curcumin were partially reversed by 3-MA, but not to control levels (Supplementary Fig 4B), suggesting curcumin-induced autophagy results in both survival/apoptosis. 3-MA had insignificant effects on cells treated with DCLK1-siRNA or DCLK1-siRNA+curcumin (Supplementary Fig 4B), confirming that autophagy in DCLK1-siRNA+curcumin treated cells is mainly linked to apoptosis.
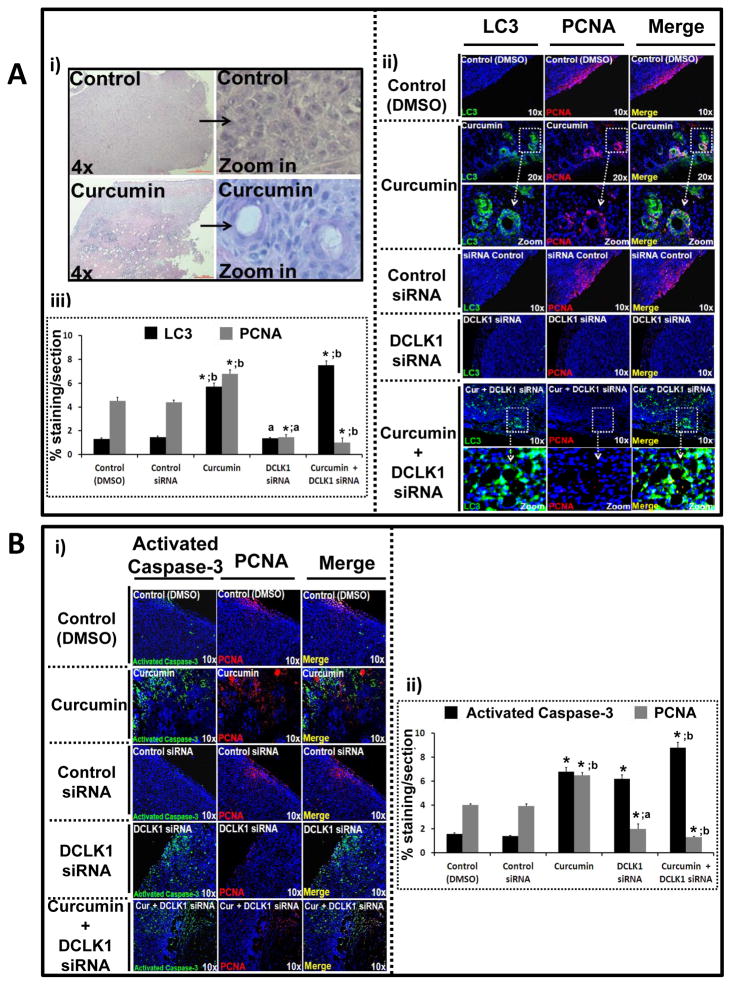
HCT-116 xenografts, harvested from control/treated mice, were processed for H&E/IF (Fig 5). The H&E sections from curcumin-treated tumors demonstrated unique hollow circular areas, surrounded by concentric layers of cells (Fig 5Ai), not seen in other groups. As observed in 2D/3D cells in vitro (Figs 4B,C), control/DCLK1-siRNA treated tumor sections were largely negative for LC3 (Figs 5Aii–iii). Curcumin treated tumor sections demonstrated strong LC3 staining in concentric layers of cells surrounding the hollow areas, which appeared free of nucleated cells; combined treatment with curcumin+DCLK1-siRNA significantly augmented LC3 staining, but the hollow areas, surrounded by concentric layers of cells, were not evident any longer (Fig 5Aii). PCNA+ve cells were present along the edges of control tumors, but relatively absent in tumors treated with DCLK1-siRNA or curcumin+DCLK1-siRNA (Fig 5Aii). Curcumin treated tumor sections, on the other hand, demonstrated PCNA staining in concentric layers of cells surrounding the hollow areas (Fig 5Aii), similar to PCNA staining pattern seen in curcumin treated spheroids (Fig 4C). Enhanced images from figure 5Aii are presented in Supplementary Figure 5A, to present the staining of LC3 and PCNA more clearly. A significant number of LC3 expressing cells co-expressed PCNA (Supplementary Fig 5A, yellow color in merged images), while cells positive for LC3 and PCNA in control tumor sections were distinct and separate (Supplementary Fig 5A). Percent staining for LC3/PCNA per tumor section from 10–15 sections/4–6 tumors/3 mice was quantified as described in legend of Supplementary Figure 5, and presented as bar graphs in Figure 5Aiii. Control and DCLK1-siRNA treated tumors were minimally (~1%) LC3+ve, while LC3 staining of tumor sections treated with curcumin/curcumin+DCLK1-siRNA increased several fold. Control tumor sections were positive for PCNA staining in 4–5% of the area, while <1–1.5% area of DCLK1-siRNA treated tumors were PCNA+ve (Fig 5Aiii). An unexpected finding was that the percentage of PCNA+ve cells in curcumin treated tumors increased 2-fold from control levels; DCLK1-siRNA+curcumin attenuated PCNA staining to <1% (Fig 5Aiii). Activation of caspase-3 was minimal in control tumors (~1%) but significantly increased in tumors treated with either curcumin (~7%), DCLK1-siRNA (~6–7%), or DCLK1-siRNA+curcumin (~9%) (Fig 5Bi,ii). Importantly, none of the PCNA+ve cells were positive for activated caspase-3 (Fig 5Bi), unlike co-staining of LC3 with PCNA in curcumin-treated samples (Fig 5Aii).
Fig. 5.
Figure 5A–B: Effect of curcumin±DCLK1-siRNA on proliferation/apoptosis/autophagy of HCT-116 xenografts, in vivo.. Tumors from Figure 3 experiments were processed for paraffin embedding/staining (Fig 5) or WB (Fig 6B). 5Ai: Representative H&E sections from control/curcumin-treated xenografts. 5Aii: Representative tumor sections stained for LC3/PCNA (magnified images presented in Supplementary Fig 5A). 5Aiii: % area stained for indicated proteins. Data=Mean±SEM of % staining of sections from 3–6 tumors/group (described in Supplementary Methods-E). 5Bi: Representative sections stained for activated caspase-3/PCNA. 5Bii: % staining/section, analyzed as described above. *=P<0.05 vs corresponding control values; a=P<0.05 vs corresponding curcumin values; b=P<0.05 vs corresponding DCLK1-siRNA values.
Treatment of tumors with curcumin±DCLK1-siRNA reverses transformed phenotype of CSCs
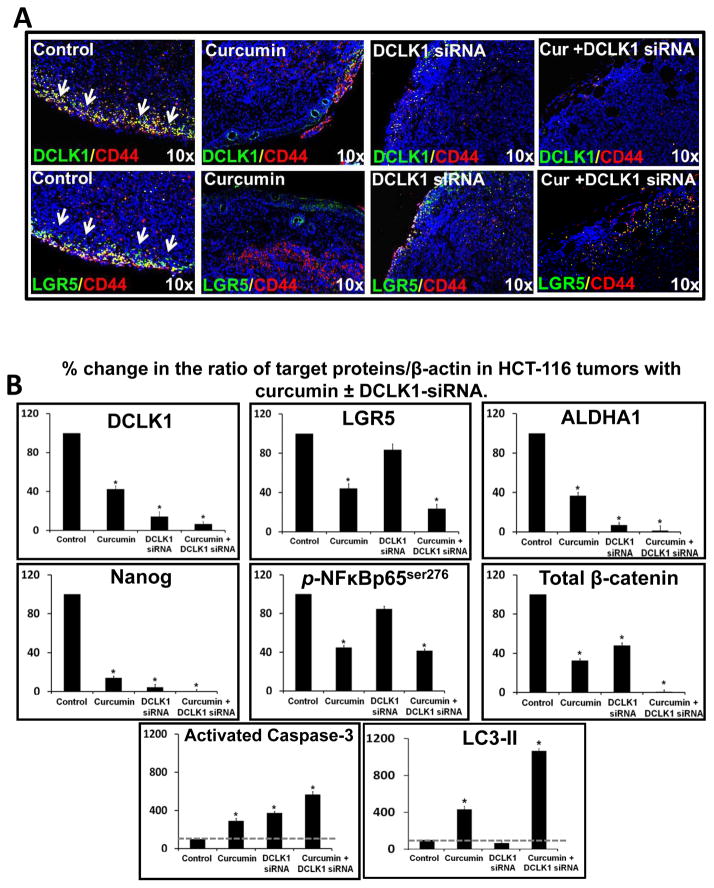
Tumor sections presented in figures 5Aii and 5Bi were also processed for DCLK1/Lgr5/CD44 staining. Representative data from one tumor/group of a total of 4–6 tumors/group are presented in Figure 6A and Supplementary Figure 5B. Intensity of staining for all three stem cell markers was strongest at the edges of tumors. Control tumors demonstrated significant co-expression of DCLK1/CD44 and Lgr5/CD44 (Fig 6A, Supplementary Fig 5B), confirming transformed phenotype of CSCs. Treatment with curcumin±DCLK1-siRNA caused complete attenuation of transformed phenotype, with negligible co-expression of CD44 with DCLK1/Lgr5 (Fig 6A, Supplementary Figs 5Bi–ii). Surprisingly, curcumin treated tumor sections demonstrated DCLK1 staining in concentric layers of cells surrounding the hollow areas (Fig 6A, Supplementary Fig 5Bi); these layers of cells were also positive for LC3 and PCNA staining, as described above for images presented in figure 5Aii. The latter findings suggest the novel possibility that autophagic cells present amongst the concentric layers of cells, surrounding the hollow areas may represent a subpopulation of DCLK1+ve cells, which retain the potential to proliferate (as suggested by PCNA-labeling of these cells) (Fig 5Aii, Supplementary Fig 5A). Curcumin treated tumor cells also continued to express CD44, unlike DCLK1-siRNA treated tumors (Fig 6A, Supplementary Fig 5B). Curcumin significantly reduced expression of Lgr5, while DCLK1-siRNA was much less effective (Fig 6A, Supplementary Fig 5B). Curcumin+DCLK1-siRNA was most effective in attenuating DCLK1/Lgr5 staining along with significant loss in CD44 staining (Figs 6A–B, Supplementary Fig 5B). Tumors were also processed for WB analysis. Data from all tumors is presented as % change in the ratio of target protein/β-actin, wherein ratio for control samples was arbitrarily assigned 100% value (Fig 6B). Values from control groups (vehicle or control siRNA) were almost identical; therefore a single bar for control values is shown in Figure 6B. Curcumin significantly reduced relative levels of stem cell markers (DCLK1/Lgr5/ALDHA1) and pluripotent marker (Nanog), associated with a significant loss in levels of activated NF Bp65s276 and total β-catenin, with a three-fold increase in levels of activated-caspase-3 and LC3-II (Fig 6B; only changes in LC3-II are shown). DCLK1-siRNA, attenuated relative levels of DCLK1/ALDHA1/Nanog, but had insignificant effects on Lgr5. DCLK1 had insignificant effects on activated NFκB, but significantly reduced total β-catenin, resulting in significantly increasing activated-caspase-3 (apoptotic pathway), with no LC3II (autophagy). Curcumin+DCLK1-siRNA was most effective in attenuating relative levels of DCLK1/Lgr5/ALDHA1/Nanog/cellular β-catenin; resulting in a robust activation of both caspase-3 and LC3, suggesting autophagic response to DCLK1-siRNA+curcumin likely leads to cell death rather than survival, unlike the response to curcumin alone.
Fig. 6.
Figure 6A–B: Effect of curcumin±DCLK1-siRNA on stem cell populations in HCT-116 xenografts.. Xenograft sections were processed as described in Figure 5. (6A) Representative merged images of sections co-stained with DCLK1/CD44 or Lgr5/CD44. Images with single antibodies are presented in Supplementary-Fig 5Bi,ii. DAP1=blue; yellow=stem cell populations co-expressing indicated markers. (6B) Mean±SEM of WB data from 3–6 tumors/group, presented as % change in ratio of target protein/β-actin. Ratio of control samples arbitrarily assigned 100% values; ratio of treated samples expressed as % of control. *=P<0.05 vs control values.
DISCUSSION
A novel finding of the current study is that a subset of DCLK1+ve colon cancer stem cells (CSCs), are resistant to inhibitory effects of curcumin, and that DCLK1-siRNA is more effective than curcumin in reducing tumor mass in vivo. Conventional anti-cancer therapies (radiation/chemotherapy), primarily kill rapidly proliferating cancer cells which form bulk of the tumors, but are believed to spare relatively quiescent CSCs. Dietary agents such as curcumin, are believed to suppress self-renewal of CSCs thus sensitizing drug-resistant tumors (2,23). A curcumin analog, G0-Y030, inhibited tumorosphere formation from ALDHA1+ve/CD133+ve colon CSCs (22). We used DCLK1/Lgr5/CD44 as markers of colon CSCs, since they mark both normal and cancer intestinal/pancreatic stem cells (4–6,32,33). The spheroid relapse assay provided the first evidence that a subset of DCLK1+ve cells may be resistant to curcumin, becoming quiescent/dormant for a period of time before re-forming spheroids (Figs 2A,B), which is a hallmark of stem cells. Resistance of a subset of esophageal squamous-carcinoma cells to curcumin has been reported; but the authors concluded that curcumin eliminates CSCs since ALDHA1+ve/CD44+ve CSCs were eliminated by curcumin (24). Another dietary agent was reported to significantly target DCLK1+ve CSCs, via the Notch signaling pathway (34). Our findings, however, suggest that DCLK1+ve CSCs are not eliminated by curcumin.
Multiple signaling pathways are inhibited by curcumin in epithelial cancers, resulting in apoptotic death (10). Besides apoptosis, curcumin induces caspase-3 independent apoptosis (autophagy) in cancer cells (15). Autophagy culminates in either cell death or survival/quiescence/differentiation of tumor cells (15,21,35). Curcumin induced autophagy in cancer cells is mainly reported to result in cell death (12,18). Our results, however, suggest that while curcumin induces apoptotic/autophagic cell death of many cancer cells/CSCs, it appears to induce autophagic survival/quiescence of a subset of DCLK1+ve CSCs (Figs 2A,2B,5Aii and 6A), representing a novel aspect of our findings.
Autophagy has been described as a pro-survival mechanism which acts as a cellular switch between apoptosis and quiescence/senescence (21). Specific pathways, activated in autophagic cells in response to curcumin, protect cells from cell death and allow cells to differentiate or become quiescent (19,20). Curcumin induces differentiation of autophagic glioma-initiating/embryonic stem cells (19,36). Similar to our findings, Mosieniak et al (20) observed that a sub-population of curcumin treated autophagic colon cancer cells survived, becoming senescent/quiescent; the authors, however, did not examine possible regrowth of cells beyond 72h. Our results suggest that curcumin induced quiescent colon cancer cells may represent DCLK1+ve CSCs, which become dormant for a period of time, followed by reformation of spheroids.
Apoptotic and autophagic cell death are not mutually exclusive but can induce cell death simultaneously and cooperatively (37), which may explain co-expression of apoptotic/autophagic markers within same cells in response to curcumin (Fig 4B). Even though autophagy and apoptosis can occur in same cells, autophagy in response to curcumin is believed to be due to ER stress, independent of apoptosis (38). Autophagy either allows ER stressed cells to survive or drives them towards apoptosis (38); it remains to be determined if ER stress plays a role in survival of a subset of DCLK1+ve colon cancer cells, in response to curcumin. Besides ER stress, curcumin also induces ROS which can result in autophagy (17). It is thus possible that a subset of DCLK1+ve cells are resistant to curcumin induced ROS and/or ER stress, and spared from autophagic cell death. A complex cross-talk between several signaling pathways is believed to dictate the outcome of autophagy in cancer cells, which can either survive or proceed to cell death, as recently reviewed (39). In future studies we will examine the role of some of these pathways in allowing a sub-population of DCLK1+vecells to go through autophagy, associated with survival, rather than apoptosis.
Results of our in vivo studies also suggest that long-term treatment of tumors with curcumin perhaps allows a subset of DCLK1+ve CSCs to proliferate and maintain tumor mass, which may explain the disconnect between potent inhibitory effects of curcumin against multiple growth-promoting pathways (10,13,14, current studies), but less than optimal inhibitory effects against tumor growth (Figs 3B,C). Previously also, curcumin was reported to increase a subset of colon cancer cells in S phase (20); based on our results, we believe that the PCNA+ve cells likely represent DCLK1+ve CSCs, which survive curcumin. Results of our preliminary studies further suggest that DCLK1+ve CSCs also survive other insults, such as radiation/chemo therapy (unpublished data from our laboratory), strongly suggesting that DCLK1 expression in CSCs is perhaps linked to chemo-resistance of CSCs, allowing cells to survive autophagy by as yet unknown mechanisms.
Since DCLK1+ve cells appeared to be resistant to curcumin and other chemotherapeutic insults as well, we examined inhibitory effects of DCLK1-siRNA. Down-regulation of DCLK1 expression significantly reduces growth of colon cancer cells in vitro and in vivo (7,40), as confirmed by us (Fig 3). A surprising and unexpected finding was that DCLK1-siRNA was more effective than curcumin in reducing size/weight of colon cancer tumors growing as sub-cutaneous xenografts (Fig 3C). Combination of DCLK1-siRNA+curcumin was even more effective than either agent alone, and caused pre-formed tumors to lose tumor-mass (Fig 3C). Synergistic inhibitory effects of curcumin against cancer cells/CSCs from many different organs have been previously reported with chemotherapeutic agents (reviewed in 23). In here, we demonstrate for the first time, synergistic inhibitory effects of curcumin with siRNA molecules against a stem cell marker, DCLK1. While curcumin, treatment resulted in both apoptotic cell death and autophagic survival, DCLK1-siRNA caused only apoptotic cell death of colon cancer cells (Figs 4B,5B). RNAi against other CSC markers, such as CD44, also inhibit proliferation and induce apoptosis of colon cancer cells (3), suggesting that DCLK1 and CD44 may represent functional CSC markers which play an important role in maintaining proliferative potential of cancer cells. Many reports strongly suggest that DCLK1 expression is critically required for maintaining tumorous growths in many different organs, including intestines and pancreas (6,7,33,40,41), as further confirmed in the current studies.
Combined regimen of curcumin+DCLK1-siRNA augmented both apoptotic/autophagic cell death pathways, with no sign of proliferation/survival of CSC populations. It is therefore speculated that addition of DCLK1-siRNA overcomes resistance of a subset of DCLK1+ve cells to curcumin, resulting in possible elimination of CSCs as suggested by the results of the relapse assay (Fig 2Aii); however it remains possible that a subset of quiescent CSCs, positive for other stem cell markers such as Lgr5 remain dormant/undetectable. Our results suggest that while curcumin targets Lgr5+ve CSCs, DCLK1-siRNA does not eliminate LGR5+ve CSCs (Fig 6). Based on our results, it is proposed that combination of curcumin+DCLK1-siRNA may therefore be effective towards eliminating CSCs positive for Lgr5/DCLK1/CD44. In a previous study, we had reported that addition of p38MAPK may overcome resistance of IGF-II expressing colon cancer cells to curcumin (14). Based on recent findings, as discussed above, it may be possible to overcome resistance of a subset of DCLK1+ve CSCs against curcumin by adding inhibitors of ROS, and/or autophagy, in order to avoid possible deleterious effects of down-regulating DCLK1 in normal intestinal stem cells and neuroprogenitor cells (6,42,43). Thus, in the future, it may be possible to avoid toxic effects of radiation/chemotherapy by utilizing combinatorial strategies with non-toxic agents (such as curcumin and DCLK1-siRNA), which target CSCs.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by NIH grants CA97959 and CA114264 to PS and NASA grants NNX09AM08G and NNJ04HD83G to RU.
Abbreviations
- Abs
antibodies
- CD44
Cluster of differentiation 44
- CRC
colorectal cancer cells
- CSCs
cancer stem cells
- DCLK1
doublecortin-CAM-kinase-like1
- Cu or Cur
Curcumin
- DLD-1
human colorectal adenocarcinoma cell line isolated by D.L. Dexter
- FACSorting
Fluorescence-activated cell sorting
- FACScan
Fluorescence-activated cell scanning
- FCS
fetal calf serum
- HCT-116
human colorectal cancer cell line
- HEK-293
human kidney embryonic epithelial cells
- HEK-C cells
HEK-293 cells expressing the control empty vector
- HEKmGAS
HEK-293 clones overexpressing mutant gastrin gene resulting in expression of full-length progastrin
- HT-29
human colorectal adenocarcinoma
- IF
Immunofluorescence
- IHC
immunohistochemistry
- LC3A/B
Microtubule-associated-protein-I like Light Chain-3 Isoforms A and B
- Lgr5
Leucine-rich repeat-containing G protein coupled receptor 5
- 3-MA
3-methylalanine, autophagy inhibitor
- NF-κB
nuclear factorκB
- p65
p65NF-κB
- siRNA
small inhibitory double stranded RNA oligonucleotides
- Sup
Supplementary
- vs
versus
- WB
western blot
Footnotes
Disclosures: No conflict of interest to report.
Author contributions: CK generated majority of the data for this study; MO and SS generated data presented in Figure 4A and Supplementary Figure 4; SM assisted CK with some of the experiments; RU provided expertise and facilities for some of the studies and PS designed and supervised the experiments and wrote the paper with the assistance of all the co-authors.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ning X, Shu J, Du Y, Ben Q, Li Z. Therapeutic strategies targeting cancer stem cells. Cancer Biol Ther. 2013;14:295–303. doi: 10.4161/cbt.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park YS, Huh JW, Lee JH, Kim HR. shRNA against CD44 inhibits cell proliferation, invasion and migration, and promotes apoptosis of colon carcinoma cells. Oncol Rep. 2012;27:339–46. doi: 10.3892/or.2011.1532. [DOI] [PubMed] [Google Scholar]
- 4.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 5.Kemper K, Prasetyanti PR, De Lau W, Rodermond H, Clevers H, Medema JP. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells. 2012;30:2378–86. doi: 10.1002/stem.1233. [DOI] [PubMed] [Google Scholar]
- 6.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–7. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98–103. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Kantara C, Ortiz I, Swiercz R, Kuo J, Davey R, et al. Progastrin overexpression imparts tumorigenic/metastatic potential to embryonic epithelial cells: phenotypic differences between transformed and nontransformed stem cells. Int J Cancer. 2012;131:E1088–99. doi: 10.1002/ijc.27615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar S, Swiercz R, Kantara C, Hajjar KA, Singh P. Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells. Gastroenterology. 2011;140:583–95.e4. doi: 10.1053/j.gastro.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–55. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helson L, Bolger G, Majeed M, Vcelar B, Pucaj K, Matabudul D. Infusion pharmacokinetics of Lipocurc™ (liposomal curcumin) and its metabolite tetrahydrocurcumin in Beagle dogs. Anticancer Res. 2012;32:4365–70. [PubMed] [Google Scholar]
- 12.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 14.Singh P, Sarkar S, Umar S, Rengifo-Cam W, Singh AP, Wood TG. Insulin-like growth factors are more effective than progastrin in reversing proapoptotic effects of curcumin: critical role of p38MAPK. Am J Physiol Gastrointest Liver Physiol. 2010;298:G551–62. doi: 10.1152/ajpgi.00497.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Sullivan-Coyne G, O’Sullivan GC, O’Donovan TR, Piwocka K, McKenna SL. Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br J Cancer. 2009;101:1585–95. doi: 10.1038/sj.bjc.6605308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–16. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH, et al. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol. 2012;57:1018–25. doi: 10.1016/j.archoralbio.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Li B, Takeda T, Tsuiji K, Wong TF, Tadakawa M, Kondo A, et al. Curcumin induces cross-regulation between autophagy and apoptosis in uterine leiomyosarcoma cells. Int J Gynecol Cancer. 2013;23:803–8. doi: 10.1097/IGC.0b013e31828c9581. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang W, Long L, Zheng B, Ji W, Yang N, Zhang Q, et al. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012;103:684–90. doi: 10.1111/j.1349-7006.2011.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosieniak G, Adamowicz M, Alster O, Jaskowiak H, Szczepankiewicz AA, Wilczynski GM, et al. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech Ageing Dev. 2012;133:444–55. doi: 10.1016/j.mad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Patschan S, Chen J, Polotskaia A, Mendelev N, Cheng J, Patschan D, et al. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am J Physiol Heart Circ Physiol. 2008;294:H1119–29. doi: 10.1152/ajpheart.00713.2007. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer. 2011;105:212–20. doi: 10.1038/bjc.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha S, Adhikary A, Bhattacharyya P, DAS T, Sa G. Death by design: where curcumin sensitizes drug-resistant tumours. Anticancer Res. 2012;32:2567–84. [PubMed] [Google Scholar]
- 24.Almanaa TN, Geusz ME, Jamasbi RJ. Effects of curcumin on stem-like cells in human esophageal squamous carcinoma cell lines. BMC Complement Altern Med. 2012;12:195. doi: 10.1186/1472-6882-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2:321–8. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996;56:4111–5. [PubMed] [Google Scholar]
- 27.Singh P, Sarkar S, Kantara C, Maxwell C. Progastrin Peptides Increase the Risk of Developing Colonic Tumors: Impact on Colonic Stem Cells. Curr Colorectal Cancer Rep. 2012;8:277–89. doi: 10.1007/s11888-012-0144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umar S, Sarkar S, Cowey S, Singh P. Activation of NF-kappaB is required for mediating proliferative and antiapoptotic effects of progastrin on proximal colonic crypts of mice, in vivo. Oncogene. 2008;27:5599–611. doi: 10.1038/onc.2008.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umar S, Sarkar S, Wang Y, Singh P. Functional cross-talk between beta-catenin and NFkappaB signaling pathways in colonic crypts of mice in response to progastrin. J Biol Chem. 2009;284:22274–84. doi: 10.1074/jbc.M109.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140–9. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–44. [PubMed] [Google Scholar]
- 32.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 33.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, et al. DCLK1 Marks a Morphologically Distinct Subpopulation of Cells With Stem Cell Properties in Preinvasive Pancreatic Cancer. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponnurangam S, Mammen JM, Ramalingam S, He Z, Zhang Y, Umar S, et al. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Cancer Ther. 2012;11:963–72. doi: 10.1158/1535-7163.MCT-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Takeda T, Tsuiji K, Wong TF, Tadakawa M, Kondo A, et al. Curcumin induces cross-regulation between autophagy and apoptosis in uterine leiomyosarcoma cells. Int J Gynecol Cancer. 2013;23:803–8. doi: 10.1097/IGC.0b013e31828c9581. [DOI] [PubMed] [Google Scholar]
- 36.Mujoo K, Nikonoff LE, Sharin VG, Bryan NS, Kots AY, Murad F. Curcumin induces differentiation of embryonic stem cells through possible modulation of nitric oxide-cyclic GMP pathway. Protein Cell. 2012;3:535–44. doi: 10.1007/s13238-012-2053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 38.Basile V, Belluti S, Ferrari E, Gozzoli C, Ganassi S, Quaglino D, et al. bis-Dehydroxy-Curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy. PLoS One. 2013;8:e53664. doi: 10.1371/journal.pone.0053664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Yu Q, Zhang R, Liu Bo. Core Signaling pathways of survival/death in autophagy-related cancer networks. Int J Biochem Cell Biol. 2011;43:1263–66. doi: 10.1016/j.biocel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, et al. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnology. 2011;9:40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verissimo CS, Molenaar JJ, Meerman J, Puigvert JC, Lamers F, Koster J, et al. Silencing of the microtubule-associated proteins doublecortin-like and doublecortin-like kinase-long induces apoptosis in neuroblastoma cells. Endocr Relat Cancer. 2010;17:399–414. doi: 10.1677/ERC-09-0301. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto N, Pilz DT, Ledbetter DH. Genomic structure, chromosomal mapping, and expression pattern of human DCAMKL1 (KIAA0369), a homologue of DCX (XLIS) Genomics. 1999;56:179–83. doi: 10.1006/geno.1998.5673. [DOI] [PubMed] [Google Scholar]
- 43.Liu JS, Schubert CR, Fu X, Fourniol FJ, Jaiswal JK, Houdusse A, et al. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol Cell. 2012;47:707–21. doi: 10.1016/j.molcel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.