Abstract
Immune responses against oncolytic adenovirus (Ad) vectors are thought to limit vector anti-tumor efficacy. In Syrian hamsters, which are immunocompetent and whose tumors and normal tissues are permissive for replication of Ad5-based oncolytic Ad vectors, treating with high-dose cyclophosphamide to suppress the immune system and exert chemotherapeutic effects enhances Ad vector anti-tumor efficacy. However, long term cyclophosphamide treatment and immunosuppression can lead to anemia and vector spread to normal tissues. Here we employed three cycles of transient high-dose cyclophosphamide administration plus intratumoral injection of the oncolytic Ad vector VRX-007 followed by withdrawal from cyclophosphamide. Each cycle lasted 4-6 weeks. This protocol allowed the hamsters to remain healthy so the study could be continued for ~100 days. The tumors were very well suppressed throughout the study. With immunocompetent hamsters, the vector retarded tumor growth initially, but after 3-4 weeks the tumors resumed rapid growth and further injections of vector were ineffective. Preimmunization of the hamsters with Ad5 prevented vector spillover from the tumor to the liver yet still allowed for effective long term anti-tumor efficacy. Our results suggest that a clinical protocol might be developed with cycles of transient chemotherapy plus intratumoral vector injection to achieve significant anti-tumor efficacy while minimizing the side effects of cytostatic treatment.
Keywords: Oncolytic, Adenovirus, Cyclophosphamide, Immunosuppression, Syrian hamster
INTRODUCTION
Oncolytic adenovirus (Ad) vectors are emerging as a promising form of cancer therapy (reviewed in(1-5)). To be effective, oncolytic viruses need to infect and lyse cancer cells and, in theory, spread efficiently throughout the tumor. Two major barriers for efficient spread of the vector inside the tumor are hypothesized to include the tumor architecture (physical barrier)(6;7) and the immune system (which clears the vector from the tumor site).(8;9) We and others have employed the Syrian hamster as a model to study the anti-tumor efficacy, biodistribution, and toxicity of oncolytic Ad Serotype 5 (Ad5)-based vectors (reviewed in(10;11)). The virtues of this model are that numerous Syrian hamster tumor cell lines exist that are permissive for Ad5 and that form tumors in hamsters, the tissues of the hamsters are permissive for Ad5 replication, and the hamsters are immunocompetent. Our long term goals are to use this model to establish general principles and to develop protocols by which oncolytic Ad vectors can be used to treat cancer in humans.
We(9;12;13) and others(8;14-18) have shown that cyclophosphamide (CP) treatment of animals bearing tumors significantly increases vector anti-tumor efficacy following intratumoral injection of the vector as compared to the untreated counterpart. We used high-dose CP in our studies; the increased efficacy observed might be due to the immunosuppressive effect of CP that facilitates prolonged persistence and efficient spread of the vector inside the tumor.(8;9;14) Further, noting that CP is a chemotherapeutic alkylating agent, we have reported that high-dose CP acts additively with the Ad vector to control tumor growth.(12) In our studies, long term treatment with CP leads to various side effects such as anemia and increased vector toxicity due to systemic vector spread and replication in normal tissues.(9)
To minimize the drawbacks of long term treatment with CP and still achieve maximum anti-tumor efficacy, in the current study we have employed repeated cycles of transient treatment with CP and vector administration. Subcutaneous tumors were developed in Syrian hamsters using a hamster renal cancer cell line (HaK), then the hamsters were administered high-dose CP and the tumors were injected with the Ad5-based oncolytic vector VRX-007. Red blood cell (RBC) and white blood cell (WBC) levels were monitored, and the CP treatment was discontinued temporarily until the RBC recovered. Tumor growth was measured throughout the study. This protocol mitigated the adverse side effects of CP (e.g. anemia) and allowed the study to proceed for ~100 days. VRX-007 is being evaluated in a Phase I dose escalation clinical trial: intratumoral injection of VRX-007 into advanced solid tumors of any indication (Protocol #510-732). VRX-007 retains all Ad5 genes except those in the E3 region.
To investigate the role of pre-existing immunity to Ad5 on vector anti-tumor efficacy and to restrict vector spillover from the tumor to normal tissues in hamsters immunosuppressed by CP in this long-term study, we immunized the hamsters with Ad5 prior to CP treatment. Pre-existing immunity in the hamster model resembles the situation in many cancer patients inasmuch as most of the human population in the world has immunity to Ad5.(19) We found that tumor growth was effectively suppressed in the preimmunized hamsters and that preimmunization successfully prevented vector spillover and infection of the liver.
MATERIALS AND METHODS
Animals
Syrian (Golden) hamsters (Mesocricetus auratus; 4-5 weeks old) were obtained from Harlan Sprague Dawley (Indianapolis, IN). Saint Louis University Institutional Animal Care and Use Committee approved the studies. The studies were conducted in accordance with institutional and federal regulations.
Viruses and vectors
The oncolytic Ad vector VRX-007 is based on Ad5. VRX-007 lacks most of the E3 genes and overexpresses the E3-11.6K Adenovirus Death Protein (ADP).(20;21) All other genes in VRX-007 are intact; despite this, VRX-007 replicates with about 100-fold greater yield in human A549 human lung cancer cells than in primary bronchial epithelial cells (22) and it replicates poorly in quiescent versus proliferating primary human bronchial epithelial cells (23). Introgen Therapeutics, Inc. (Houston, TX) kindly provided us with the purified stocks.(13;24) VRX-007 is named INGN 007 in some previous publications.
Immunization
Single intramuscular injection of Ad5 (1×1010 plaque forming units [pfu]) was used to generate pre-existing immunity in some groups of hamsters 15 days before injecting HaK cells to form tumors.(13)
Antitumor therapy: Cycles of treatment with CP, intratumoral injection with VRX-007, and recovery from CP
Subcutaneous tumors were established in the hind flank of hamsters by injecting 2×107 hamster kidney cancer cells (HaK) in 200 μl of serum-free DMEM.(13) When the mean tumor volumes reached ~600-700 μl, the treatment procedures started. Tumors of ~600-700 μl are considerably larger than tumors typically studied in animal models using oncolytic viral vectors. The experimental groups are shown in Table 1. Some groups of hamsters were subjected to the cycles of CP treatment, intratumoral VRX-007 injection, then withdrawal from CP (see Figs. 2 and 3). These hamsters were treated with CP (Sigma-Aldrich, St. Louis, MO), and were housed in sterile caging and fed irradiated chow and antibiotic (Baytril Bayer HealthCare [Shawnee Mission, KS]) treated water.(9;13;24) The initial dose of CP was 140 mg/kg body weight and the subsequent doses were 100 mg/kg twice weekly. About one week following the first injection of CP and coinciding with the third CP injection, when the hamsters were severely immunosuppressed, the tumors were administered 5 daily injections of VRX-007, 1×1010 pfu/injection. The levels of RBC and WBC including lymphocytes were monitored by periodic analysis of complete blood counts through the length of the study. When the hamsters started to show signs of anemia (less than normal RBC), the CP was withdrawn and the hamsters were allowed to recover to normal RBC levels. This withdrawal period completed the cycle. Three cycles of CP treatment-VRX-007 intratumoral injection–withdrawal from CP were completed. The CP-VRX-007 treatment immunosuppression/chemotherapy periods were ~18-24 days and the recovery periods were ~14-22 days. Tumor volumes were measured using digital calipers twice every week.
TABLE 1.
Groups of hamsters involved in the experiment described in Fig. 3
| Immune Status | Addition of CP | Group Name |
|
|---|---|---|---|
| Buffer Groups | VRX-007 Groups | ||
| Immune | + | Imm+CP+Buffer | Imm+CP+007 |
| - | Imm+Buffer | Imm+007 | |
| Non Immune | + | ---------- | NonImm+CP+007 |
| - | ---------- | NonImm+007 | |
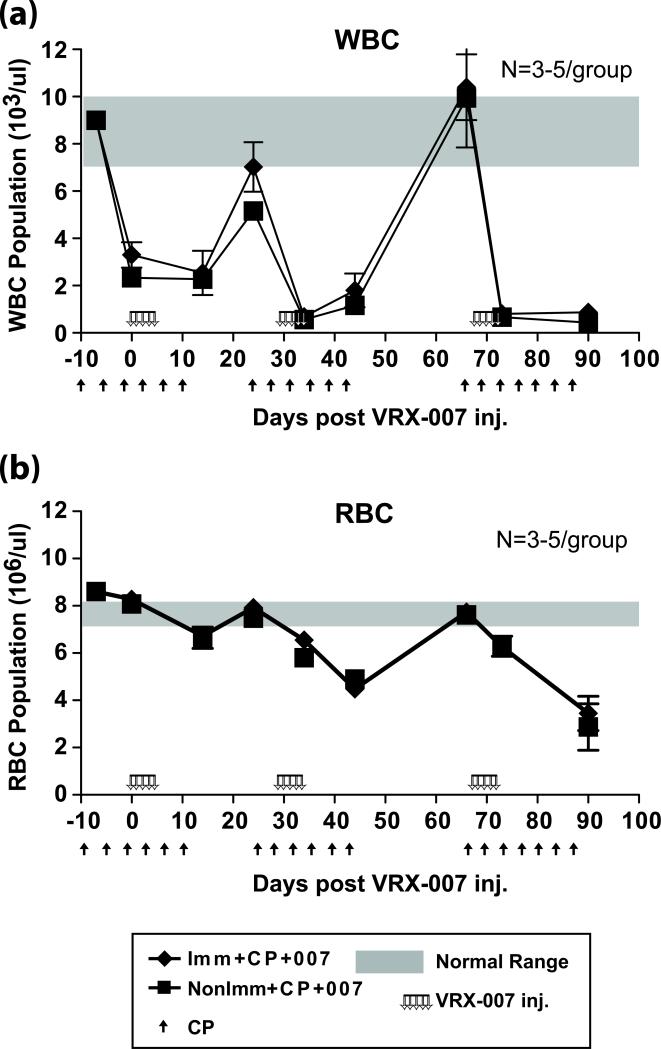
Figure 2. Suppression and recovery of WBC and RBC during the tumor efficacy study shown in Fig. 3.
Blood was collected from the hamsters periodically throughout the tumor efficacy study (Fig. 3) and WBC (a) and RBC (b) levels were determined.
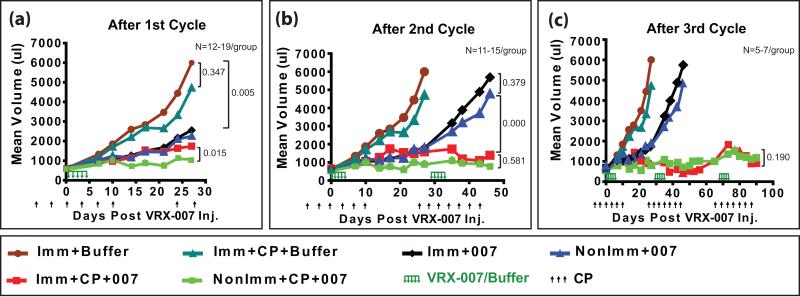
Figure 3. VRX-007 effectively suppresses subcutaneous tumor growth for 3-4 weeks in immunocompetent hamsters. Cycles of cyclophosphamide plus intratumoral VRX-007 administration plus recovery allow for long term tumor growth control.
Established HaK tumors in immunocompetent hamsters or hamsters undergoing cycles of CP treatment and recovery were injected with buffer or VRX-007. Both buffer groups were preimmunized by intramuscular injection with Ad5; then, one group was treated with CP (Imm+CP+Buffer) while the other group was not treated with CP (Imm+Buffer). With the VRX-007 groups, one was previously immunized with Ad5 (Imm+007) while the other was immunized, treated with CP, then the tumors were injected with VRX-007 (Imm+CP+007). Another group was not preimmunized prior to intratumoral injection of VRX-007 (NonImm+007). The final group was not preimmunized, was treated using CP, then was injected intratumorally with VRX-007 (NonImm+CP+007). A total of three cycles of CP treatment-recovery-vector injection were performed, as follows. Hamsters bearing subcutaneous HaK tumors were treated with CP at various time points as indicated. At day 0, VRX-007 was injected intratumorally (five consecutive daily injections, 1×1010 pfu/injection). The CP treatment was stopped at day 10; at this time the WBC and RBC levels had dropped below normal and the hamsters were severely immunosuppressed (Fig. 2). The hamsters were left without CP treatment for 14 days, at which time they mostly had normal RBC and WBC levels (day 24) (Fig. 2). The first cycle was completed at this point. Then, the hamsters were treated with CP for a second cycle (days 24 to 42) and the vector was injected again (days 29-33), followed by recovery to near normal RBC and WBC levels (days 43-66) (Fig. 2). At this point, two cycles of “CP treatment-vector injection-recovery” had been performed. Then, a third cycle of CP treatment (days 66-87) and vector injection (days 68-72) was administered but without the final recovery period. The tumors were measured with digital calipers twice every week and the mean tumor volume for each group is shown after the first cycle (a), second cycle (b), and the third cycle (c). Other data from this experiment are shown in Figs. 2, 4, and 5.
Histopathology
The tumors from each animal were fixed in 10% neutral-buffered formalin. After fixation, the tumors were trimmed, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin-eosin (H&E). A Nikon Optiphot microscope (Nikon, Melville, NY) equipped with a Nikon DXM1200 digital camera and ACT-1 software (Nikon) was used to capture the images. To obtain a comprehensive view of the tumor, several images (7-15) were taken from one end of the tumor section to the other end traversing through the center of the tumor. The images were then stitched together using Adobe Photoshop and Illustrator softwares.
Neutralization assay
Neutralization assay was performed as described previously.(13;24) The NAb titers were determined by the highest dilution of serum/tissue extract that resulted in at least 50% inhibition of cytopathic effect by 100 pfu of VRX-007 assayed on A549 cells.
Virus quantification in tissues
The whole tumor and part of the right lateral lobe of the liver were collected at the time of sacrifice. All the tissues were frozen immediately in liquid nitrogen and stored at –80°C. The tissues were weighed and processed as described elsewhere. (13;25) The infectious virus titers were determined by tissue culture infectious dose 50% (TCID50) assay on A549 cells.(13;25)
Statistical analysis
The overall treatment effect was determined by the Kruskal–Wallis test and the Mann–Whitney U test was used for pairwise comparisons. P≤0.05 was considered to be significant.
RESULTS
Kinetics of red blood cell, white blood cell, and lymphocyte decline after treatment with cyclophosphamide, the recovery kinetics after withdrawal from cyclophosphamide, and the generation of neutralizing antibodies against VRX-007
Prior to beginning the tumor growth control study, namely cycles of CP and VRX-007 administration, we conducted an experiment to define the kinetics of RBC, WBC, and lymphocyte decline after treatment with CP, the recovery kinetics of these cells after the hamsters were taken off CP, and the generation of NAb against VRX-007. One group of hamsters was subjected to one round of CP treatment (Fig. 1a) and the second group received a second and third round of CP (Fig. 1c). VRX-007 was injected intramuscularly after the first (Fig. 1a) or the second (Fig. 1c) round of CP and the response of NAb at various days was determined (Fig. 1b, d).
Figure 1. Cycle(s) of transient treatment with high-dose cyclophosphamide and recovery, and NAb response by the recovering WBC.
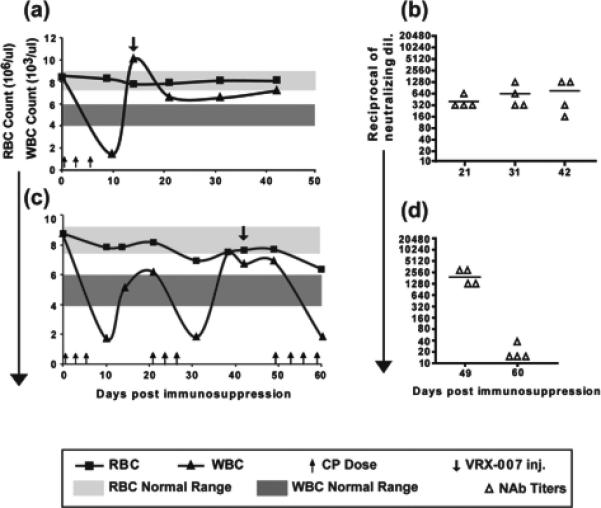
(a,c) Kinetics of RBC and WBC suppression and recovery in hamsters undergoing cycles of CP treatment and recovery. (b,d) Serum NAb titers. VRX-007 was injected intramuscularly (1×1010 pfu/hamster) during the initial phase of recovery after either the first (a) or second (c) cycle of CP treatment. Serum NAb titers were plotted as the reciprocal of the highest dilution of serum that resulted in at least 50% inhibition of cytopathic effect when incubated with 100 pfu of VRX-007. The bar through the triangles indicates the mean NAb value.
The WBC (Fig. 1a) and lymphocyte (not shown) counts declined very rapidly by day 10 following the first CP treatment, and the hamsters became severely immunosuppressed. The RBC counts remained within normal range at 10 days (Fig. 1a).
The hamsters from the first group were allowed to recover partially from immunosuppression, then VRX-007 was injected at day 14 (i.e. 14 days after the first injection of CP) (Fig. 1a). Serum was collected at days 21, 31, and 42, and NAb titers were determined. As shown in Fig. 1b, there was a moderate NAb response (~1:400) at day 21 that increased to 1:760 at day 42. Thus, the naïve lymphocytes that developed following withdrawl from CP were capable of mounting a NAb response against the vector after only 7 days following withdrawl from CP.
The second group of hamsters was subjected to three rounds of CP (Fig. 1c). VRX-007 was injected at day 42, during the lymphocyte recovery period following the second round of CP (Fig. 1c). At 7 days following VRX-007 injection the hamsters were treated a third time with CP, and the lymphocytes declined again. A good NAb response was observed at day 49 (~1:1920) that probably was generated by lymphocytes between day 41 and 49 when CP was administered again. Little or no NAb was detected by day 60 (Fig. 1d).
Importantly, the RBC levels for the most part recovered when the CP was withdrawn (Fig. 1a,c). As such, the hamsters did not become anemic.
In summary, the levels and function of RBC and lymphocytes can be controlled by using rounds of CP treatment. Lymphocytes formed following CP withdrawal are able to generate NAb within 7 days of VRX-007 intramuscular injection.
Design of the long term tumor growth control study
We next conducted the tumor growth control study being guided in part by the findings from Fig. 1. As part of the study, we wished to evaluate the anti-tumor efficacy of intratumoral VRX-007 in immunocompetent hamsters, the anti-tumor efficacy of cycles of treatment with CP and VRX-007, whether the cycling of CP mitigated anemia, whether cycling controlled the levels of WBC, and the role of pre-existing NAb to the vector. Six groups of hamsters bearing subcutaneous HaK tumors were established. The nature of these groups is depicted in Table 1. These groups and the results obtained will be described in the following sections.
The procedure used for the multiple cycles of transient treatment with CP followed by VRX-007 intratumoral administration is shown in Figs. 2 and 3 and is described in the legend to Fig. 3. The study lasted ~100 days following the first treatment with CP. During the entire study the RBC and WBC levels were monitored closely (Fig. 2), and were used to determine the duration of CP treatment and the recovery period. The tumor growth measurements are in Fig. 3. For ease of presentation and discussion, the cumulative tumor growth is shown after the first (Fig. 3a), second (Fig. 3b), and third (Fig. 3c) cycles of CP and vector administration. Tumors from an arm of the experiment were harvested at days 43, 74, and 90 and the NAb titers and the amount of VRX-007 in the tumors were determined (Fig. 4). Histopathology of typical H&E stained tumors at day 46 is in Fig. 5.
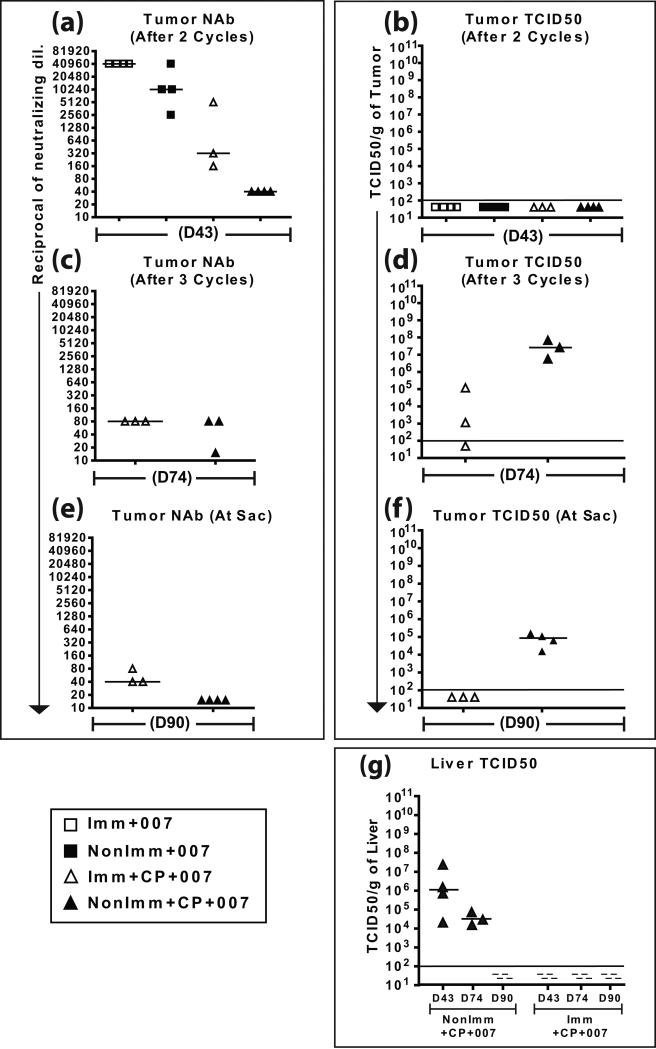
Figure 4. Tumor NAb and TCID50 titers.
NAb titers present in the tumor were determined by a neutralization assay with the tumor lysate after two cycles (a), after three cycles (c) and at sacrifice (e). Infectious virus in the tumor was determined by TCID50 assay with tumor lysates after two cycles (b), after three cycles (d), and at sacrifice (f). These data are from an arm of the experiment shown in Fig. 3. (g) Preexisting immunity prevents vector spillover from the site of injection to the liver. Liver was collected from the vector-injected hamsters undergoing cycles of CP-treatment-recovery (Fig. 3) at various time points and TCID50 titers were determined.
Figure 5. Most of the tumor is non-viable in VRX-007-injected hamsters undergoing cycles of CP treatment-recovery and vector injection.

H&E stained tumor sections from the experiment in Fig. 3 are shown after the second cycle (day 46). Each panel shows a series of photographs stitched together to show the area through the center axis of the tumor starting from one end of the tumor to the other. Representative tumors from the groups that were not immunosuppressed but underwent two cycles of vector injection are shown in panels (a) (NonImm+007) and (b) (Imm+007). Representative tumors from groups that underwent two cycles of CP treatment-vector injection are shown in panels (c) (NonImm+CP+007) and (d) (Imm+CP+007). Viable nuclei show up as purple dots, while the homogenous pink staining represents necrotic areas in the tumor.
VRX-007 is effective for a period in immunocompetent hamsters, but then tumor regrowth occurs and a second series of vector injection is not effective
As shown in Fig. 3a, growth of tumors was rapid in preimmunized hamsters that were immunocompetent and treated with buffer alone (Imm+Buffer) or hamsters that were immunosuppressed with CP and treated with buffer (Imm+CP+Buffer). The CP treatment retarded tumor growth slightly, but it was not statistically significant (P=0.347) (Fig. 3a). The buffer-injected groups were terminated at 28 days because of their large tumor burden.
Injection of VRX-007 into tumors of immunocompetent hamsters (NonImm+007, Imm+007) retarded tumor growth for 3-4 weeks compared to the buffer control (P=0.005) (Fig. 3a). However, the vector-injected tumors then began to grow rapidly (Fig. 3b). Notably, a second round of intratumoral vector injection (5 consecutive daily injections of 1×1010 pfu/injection, at day 29 to 33) did not affect the increased tumor growth rate (Fig. 3b). These hamsters were terminated at day 46.
It is important to understand why VRX-007 ceased to be effective in these immunocompetent hamsters. One reason could be vector elimination by NAb. Indeed, anti-Ad5 NAb were very high in the tumors at day 43 (~1:40960 in the Imm+007 group, ~1:10240 in the Non-Imm+007 group) (Fig. 4a). Not surprisingly from these high NAb levels, vector could not be detected in the tumors of these groups (Fig. 4b). Those tumors from animals that were not treated with CP contained mostly viable cells (Fig. 5, top two panels), indicating that the tumors were growing rapidly.
Cyclophosphamide plus VRX-007 can be administered in multiple cycles and are strongly suppressive of tumor growth
The following discussion pertains to the groups treated with CP plus vector. First, considering the preimmunized group (Imm+CP+007), at 28 days, the tumors grew faster than for the NonImm+CP+007 group (p=0.015) (Fig. 3a). This lesser vector efficacy in the Imm+CP+007 group was likely due to the presence of NAbs inside the tumor at the time of vector administration as described in our previous studies.(13)
After the second cycle of CP treatment-vector injection-recovery, tumors in both CP groups (Imm+CP+007, Non-Imm+CP+007) were controlled much better than the tumors in the non-CP groups injected with VRX-007 (p=0.000) (Fig. 3b). Clearly, the combination of high-dose CP treatment plus vector is much superior to either CP alone or vector alone. The question arises whether CP is enhancing vector replication and persistence though CP's immunosuppressive activity and/or if CP is acting as a chemotherapeutic agent.
It is noteworthy that after the second cycle of CP-vector injection-recovery the tumors in the Imm+CP+007 group were controlled equally as well as in the NonImm+CP+007 group (P=0.581) (Fig. 3b). As shown in Fig. 4a, at day 43, NAb were found in the tumors of the Imm+CP+007 group (~1:320) and the NonImm+CP+007 group (~1:40). No vector was detected in the tumors of these groups. It should be noted that the data in Figs. 4a and 4b are from tumors collected 14 days after the last vector injection, and also that the tumors at this point consisted mostly of necrotic areas and with limited patches of viable cells (see Fig. 5c, d). No doubt there was vector in the tumors at the time of injection; the lack of vector in the tumors at day 43 is probably due to a combination of vector neutralization by NAb and a paucity of viable cells in the tumors from the CP-treated hamsters in which the vector can replicate. In any case, the suppression of tumor growth in the absence of vector suggests that CP is having a chemotherapeutic effect over the period days 28 to 46.
Starting at about day 65, tumors in both CP+vector groups started to grow again (Fig. 3c). This could be because the tumors were devoid of vector (i.e. there was no vector at day 43, see Fig. 4b). Also, the hamsters had been off CP for 18 days, so any chemotherapeutic effect due to CP might be waning. A third cycle of CP treatment was administered at days 63 to 88, and vector was injected for a third round at days 69-73 (Fig. 3c). This CP+vector treatment seemed to stop the re-growth of tumors and cause a decrease in tumor size (Fig. 3c). Measurement of tumors continued until day 90 following the first injection of vector. Remarkably, the tumors in both CP-treated groups were controlled very well and equally well (P=0.190) with a tumor volume almost equal to the starting volume. Again, the question arises, what is responsible for retarding the tumor growth: the vector, CP, or both? At day 74, one day after the last injection of vector in the third cycle of vector injection, high levels of vector (108 TCID50 units) were detected in the tumors of the NonImm+CP+007 group, and much lower levels (~103 TCID50 units) in the Imm+CP+007 group (Fig. 4d). At day 90, 18 days after the last injection of vector, vector (~ 105 TCID50 units) continued to be isolated from tumors in the NonImm+CP+007 group, but not from the Imm+CP+007 group (Fig. 4f). The tumors of the Imm+CP+007 group seemed to have more NAb than the NonImm+CP+007 group at day 74 (Fig. 4c) and day 90 (Fig. 4e), and that could explain why the Imm+CP+007 tumors had less vector. In any case, considering that the NonImm+CP+007 group had the most vector in the tumor yet had similar growth suppression as the Imm+CP+007 group, it seems possible that the tumor growth suppression may be due to CP chemotherapy. However, the vector injected at days 69-73 likely contributed as well to suppression of tumor growth.
Even though the tumors in the hamsters were very well controlled, some animals died during the late phases of the study. Upon necropsy, it was found that the subcutaneous tumor had metastasized to several tissues such as lungs and kidney which might be the reason for the deaths (Supplementary Fig. 1). Therefore, we terminated the experiment at day 90 post first vector injection.
Figure 5 shows the histopathology analysis of representative tumors at day 46 of the experiment. The tumors from the non-CP-treated groups appeared to have viable cells (purple stained cells) throughout much of the tumor (Fig. 5a, b), which explains why these tumors were growing rapidly (Fig. 3b). As discussed, the high NAb in these tumors likely eliminated the vector. The tumors from the CP+VRX-007 groups had very few viable cells and large necrotic areas after the 2nd cycle (Fig. 5c, d) and also after the 3rd cycle (data not shown). The paucity of viable cells in these latter tumors may explain why these tumors had little vector remaining (Fig. 4b) from the last injection of vector 10 days previously, and why these tumors were growing slowly, if at all. Notably, the tumor mass that we were measuring using digital calipers (Fig. 3) in the hamsters that underwent cycling comprised mainly dead tissue.
Serum neutralizing antibodies prevent VRX-007 spillover from the tumor to the liver
We investigated whether serum NAb could minimize vector spillover from tumors. As shown in Fig. 4g for the Imm+CP+007 group, preimmunization effectively prevented vector spillover from the tumor to the liver at all time points (days 43, 74, 90). The naïve hamsters (NonImm+CP+007), on the other hand, had vector in the liver: ~106 TCID50/g of liver at day 43 and ~3×104 TCID50/g of liver at day 74 (Fig. 4g). No vector was detected in the liver at day 90 (sacrifice).
DISCUSSION
A notable aspect of our results is that, in immunocompetent hamsters, VRX-007 injected intratumorally is able to retard tumor growth for 3-4 weeks, but then tumors start to grow rapidly. A second round of vector injection has no effect on tumor growth, probably because there are high levels of NAb in the tumor. These results are important considering that clinical protocols usually recommend multiple rounds of vector injection into patients that are for the most part immunocompetent.(26;27).
Another notable aspect of our results is that the combination of intratumoral injection with VRX-007 plus high-dose CP treatment is much more effective at suppressing tumor growth than either VRX-007 alone or CP alone. With our cycling protocol, we were able to achieve very effective long-term control of tumor growth due to aggressive virotherapy (high dose of VRX-007) and chemotherapy (high dose of CP), while mitigating, at least to some degree, the toxic effects of high-dose CP. This tumor growth suppression was achieved even though the starting tumors were large (600-700 μl) and vascularized.
Another aspect of our results is that pre-immunization of the hamsters with Ad5 did not affect the long-term anti-tumor efficacy of the VRX-007+CP combination. Importantly, preimmunization was able to prevent spillover of VRX-007 from the tumors to the liver over the course of 90 days following first intratumoral injection of vector in the hamsters that were treated with CP.
CP has been employed by a number of laboratories to increase the anti-tumor efficacy and/or to decrease the immune response to oncolytic vectors including those based on Ad(9;12;13;15;24;28;29), herpes simplex virus type 1(16;30), reovirus(31), measles virus(17;32), vesicular stomatitis virus(32), and vaccinia virus.(18) A variety of CP doses and dosing regimes have been used, usually in mouse models. Peng et al(32) concluded that 120 mg/kg of CP given daily by intraperitoneal injection for 4 days was a good regime to control the humoral response to intravenously administered oncolytic measles virus or vesicular stomatitis virus, but they did not study suppression of tumor growth. They noted that this regime was similar to CP dosing regimes used in humans for chemotherapy of cancer. With oncolytic Ads in the Syrian hamster tumor model, low-dose CP, e.g. 20 mg/kg intraperitoneally twice per week(33) or 300 mg/kg or 500 mg/kg given once intraperitonealy(28), has been used as a means to specifically delete regulatory T cells. Low-dose CP, e.g. metronomic dosing of 50 mg/kg, has been used in cancer patients in combination with oncolytic Ad vectors.(28;33) Another laboratory(15) working with an oncolytic Ad vector in the Syrian hamster model reported the successful use of intermediate-dose CP (37 mg/kg, twice weekly) as well as the high dose regime used in our current study. As discussed above, high-dose CP administered to animal models is toxic. However, our results reported here suggest that cycles of transient chemotherapy (and immunosuppression) using CP and vector intratumoral administration is a promising approach, one that has not been attempted previously in an immunocompetent animal model that is permissive for replication of oncolytic Ad vectors. The procedure allowed us to control the tumor growth and to keep the hamsters healthier for a much longer time than in previous studies.
We speculate from our results that a clinical protocol might be developed with cycles of transient chemotherapy coupled with multiple rounds of intratumoral vector injection to achieve significant antitumor efficacy while minimizing the side effects of chemotherapy and vector. Chemotherapy regimens involve repeated cycles of dosing of the chemotherapy drug followed by recovery similar to the protocol employed by us in the current study. We used high-dose CP, but an intermediate dose such as 37 mg/kg, twice weekly, might be sufficient.(15) Also, other chemotherapeutic agents could work as well as many of these cause transient immunosuppression. To prevent vector spread, patients could either be immunized with a replication-defective Ad prior to the treatment, screened for pre-existing anti-Ad NAb, or patients could be passively immunized with anti-Ad NAb.(24) Chemotherapy has shown to increase the vector efficacy when used together.(34)
Supplementary Material
Figure S1. Subcutaneous HaK tumors metastasize to various organs at late phases. Lungs (a) and kidney (b) from a representative animal from the experiment in Fig. 3 undergoing CP treatment-recovery and vector injection showing metastatic nodules. The animal was sacrificed at termination of the experiment (day 90), tissues were isolated and photographed.
ACKNOWLEDGEMENTS
We thank Jacqueline F. Spencer for her help in bleeding the animals. This research was supported by grant CA118022 to W.S.M.W. from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The VRX-007 vector, which is owned by VirRx, Inc., is being evaluated in a Phase I clinical trial. WSMW and KT own stock in VirRx, Inc.
REFERENCES
- 1.Wold WSM, Ison MG. Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 1732–1767. [Google Scholar]
- 2.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18(5):305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 3.Toth K, Dhar D, Wold WSM. Oncolytic (replication-competent) adenoviruses as anticancer agents. Expert Opinion on Biological Therapy. 2010;10(3):353–368. doi: 10.1517/14712590903559822. [DOI] [PubMed] [Google Scholar]
- 4.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011;8(1):12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Zheng D. An update on gene therapy in China. Curr Opin Mol Ther. 2009;11(5):547–553. [PubMed] [Google Scholar]
- 6.Ganesh S, Gonzalez EM, Idamakanti N, Abramova M, Vanroey M, Robinson M, et al. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res. 2007;67(9):4399–4407. doi: 10.1158/0008-5472.CAN-06-4260. [erratum appears in Cancer Res. 2007 Jun 15;67 12:5998].
- 7.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 8.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips N, Wold WSM. Immunosuppression enhances oncolytic adenovirus replication and anti tumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wold WS, Toth K. Chapter three--Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res. 2012;115:69–92. doi: 10.1016/B978-0-12-398342-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Dhar D, Toth K, Wold WSM. Syrian hamster tumor model to study oncolytic Ad5 based vectors. In: Kirn D, Liu TC, Thorne S, editors. Oncolytic Viruses: Methods and Protocols. Humana Press; New York: 2012. pp. 53–63. [DOI] [PubMed] [Google Scholar]
- 12.Young BA, Spencer JF, Ying B, Tollefson AE, Toth K, Wold WSM. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther. 2013;20:521–530. doi: 10.1038/cgt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhar D, Spencer JF, Toth K, Wold WSM. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83(5):2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14(1):259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa N, Abei M, Yokoyama KK, Fukuda K, Seo E, Kawashima R, et al. Cyclophosphamide enhances antitumor efficacy of oncolytic adenovirus expressing uracil phosphoribosyltransferase (UPRT) in immunocompetent Syrian hamsters. Int J Cancer. 2013;133(6):1479–1488. doi: 10.1002/ijc.28132. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 17.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ, et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther. 2007;15(11):1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- 18.Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15(8):2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 19.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 20.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WSM. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 21.Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WSM. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuppuswamy M, Spencer JF, Doronin K, Tollefson AE, Wold WS, Toth K. Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Ther. 2005;12(22):1608–1617. doi: 10.1038/sj.gt.3302581. [DOI] [PubMed] [Google Scholar]
- 23.Toth K, Kuppuswamy M, Shashkova EV, Spencer JF, Wold WSM. A fully replication- competent adenovirus vector with enhanced oncolytic properties. Cancer Gene Ther. 2010;17(11):761–770. doi: 10.1038/cgt.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhar D, Spencer JF, Toth K, Wold WSM. Pre-existing Immunity and Passive Immunity to Adenovirus 5 Prevents Toxicity Caused by an Oncolytic Adenovirus Vector in the Syrian Hamster Model. Mol Ther. 2009;17:1724–1732. doi: 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas MA, Spencer JF, Wold WSM. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods in Molecular Medicine. 2007;130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
- 26.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. PhaseI-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- 28.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological Effects of Low-dose Cyclophosphamide in Cancer Patients Treated With Oncolytic Adenovirus. Mol Ther. 2011;19(9):1737–1746. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14(6):779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14(1):259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng KW, Myers R, Greenslade A, Mader E, Greiner S, Federspiel MJ, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20(3):255–261. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18(10):1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Subcutaneous HaK tumors metastasize to various organs at late phases. Lungs (a) and kidney (b) from a representative animal from the experiment in Fig. 3 undergoing CP treatment-recovery and vector injection showing metastatic nodules. The animal was sacrificed at termination of the experiment (day 90), tissues were isolated and photographed.