Abstract
Natural killer T (NKT) cells represent an innate-like lymphocyte population endowed with unique antigen recognition and tissue distribution features. Their abundance in the microvascular compartments of the liver allows NKT cells to immediately respond to lipid antigens and soluble factors circulating through the portal vein system by releasing tremendous amounts of different cytokines and chemokines. Subsequently, dependent on the nature of the lipid antigen encountered as well as the accessory signal(s) provided, NKT cells not only contribute to the maintenance of immune tolerance, but also direct adverse immune reactions locally and systemically. Focusing on their potent immunomodulatory features and their interactions with various innate and adaptive immune cells, the role of NKT cells in perpetuating the loss of liver-specific immune tolerance will be discussed.
Introduction
The liver commands various specific functions in the carbohydrate, protein and lipid metabolism in the body. Among those are the synthesis of hormones and plasma proteins, the detoxification of harmful substances, the metabolism of different drugs, the decomposition of red blood cells, the storage of glycogen, the synthesis of fatty acids and the control of the systemic lipid circulation through the synthesis of lipoproteins.
The exposure to all of these metabolic products as well as to multiple other molecules absorbed in the intestinal system renders the liver an organ that needs to distinguish between harmless and dangerous antigens in order to not only maintain immune tolerance on the one hand, but also to mount appropriate immune responses against pathogens on the other. Different T cell populations play a pivotal role in these processes. Among the liver-specific tolerogenic immune mechanisms the induction of T cell tolerance or the generation of regulatory T cells due to the cross-presentation of antigens by liver sinusoidal cells, T cell apoptosis mediated by hepatic stellate cells and the inactivation of T cells by antigenic priming havebeen discussed [1]. In contrast, altered pathways of T cell survival and antigen presentation [2] as well as modified cytokine milieus or costimulatory signals [3] might drive tissue damage due to an aberrant activation of the immune system.
The liver as an innate immune organ
The cellular composition of the liver is unusual compared to other secondary immune organs as components of the innate immune system such as Kupffer and stellate cells, dendritic cells (DCs), natural killer (NK) and NKT cells constitute the majority of immune cells [4]. In contrast, B cells are underrepresented [5] and CD8-positive T cells that frequently display memory and innate (-like) features outnumber CD4-positive T cells [6].
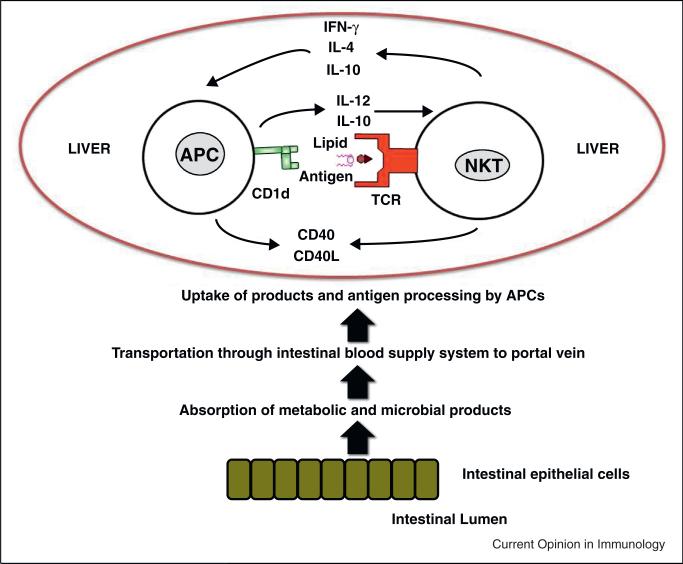
The specific metabolic features of the liver as well as their location at the interface between the intestinal and systemic blood circulation might be involved in the selection of the hepatic immune cell repertoire as well as the control of the respective immune reactions (Figure 1). The concentration of lipids in hepatic DCs, for example, defines the initiation and generation of immunogenic versus tolerogenic immune responses in mice and humans [7•]. Furthermore, scavenger receptors [8] expressed on liver sinusoidal endothelial and Kupffer cells mediate the uptake of glycolipids [9] and might subsequently shape NKT cell responses through controlling the availability of lipid antigens.
Figure 1.
Lipid antigens as well as accessory signals provided by different antigen-presenting cell subsets define the reactivity and mobility of NKT cells in the liver. Metabolic and microbial compounds absorbed from the intestinal tract circulate into the hepatic blood circulation through the portal vein system. Different antigen presenting cells (APCs) lining the microvascular compartments of the liver take up and process these compounds. Dependent on the type of the APC lipid antigens as well as accessory signals such as cytokines or costimulatory molecules are provided to NKT cells. NKT cells patrolling the liver sinusoids release immediately cytokines and/or chemokines upon antigen encounter and shape dependent on the nature of the antigen and accessory signals provided the subsequent immune response.
Thus, although being an overall tolerogenic organ, the liver can become the target of adverse immune reactions in liver-specific and systemic immune-mediated diseases dependent on the triggers involved and the subsequent cellular and molecular immune responses elicited.
NKT cells
NKT cells can be divided into two distinct subpopulations, namely type I and type II NKT cells [10]. Whereas conventional T lymphocytes exhibit diverse T cell receptors (TCRs), clonally expand upon antigen encounter and react to peptide antigens, type I NKT cells express a semi-invariant TCR (Vα14Jα18/Vβ2,7,8 in mice and Vα24Jα18/Vβ11 in humans) and recognize — in an innate pattern recognition like manner — a broad range of self-lipid and microbial-lipid antigens including glycosphingolipids, glycerophospholipids, lysophospholipids and cholesterol esthers presented by the atypical MHC-I (-like) molecule CD1d on antigen presenting cells (APCs) [11–14]. In contrast, type II NKT cells exhibit a more diverse T cell receptor repertoire and react to mammalian and microbial phospholipids [15] and sulfatides [16,17].
Both NKT cell populations are endowed with potent immunomodulatory functions and bridge the innate with the adaptive immune response [18]. While the type I NKT cell subset represents up to 30% of the T cells in the liver of mice [19], the majority of NKT cells in humans consists of type II NKT cells [10]. TCR-dependent and TCR-independent activation signals contribute to the distribution of type I NKT cells and their phenotypic presentation in the liver [20]. For example, type I NKT cells immediately arrest upon engagement of their TCRs by glycolipids, while they continue to migrate upon exposure to soluble factors such as IL-18 [21]. Accordingly, type I NKT cells activated by microbial glycolipid antigens contribute to bacterial clearance (cognate activation), while toll-like receptor (TLR)-elicited cytokine responses of myeloid cells infected by bacteria that do not contain lipids in their cell wall recruit type I NKT cells as part of an inflammatory cellular network (bystander activation) [22••]. Thus, the CD1d-mediated presentation of lipid antigens and the provision of accessory signals by stellate [23], Kupffer [24], sinusoidal endothelial [25••] or myeloid suppressor cells [26] as well as hepatocytes [27] shape the NKT cell response in the liver (Figure 1). Furthermore, the sympathetic nervous system modulates the behavior of hepatic type I NKT cells independent of CD1d presented lipid antigens [28]. In contrast, chemokines such as CXCR6, LFA-1 or ICAM-1 [25••,29,30] promote the accumulation of type I NKT cells in the liver. As there exists only sparse evidence for NKT cell trafficking to diseased and/or inflamed tissues, the reactivity of the NKT cell TCR that is specific for lipid antigens combined with the abundance of NKT cells in the liver and their potent immunomodulatory features suggests NKT cells as critical mediators of hepatic immune homeostatic mechanisms.
Autoimmune liver diseases
Autoimmune hepatitis (AIH) represents next to primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) one of the three main pathogenic entities of idiopathic autoimmune diseases of the liver with distinct clinical, serologic and histologic profiles [31]. In contrast to the immune-mediated damage of small and large intrahepatic bile ducts that are associated with mixed cellular infiltrates in PBC [32] and PSC [33], AIH is characterized by a plasma cell-dominated inflammation of the liver parenchyma [34]. While anti-mitochondrial antibodies represent the hallmark of PBC [32], dominant pathogenic autoantigens specific for PSC and AIH have not yet been identified.
The identification of pathways underlying the generation and propagation of autoimmune lymphocytes [35] in all three disorders has been difficult, as complex interactions between environmental factors and genetic traits are thought to contribute. Interestingly, the immune-mediated damage is specific for defined cell subsets in the liver, although the autoantigens described are ubiquitously expressed in other intra-hepatic and extra-hepatic tissues. Thus, distinct triggers and cell-specific/tissue-specific pathogenic pathways likely initiate the compartment-specific hepatic damage.
The difficulty to induce autoreactive immune responses to liver antigens in animal models reflects one of the problems contributing to the lack of understanding the underlying pathogenic mechanisms. It is even unclear to date whether the antigens that trigger the generation and propagation of autoreactive lymphocytes originate from the liver or extra-hepatic tissue sites. Frequently, liver-specific tolerance can only be broken through the introduction of vectors expressing ectopic antigens under experimental conditions. For example, the infection with a liver-tropic adenovirus expressing human cytochrome P450 2D6 (Ad-2D6), an autoantigen of AIH, triggers significantly more severe liver damage in wild-type mice when compared with transgenic mice expressing the identical human CYP2D6 protein in the liver [36]. Accordingly, the xeno-immunization of mice with human antigens and IL-12 suggested that peripheral tolerance can be directly broken [37] and pathogenic lymphocyte populations propagated/generated in the liver. In contrast, the migration of dysregulated T cells primed in the spleen (along the CCR6-CCL20 axis) is responsible for the induction of fatal hepatic tissue damage in thymectomized PD-1 –/– mice [38].
Different bacteria have been linked to the pathogenesis of PBC and PSC [39,40]. The induction of biliary inflammation in an infection-induced model resembling PBC, for example, was strictly dependent on chronic bacterial persistence [41]. Once disease was established and bacterial infection cleared, however, conventional donor T cells adoptively transferred liver lesions into recipient mice suggesting an autoimmune component. Although the antigens these T cells are reacting to are unknown, molecular mimicry might be critical for the induction and maintenance of biliary liver disease as phylogenetically related proteins of the infectious agent utilized in this model exhibit a striking homology to the mitochondrial hallmark antigens of PBC patients [42,43].
Although multiple triggers including microbial and environmental antigens, genetic predisposition and dys-regulated immune responses have been implicated in the pathogenesis of all three disorders, the exact mechanisms leading to the breakdown of tolerance specifically in the liver environment have remained unknown.
Role of NKT cells
While type II NKT cells protect from AIH [44,45], type I NKT cells promote autoimmune liver diseases: firstly PBC patients reveal a striking redistribution of NKT cells from the blood to the liver [46] and the genetic deletion of NKT cells in mouse models of PBC ameliorates biliary liver pathology [41,47]; secondly NKT cell-deficient mice or animals with reduced hepatic NKT cell numbers exhibit less severe liver pathology than wild-type controls in response to concanavalin A (ConA) in animal models of AIH [25••,48] and the adoptive transfer of hepatic NKT cells sensitized NKT cell-deficient mice to ConA-mediated hepatitis [48]. Albeit there exist no data for PSC in patients or animal models, these studies collectively suggest that type I NKT cells might promote the breakdown of hepatic tolerance and thus play a central role in initiating and perpetuating autoimmune liver damage.
Different cytokines such as TNF-α, IFN-γ and IL-4 that are released upon application of ConA or α-GalCer have been suggested to mediate hepatic inflammation and parenchymal necrosis [49–51]. TNF-α and IFN-γ exacerbate liver injury through the recruitment of dysregulated T cells to the liver [52] or the induction of hypercoagulation [53]. IL-4 promotes the infiltration of the liver parenchyma by neutrophils upon type I NKT cell activation [54]. In contrast, the role of IL-17 has remained controversial [55,56], while IL-15 inhibits ConA-mediated liver injury via type I NKT cell-dependent mechanisms [57].
Type I NKT cells exhibit their pathogenic effects also due to cognate or costimulatory interactions with other tissue-destructive and/or auto-reactive immune cell populations: CD1d-restricted NKT cells, for example, promote the expansion and generation of autoreactive B cell responses [41] and stimulate intrahepatic CD8 T-cell effector responses to antigens expressed in the liver [58]. As NKT cells cross-talk to many other immune cell populations [59], there are likely more cellular circuits involved in these pathogenic mechanisms that need to be elucidated in the future.
Profibrotic effects of NKT cells in liver pathology
NKT cells do not only promote inflammatory immune reactions in the liver, but also contribute to hepatic fibrogenesis: profibrotic effects of NKT cells were observed after the application of xenobiotic or chemical compounds, methionine-choline-deficient diet, in Hepatitis B Virus-transgenic mice as well as in models of PBC and nonalcoholic steatohepatitis [60–67]. Inflammatory mediators drive chronic liver inflammation and progression to liver fibrosis in these models. However, type I NKT cells are not always fibrinogenic: particularly in CCl4-induced liver injury [61] the nature of the lipid antigen presented to the NKT cells appears to be critical whether NKT cells exhibit pro-fibrotic or anti-fibrotic effects.
Regulation of NKT cell activation
Various soluble mediators affect the activation of NKT cells. For example, oxygen radicals released by Kupffer cells promote ConA-hepatitis without affecting the production of cytokines [68]. Signaling through the IL-12 receptor that is constitutively expressed on type I NKT cells enhances the release of Th1 and Th2 cytokines during ConA-mediated hepatitis [69]. In contrast, IL-10 produced by Kupffer cells and regulatory T cells ameliorates ConA-mediated hepatitis [70]. IL-33, a member of the IL-1 family produced by hepatocytes, has been recently associated with protective effects in ConA-mediated liver damage, as it inhibits the systemic release of IFN-γ, IL-17 and TNF-α [71]. Prostaglandin E2 and retinoic acid also suppress the activation of or cytokine production by NKT cells in vivo [72,73].
Furthermore, various cell surface molecules, particularly NK cell receptors and signaling pathways have been implicated in the regulation of NKT cell activation. Qa-1b-CD94/NKG2A interactions inhibit the activation of type I NKT cells as well as ConA-mediated and α-GalCer-mediated hepatic injury [74]. The protective effects of type II NKT cells in a transgenic hepatitis B virus model were mainly related to NKG2D [75] that is expressed by about 50% of NKT cells. NKG2D as well as NK1.1 have been also associated with changes in the cytokine expression profile [76].
In contrast, thioredoxin binding protein modulates 2 (TBP2) promotes the susceptibility to ConA-mediated hepatitis by affecting the lipid metabolism and subsequently NKT cell activity [77•]. Wnt/β-catenin signaling pathways [78••] or CD28/CD80 and PD-1/PD-L1 costimulatory signals [79••] contribute to the modulation of NKT cell anergy. In addition, altered pathways of antigen presentation [80] or the expression of complement receptors during sepsis [81] might drive tissue damage due to an aberrant activation of type I NKT cells.
Furthermore, genetic variations [82] as well as the composition of the intestinal microbiota [83••,84••] influence the NKT cell response in the liver. Interestingly, strain-specific alterations even occur under germ-free conditions [85••].
Conclusions
The abundance of NKT cells in the liver and their remarkable immunomodulatory properties together with their role as critical mediators of hepatic damage in different animal models characterize NKT cells as promising targets for therapeutic intervention in autoimmune liver diseases. Although NKT cells have been characterized as direct effectors of liver damage or adjuvant bystanders under the respective disease conditions, further studies are required for the identification and characterization of firstly, the molecular and cellular mechanisms precipitated by NKT cells that contribute to the break of peripheral tolerance; secondly, the relative and absolute defects of NKT cells in the respective hepatic compartments during different disease stages; thirdly, the tissue-migratory and vessel-evasive capacities of NKT cells upon lipid antigen encounter and massive cytokine exposure and finally, the ability of NKT cells to recruit different immune cells to defined hepatic compartments. Consequently, the analysis of the circuits of these cell–cell interactions and the underlying pathogenic molecular mechanisms will identify therapeutic targets and approaches for clinical intervention in the future.
Acknowledgements
This work is supported by Award R01DK084054 from the National Institute of Diabetes and Digestive and Kidney Diseases, the German Research Foundation Deutsche Forschungsgemeinschaft (MA 2621/2-1 and MA 2621/3-1), and by the Interdisciplinary Center for Clinical Research of the Universitätsklinikum Erlangen (IZKF_JB10_A48).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
of special interest
of outstanding interest
- 1.Hardtke-Wolenski M, Jaeckel E. Mouse models for experimental autoimmune hepatitis: limits and chances. Dig Dis. 2010;28:70–79. doi: 10.1159/000282067. [DOI] [PubMed] [Google Scholar]
- 2.Lukacs-Kornek V, Turley SJ. Self-antigen presentation by dendritic cells and lymphoid stroma and its implications for autoimmunity. Curr Opin Immunol. 2011;23:138–145. doi: 10.1016/j.coi.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steward-Tharp SM, Song YJ, Siegel RM, O'Shea JJ. New insights into T cell biology and T cell-directed therapy for autoimmunity, inflammation, and immunosuppression. Ann N Y Acad Sci. 2010;1183:123–148. doi: 10.1111/j.1749-6632.2009.05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 5.Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol. 2009;31:333–343. doi: 10.1007/s00281-009-0173-4. [DOI] [PubMed] [Google Scholar]
- 6.Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–594. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7•.Ibrahim J, Nguyen AH, Rehman A, Ochi A, Jamal M, Graffeo CS, Henning JR, Zambirinis CP, Fallon NC, Barilla R, et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology. 2012;143:1061–1072. doi: 10.1053/j.gastro.2012.06.003. [This study defines a critical role of the lipid concentrations for the initiation of immunogenic versus tolerogenic immune responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 9.Freigang S, Kain L, Teyton L. Transport and uptake of immunogenic lipids. Mol Immunol. 2013;55:179–181. doi: 10.1016/j.molimm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 11.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 12.Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13:474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 13.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, Trott DL, Ndonye RM, Veerapen N, Besra GS, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tatituri RV, Watts GF, Bhowruth V, Barton N, Rothchild A, Hsu FF, Almeida CF, Cox LR, Eggeling L, Cardell S, et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci U S A. 2013;110:1827–1832. doi: 10.1073/pnas.1220601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blomqvist M, Rhost S, Teneberg S, Löfbom L, Osterbye Må T, Brigl M, Månsson JE, Cardell SL. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur J Immunol. 2009;39:1726–1735. doi: 10.1002/eji.200839001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 19.Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 20.Subleski JJ, Hall VL, Wolfe TB, Scarzello AJ, Weiss JM, Chan T, Hodge DL, Back TC, Ortaldo JR, Wiltrout RH. TCR-dependent and -independent activation underlie liver-specific regulation of NKT cells. J Immunol. 2011;186:838–847. doi: 10.4049/jimmunol.1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 22••.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [This report describes the two basic mechanisms of NKT cell activation during bacterial infection.] [DOI] [PubMed] [Google Scholar]
- 23.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Schmieg J, Yang G, Franck RW, Van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci U S A. 2005;102:1127–1132. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [This report describes the migration of NKT cells in the liver.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CH, Jenne CN, Lee WY, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 29.Emoto M, Mittrücker HW, Schmits R, Mak TW, Kaufmann SH. Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J Immunol. 1999;162:5094–5098. [PubMed] [Google Scholar]
- 30.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Invernizzi P, Lleo A, Podda M. Interpreting serological tests in diagnosing autoimmune liver diseases. Semin Liver Dis. 2007;27:161–172. doi: 10.1055/s-2007-979469. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 33.Bogdanos DP, Invernizzi P, Mackay IR, Vergani D. Autoimmune liver serology: current diagnostic and clinical challenges. World J Gastroenterol. 2008;14:3374–3387. doi: 10.3748/wjg.14.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oo YH, Hubscher SG, Adams DH. Autoimmune hepatitis: new paradigms in the pathogenesis, diagnosis, and management. Hepatol Int. 2010;4:475–493. doi: 10.1007/s12072-010-9183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 36.Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter J, Manns MP, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapierre P, Beland K, Alvarez F. Autoimmune hepatitis experimental model based on adenoviral infections. Hepatology. 2013 doi: 10.1002/hep.26523. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Aoki N, Kido M, Iwamoto S, Nishiura H, Maruoka R, Tanaka J, Watanabe T, Tanaka Y, Okazaki T, Chiba T, Watanabe N. Dysregulated generation of follicular helper T cells in the spleen triggers fatal autoimmune hepatitis in mice. Gastroenterology. 2011;140:1322–1333. doi: 10.1053/j.gastro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Smyk DS, Rigopoulou EI, Bogdanos DP. Potential roles for infectious agents in the pathophysiology of primary biliary cirrhosis: what's new? Curr Infect Dis Rep. 2013;15:14–24. doi: 10.1007/s11908-012-0304-2. [DOI] [PubMed] [Google Scholar]
- 40.Olsson R, Björnsson E, Bäckman L, Friman S, Höckerstedt K, Kaijser B, Olausson M. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. 28. J Hepatol. 1998:426–432. doi: 10.1016/s0168-8278(98)80316-4. [DOI] [PubMed] [Google Scholar]
- 41.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, Scanlon ST, Pendem K, Teyton L, Hart J, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 43.Padgett KA, Selmi C, Kenny TP, Leung PS, Balkwill DL, Ansari AA, Coppel RL, Gershwin ME, et al. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24:209–219. doi: 10.1016/j.jaut.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218:246–250. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 47.Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, Kronenberg M, Flavell RA, Gao B, Gershwin ME. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 48.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biburger M, Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Duan L, Xiong A, Zhang H, Zheng F, Tan Z, Gong F, Fang M. Blockade of IL-33 ameliorates Con A-induced hepatic injury by reducing NKT cell activation and IFN-gamma production in mice. J Mol Med (Berl) 2012;90:1505–1515. doi: 10.1007/s00109-012-0938-4. [DOI] [PubMed] [Google Scholar]
- 51.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwamoto S, Kido M, Aoki N, Nishiura H, Maruoka R, Ikeda A, Okazaki T, Chiba T, Watanabe N. TNF-alpha is essential in the induction of fatal autoimmune hepatitis in mice through upregulation of hepatic CCL20 expression. Clin Immunol. 2013;146:15–25. doi: 10.1016/j.clim.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Kato J, Okamoto T, Motoyama H, Uchiyama R, Kirchhofer D, Van Rooijen N, Enomoto H, Nishiguchi S, Kawada N, Fujimoto J, Tsutsui H. Interferon-gamma-mediated tissue factor expression contributes to T-cell-mediated hepatitis through induction of hypercoagulation in mice. Hepatology. 2013;57:362–372. doi: 10.1002/hep.26027. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: oppositely regulated by IL-4 and IFN-gamma. Hepatology. 2013 doi: 10.1002/hep.26471. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Longhi MS, Liberal R, Holder B, Robson SC, Ma Y, Mieli-Vergani G, Vergani D. Inhibition of interleukin-17 promotes differentiation of CD25(S) cells into stable T regulatory cells in patients with autoimmune hepatitis. Gastroenterology. 2012;142:1526–1535. e6. doi: 10.1053/j.gastro.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 56.Yan S, Wang L, Liu N, Wang Y, Chu Y. Critical role of interleukin-17/interleukin-17 receptor axis in mediating Con A-induced hepatitis. Immunol Cell Biol. 2012;90:421–428. doi: 10.1038/icb.2011.59. [DOI] [PubMed] [Google Scholar]
- 57.Li B, Sun R, Wei H, Gao B, Tian Z. Interleukin-15 prevents concanavalin A-induced liver injury in mice via NKT cell-dependent mechanism. Hepatology. 2006;43:1211–1219. doi: 10.1002/hep.21174. [DOI] [PubMed] [Google Scholar]
- 58.Sprengers D, Sillé FC, Derkow K, Besra GS, Janssen HL, Schott E, Boes M. Critical role for CD1d-restricted invariant NKT cells in stimulating intrahepatic CD8 T-cell responses to liver antigen. Gastroenterology. 2008;134:2132–2143. doi: 10.1053/j.gastro.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 60.Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, Arai K, Takeda K, Watanabe S. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol. 2011;54:1195–1204. doi: 10.1016/j.jhep.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, Zimmermann HW, Pack O, Gassler N, Hittatiya K, et al. Chemokine receptor CXCR6-dependent hepatic NK T cell accumulation promotes inflammation and liver fibrosis. J Immunol. 2013;190:5226–5536. doi: 10.4049/jimmunol.1202909. [DOI] [PubMed] [Google Scholar]
- 63.Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology. 2011;53:219–229. doi: 10.1002/hep.23983. [DOI] [PubMed] [Google Scholar]
- 64.Wu SJ, Yang YH, Tsuneyama K, Leung PS, Illarionov P, Gershwin ME, Chuang YH. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53:915–925. doi: 10.1002/hep.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233–242. doi: 10.1586/17474124.2.2.233. [DOI] [PubMed] [Google Scholar]
- 67.Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets. 2011;10:509–536. doi: 10.2174/187152811798104890. [DOI] [PubMed] [Google Scholar]
- 68.Nakashima H, Kinoshita M, Nakashima M, Habu Y, Shono S, Uchida T, Shinomiya N, Seki S. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology. 2008;48:1979–1988. doi: 10.1002/hep.22561. [DOI] [PubMed] [Google Scholar]
- 69.Zhu R, Diem S, Araujo LM, Aumeunier A, Denizeau J, Philadelphe E, Damotte D, Samson M, Gourdy P, Dy M, et al. The Pro-Th1 cytokine IL-12 enhances IL-4 production by invariant NKT cells: relevance for T cell-mediated hepatitis. J Immunol. 2007;178:5435–5442. doi: 10.4049/jimmunol.178.9.5435. [DOI] [PubMed] [Google Scholar]
- 70.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 71.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 72.Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, Liu Y, Jiang H, Zhang L, Mobley J, McClain C, et al. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J Immunol. 2013;190:3579–3589. doi: 10.4049/jimmunol.1203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee KA, Song YC, Kim GY, Choi G, Lee YS, Lee JM, Kang CY. Retinoic acid alleviates Con A-induced hepatitis and differentially regulates effector production in NKT cells. Eur J Immunol. 2012;42:1685–1694. doi: 10.1002/eji.201142322. [DOI] [PubMed] [Google Scholar]
- 74.Kawamura T, Takeda K, Kaneda H, Matsumoto H, Hayakawa Y, Raulet DH, Ikarashi Y, Kronenberg M, Yagita H, Kinoshita K, et al. NKG2A inhibits invariant NKT cell activation in hepatic injury. J Immunol. 2009;182:250–258. doi: 10.4049/jimmunol.182.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joshi SK, Lang ML. Fine tuning a well-oiled machine: influence of NK1.1 and NKG2D on NKT cell development and function. Int Immunopharmacol. 2013 doi: 10.1016/j.intimp.2013.05.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Okuyama H, Yoshida T, Son A, Oka S, Wang D, Nakayama R, Masutani H, Nakamura H, Nabeshima Y, Yodoi J. Thioredoxin binding protein 2 modulates natural killer T cell-dependent innate immunity in the liver: possible link to lipid metabolism. Antioxid Redox Signal. 2009;11:2585–2593. doi: 10.1089/ars.2009.2691. [This study suggests a connection between lipid metabolism and subsequent NKT cell activation.] [DOI] [PubMed] [Google Scholar]
- 78••.Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, Wang Q, Jiang H, Zhang L, Kronenberg M, Yan J, Miller D, Zhang HG. Intestinal mucus-derived nanoparticle-mediated activation of Wnt/beta-catenin signaling plays a role in induction of liver natural killer T cell anergy in mice. Hepatology. 2013;57:1250–1261. doi: 10.1002/hep.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Wang XF, Lei Y, Chen M, Chen CB, Ren H, Shi TD. PD-1/PDL1 and CD28/CD80 pathways modulate natural killer T cell function to inhibit hepatitis B virus replication. J Viral Hepat. 2013;20(Suppl. 1):27–39. doi: 10.1111/jvh.12061. [These two reports address potential mechanisms underlying NKT cell anergy.] [DOI] [PubMed] [Google Scholar]
- 80.Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F, Teyton L, Savage PB, Bendelac A. Distinct APCs explain the cytokine bias of alpha-galactosylceramide variants in vivo. J Immunol. 2012;188:3053–3061. doi: 10.4049/jimmunol.1102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fusakio ME, Mohammed JP, Laumonnier Y, Hoebe K, Köhl J, Mattner J. C5a regulates NKT and NK cell functions in sepsis. J Immunol. 2011;187:5805–5812. doi: 10.4049/jimmunol.1100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammed JP, Fusakio ME, Rainbow DB, Moule C, Fraser HI, Clark J, Todd JA, Peterson LB, Savage PB, Wills-Karp M, et al. Identification of Cd101 as a susceptibility gene for Novosphingobium aromaticivorans-induced liver autoimmunity. J Immunol. 2011;187:337–349. doi: 10.4049/jimmunol.1003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83••.Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, Borneman J, Kronenberg M, Braun J. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol. 2010;184:1218–1226. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84••.Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, Braun J, Mazmanian SK, Kronenberg M. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85••.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [These three studies suggest an impact of the intestinal microbiota on the distribution and function of NKT cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]