Abstract
Dendritic cells (DCs), the most potent antigen-presenting cells have been extensively applied in clinical trials for evaluation of antitumor immunity. However, the efficacy of DC-mediated cancer vaccines is still limited as they are unable to sufficiently break the immune tolerance. In this study, we constructed a recombinant adenoviral vector (AdVIL-6) expressing IL-6, and generated IL-6 transgene-engineered DC vaccine (DCOVA/IL-6) by transfection of murine bone marrow-derived ovalbumin (OVA)-pulsed DCs (DCOVA) with AdVIL-6. We then assessed DCOVA/IL-6-stimulated cytotoxic T-lymphocyte (CTL) responses and antitumor immunity in OVA-specific animal tumor model. We demonstrate that DCOVA/IL-6 vaccine up-regulates expression of DC maturation markers, secretes transgene-encoded IL-6, and more efficiently stimulates OVA-specific CTL responses and therapeutic immunity against OVA-expressing B16 melanoma BL6-10OVA in vivo than the control DCOVA/Null vaccine. Moreover, DCOVA/IL-6-stimulated CTL responses were relatively maintained in mice with transfer of CD4+25+Foxp3+ Tr-cells, but significantly reduced when treated with anti-IL-6 antibody. In addition, we demonstrate that IL-6 down-regulates Foxp3-expression of CD4+25+Foxp3+ Tr-cells in vitro. Taken together, our results demonstrate that AdV-mediated IL-6 transgene-engineered DC vaccine stimulates potent CTL responses and antitumor immunity by counteracting CD4+25+ Tr immunosuppression via IL-6-induced Foxp3 down-regulation. Thus, IL-6 may be a good candidate for engineering DCs for cancer immunotherapy.
Keywords: dendritic cells (DCs), cytotoxic T lymphocytes (CTLs), interleukin-6 (IL-6), forkhead box P3 (Foxp3), antitumor immunity
1. Introduction
Immune surveillance by CD8+ cytotoxic T lymphocytes (CTLs) represents a major mechanism for the detection and elimination of pathogen-infected cells. CTLs are also essential for effective immunity against tumors [1]. Dendritic cells (DCs) are the most potent professional antigen-presenting cells of the immune system, uniquely capable of stimulating tumor-specific CD4+ and CD8+ T cell immune responses leading to CTL tumor infiltration and tumor regression [2,3]. DC vaccines have been extensively applied in experimental animal models and clinical trials for evaluation of antitumor immunity [4,5]. While only a proportion of the tumor immunotherapy clinical trials carried out so far have yielded positive results, those using DCs as carrier of tumor antigens have obtained the highest rates of success amongst others [6]. However, in general, the efficacy of DC-mediated cancer vaccine is still limited, mostly because DC vaccines are unable to sufficiently break the suppressive tumor microenvironment and immune tolerance in cancer patients [4].
Inflammatory cytokines such as IL-2, IL-6, IL-12, IL-15 and TNF-α play an important role in inflammation, innate and adaptive immunity [7]. To improve the efficacy of DC vaccine, DCs were genetically modified to produce IL-2 or IL-12 [8,9]. These engineered DC vaccines induced potent antitumor immunity via activation of strong CTL responses. It was also demonstrated that IL-15 transgene expression of engineered DCs increased their functional effect and survival, and became resistant to tumor-induced DC apoptosis via up-regulation of DC markers and Bcl-2, respectively [10]. We previously demonstrated that inflammatory cytokine TNF-α transgene-expressing DCs underwent augmented cellular maturation and induced more robust CTL responses and antitumor immunity [11]. However, the impact of genetically modified-DCs with IL-6 transgene in antitumor vaccine has not been studied.
In this study, we cloned murine inflammatory cytokine IL-6 gene from ConA-stimulated T cells by reverse transcription-polymerase chain reaction (RT-PCR) and constructed a recombinant adenoviral vector AdVIL-6 using the cloned IL-6 cDNA. We then generated IL-6 transgene-engineered DC (DCIL-6) vaccine by transfection of murine bone marrow (BM)-derived ovalbumin (OVA)-pulsed DCs (DCOVA) with AdVIL-6 and further assessed DCOVA/IL-6-stimulated CTL responses and antitumor immunity in an OVA-specific animal tumor model.
2. Results and Discussion
2.1. AdVIL-6-Transfected DCs Upregulate Expression of Iab, CD54, CD80 and IL-6
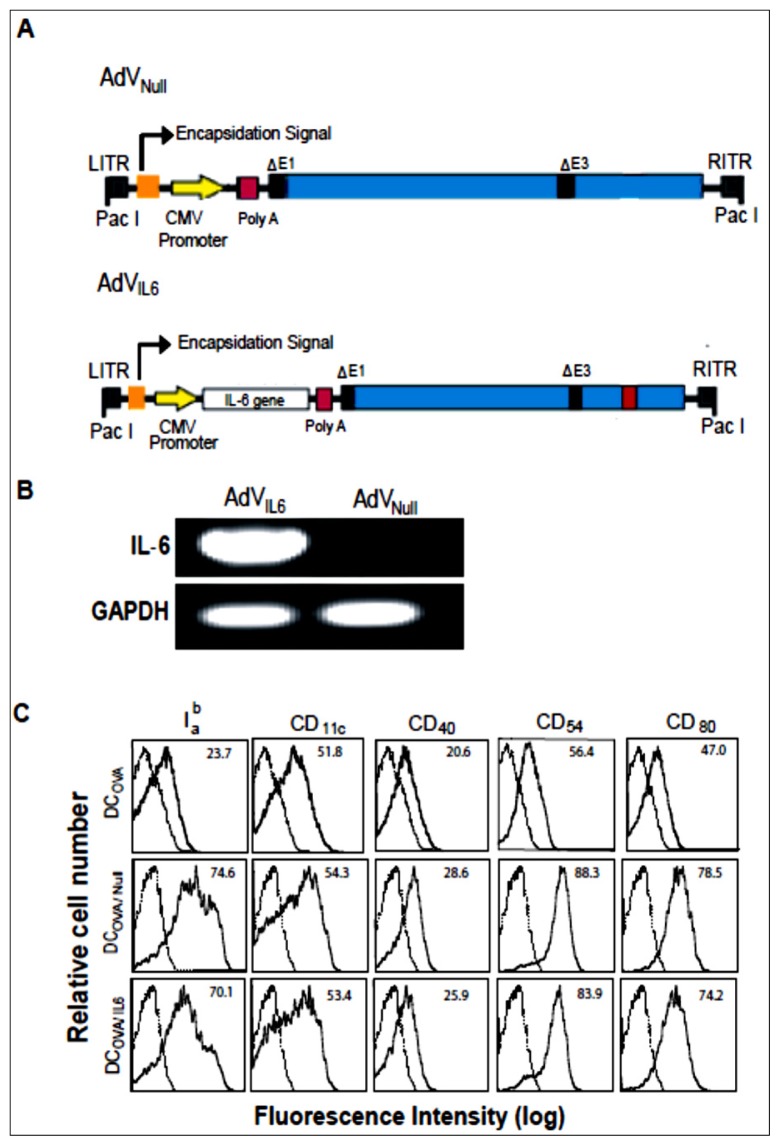
To assess the impact of genetically modified-DCs with IL-6 transgene in antitumor vaccine, we first constructed a recombinant adenoviral vector AdVIL-6 expressing transgene IL-6 under the regulation of the cytomegalovirus (CMV) early/immediate promoter/enhancer (Figure 1A). To assess the transcriptional IL-6 expression, RNA extracted from AdVIL-6 was subjected to reverse transcription-polymerase chain reaction (RT-PCR) analysis using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the loading control. As shown in Figure 1B, a significant amount of IL-6 expression was found in recombinant adenovirus AdVIL-6, but not in the control adenovirus AdVNull without any transgene insertion. We demonstrated that DCOVA expressed DC marker CD11c, adhesion molecule CD54 and DC maturation markers CD40, CD80 and Iab (Figure 1C), and secreted little amount of IL-6 (0.03 ng/mL). We also demonstrated that both AdVIL-6- and AdVNull-transfected DCOVA/IL-6 and DCOVA/Null up-regulated CD40, CD54, CD80, and Iab (Figure 1C), indicating that AdV-mediated transfection enhances DC maturation. In addition, we also found that AdVNull-transfected DCOVA/Null secreted some IL-6 (0.40 ng/mL) whereas AdVIL-6-transfected DCOVA/IL-6 secreted much more IL-6 (1.90 ng/mL), indicating that AdV transfection induces DCs to express the inflammatory cytokine IL-6.
Figure 1.
Phenotypic analysis of transgene IL-6-engineered DCOVA/IL-6 (A) Schematic representation of adenoviral (AdV) vector construct expressing IL-6 gene. The E1/E3 depleted replication-deficient AdV is under the regulation of the cytomegalovirus (CMV) early/immediate promoter/enhancer. ITR, inverted terminal repeat; (B) RT-PCR analysis of RNA obtained from AdVIL-6 and AdVNull [IL-6 Primer sequence: Forward 5′- ACCGC TATGA AGTTC CTCTC TGC -3′; Reverse 5′- AGGCA TAACG CACTA GGTTT GC -3′] [GAPDH Primer sequence: Forward 5′- CAGGT TGTCT CCTGC GACTT -3′; Reverse 5′- CTTGC TCAGT GTCCT TGCTG -3′]; (C) AdV transfected DCs were stained with a panel of Abs (solid lines) or isotype-matched control antibodies (dashed lines) followed by flow cytometric analysis. The value in each panel represents the percentage of positive cells based on the isotype control. One representative experiment of two is shown.
2.2. AdVIL-6-Transfected DCs Stimulate Potent CTL Responses
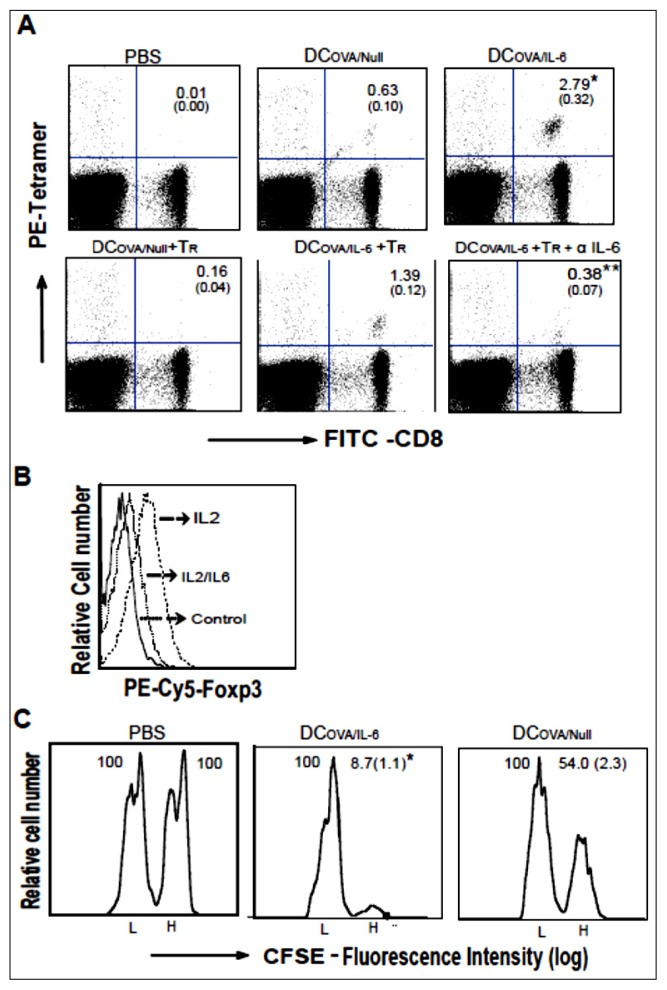
To assess DCOVA/IL-6 vaccine-stimulated CTL responses, we intravenously (i.v.) immunized C57BL/6 mice with DCOVA/IL-6. Six days later, the amount of OVA-specific CD8+ T cells in the peripheral blood was measured using PE-labeled H-2Kb/OVA257–264 tetramer and FITC-anti-CD8+ antibody staining by flow cytometry. As illustrated in Figure 2A, the percentage of double positive (PE-tetramer+ and FITC-CD8+) cells in the total CD8+ population is significantly higher in the DCOVA/IL-6-immunized mice (2.79%) compared to the control DCOVA/Null-immunized mice (0.63%) (p < 0.05), with both immunized groups showing a significant difference compared to the control PBS-immunized mice (p < 0.05), indicating that DCOVA/IL-6 immunization stimulates potent OVA-specific CD8+ T cell responses.
Figure 2.
DCOVA/IL6 stimulates potent CTL responses. (A) C57BL/6 mice were intravenously (i.v.) immunized with PBS, DCOVA/IL-6 and DCOVA/Null. On day six after immunization, mouse tail blood samples were stained with PE-labeled H-2Kb/OVA257–264 tetramer (Beckman-Coulter, Mississauga, ON, Canada) and FITC-labeled anti-CD8+ antibody, followed by flow cytometric analysis. One day after CD4+25+Foxp3+ Tr cells transfer, C57BL/6 mice were i.v. immunized with DCOVA/IL-6, DCOVA/Null and the CTL responses were analyzed by flow cytometry with or without i.v. treatment of anti-IL-6 antibody (0.5 mg/mL). The value in each panel represents the percentage of OVA-specific (tetramer-positive) CD8+ T cells vs. the total CD8+ T cell population. The value in parenthesis represents the standard deviation (SD). * p < 0.05 vs. cohorts of the DCOVA/Null group and ** p < 0.05 vs. cohorts of DCOVA/IL-6 + Tr group (student t test); (B) CD4+25+Foxp3+ Tr cells were incubated with IL-2 with or without IL-6 overnight. After fixation, the cell membranes were permeabilized and then stained with PE-Cy5-conjugated anti-Foxp3+ antibody followed by flow cytometric analysis; (C) In vivo cytotoxicity assay. Six days after immunization, the immunized mice were i.v. injected with a mixture of CFSEhigh and CFSElow-labeled splenocytes (at 1:1 ratio) that had been pulsed with OVAI and the control Mut1 peptide, respectively. After sixteen hours, spleens of immunized mice were removed and the percentages of the residual CFSEhigh (H) and CFSElow (L) target cells remaining in the recipients’ spleens were analyzed by flow cytometry. The value in each panel represents the percentage of CFSEhigh vs. CFSElow target cells remaining in spleen. The value in parenthesis represents the standard deviation (SD). * p < 0.05 vs. cohorts of the DCOVA/Null group (student t test). One representative experiment of two is shown.
2.3. AdVIL-6-Transfected DCs Counteract CD4+25+Foxp3+ Tr Immunosuppression via Transgene Encoded IL-6 Signaling
To assess the potential counteraction of CD4+25+Foxp3+ Tr immunosuppression, the CTL responses of immunized C57BL/6 mice, previously infused with naïve CD4+25+Foxp3+ Tr cells, were assessed by flow cytometry. We demonstrated that DCOVA/Null-stimulated CTL responses were significantly decreased (0.16%) in mice infused with CD4+25+Foxp3+ Tr cells (p < 0.05), whereas DCOVA/IL-6-stimulated CTL responses were relatively maintained (1.39%) in CD4+25+Foxp3+ Tr-infused mice (Figure 2A), indicating that DCOVA/IL-6 vaccine counteracts CD4+25+Foxp3+ Tr immunosuppression. To confirm it, we also blocked IL-6 signaling in DCOVA/IL-6-vaccinated mice by anti-IL-6 antibody treatment. We found that DCOVA/IL-6-stimulated CTL responses became significantly reduced (p < 0.05) in anti-IL-6 antibody-treated mice (Figure 2A), suggesting that DCOVA/IL-6 vaccine counteracts CD4+25+Foxp3+ Tr immunosuppression possibly via transgene-encoded IL-6 signaling.
2.4. IL-6 Induces Foxp3 down-Regulation of CD4+25+Foxp3+ Tr Cells
IL-6 has been reported to inhibit the generation and counteract the immunosuppression of CD4+25+Foxp3+ Tr cells [12,13]. To assess the mechanism for IL-6-induced counteraction, we cultured CD4+25+Foxp3+ Tr cells in the presence or absence of IL-6. We found that IL-6-treated CD4+25+Foxp3+ Tr cells down-regulated Foxp3 expression (Figure 2B), indicating that IL-6-induced counteraction of CD4+25+Foxp3+ Tr immunosuppression may be via Foxp3 down-regulation.
2.5. AdVIL-6-Transfected DC-Stimulated CD8+ T Cells Are Effector CTLs
To analyze the differentiation of DCOVA/IL-6-stimulated CD8+ T cells into effector CTLs, an in vivo cytotoxicity assay was performed. We adoptively i.v. transferred OVAI peptide-pulsed and strongly carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled splenocytes (CFSEhigh) as the OVA-specific target cells, as well as the control peptide-pulsed and weakly CFSE-labeled splenocytes (CFSElow) as the control non-specific target cells into recipient mice six days after immunization with DCOVA/IL-6 and DCOVA/Null. Flow cytometric analysis was performed to examine the ability of activated T cells to induce specific killing of the above target cells sixteen hours after target cell transfer. In Figure 2C, the cell killing was specifically targeted towards OVAI-pulsed CFSEhigh target cells, and the levels of CFSElow cells remain unaffected. Mice immunized with DCOVA/Null had a decrease of 46% OVAI-pulsed CFSEhigh target cells, whereas mice immunized with DCOVA/IL-6 had a significantly greater degree of loss of OVAI-pulsed CFSEhigh target cells (91.3%) (p < 0.05), indicating that DCOVA/IL-6-stimulated CD8+ T cells are effector CTLs with more efficient killing activity for OVA-specific target cells.
2.6. AdVIL-6-Transfected DCs Induce Potent Antitumor Immunity
To study whether DCOVA/IL-6 is capable of inducing therapeutic immunity against six-day-established tumor, we i.v. injected mice with the highly metastatic OVA-expressing B16 melanoma cells BL6-10OVA (1 × 106 cells). Six days later, mice were i.v. immunized with DCOVA/IL-6. Three weeks after tumor cell challenge, mice were sacrificed and numbers of lung metastatic tumor colonies were counted. As shown in Table 1, DCOVA/Null immunization only cured 50% of the mice (4/8). However, the median number of lung tumor colonies in DCOVA/Null-immunized group was 49, which is much less than that (>300) in PBS control group (p < 0.05). In contrast, DCOVA/IL-6 immunization was able to protect 100% of mice (8/8) from tumor growth, indicating that DCOVA/IL-6 vaccine stimulates potent therapeutic immunity against six-day-established B16 melanoma.
Table 1.
DCOVA/IL6 induces therapeutic antitumor immunity.
| Animal groups | Tumor cell challenge | Tumor bearing mice (%) | Median number of lung tumor colonies |
|---|---|---|---|
| DCOVA/Null | BL6-10OVA | 4/8 (50) | 49 ± 13 * |
| DCOVA/IL-6 | BL6-10OVA | 0/8 (0) | 0 |
| PBS | BL6-10OVA | 8/8 (100) | >300 |
C57BL/6 mice were i.v. injected with 1 × 106 OVA-expressing BL6-10OVA tumor cells. Six days after tumor cell injection, mice were i.v. immunized with engineered DCOVA/IL-6, DCOVA/Null, and PBS, respectively. Three weeks after tumor cell challenge, mice were sacrificed and the numbers of lung metastatic tumor colonies were counted.
p < 0.01 vs. cohorts of the DCOVA/IL-6 and PBS groups (Mann-Whitney U test). One representative experiment of three is shown.
2.7. Discussion
Dendritic cells (DCs) are a subset of white blood cells that are critical to most aspects of adaptive immunity because of their central role in initiation of T-cell responses [14–16]. As dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) [14–16], engineering DCs is likely to yield improved therapeutic vaccines [17] by inducing or promoting efficient antitumor immune responses in cancer patients [18]. Previous reports indicate that intratumoral injection of DCs, engineered to express a combination of different cytokines, such as IL-12, IL-21, or IFN-α, showed potent therapeutic effect against established tumors [19]. Vogt et al. [20] have reported that intratumoral injection of adenoviral vector-transfected DCs with IL-12 over-expression was crucial for effective tumor regression. Qu et al. [21] have demonstrated the therapeutic effectiveness of intratumorally delivered DCs engineered to express the pro-inflammatory cytokine IL-32.
The cytokine IL-6 secreted by many different cells, including the monocyte/macrophages, fibroblasts, endothelial cells, keratinocytes, mast cells, T cells, and DCs acts as a central regulator of inflammatory processes [22]. It plays a key role in progression from the initial innate immune responses to infection to adaptive immune responses [23]. IL-6 is involved in the maturation of B cells and development of a major proinflammatory T cell population, the pathogenic CD4+ Th17 cells [24,25]. IL-6 has been reported to inhibit the generation of and counteract the immunosuppression of CD4+25+ Tr cells [12,13]. We have shown that IL-6 counteracts CD4+ Th2 cell’s IL-10-mediated immunosuppression [26]. These unique characteristics of IL-6 suggest that it may be a good candidate transgene to engineer DCs for the development of new DC-based vaccines capable of overcoming immunosuppression leading to potent CTL responses and antitumor immunity.
To assess the impact of genetically modified-DCs with IL-6 transgene in antitumor vaccine, we constructed the recombinant adenoviral vector AdVIL-6 expressing transgene IL-6 and the control adenoviral vector AdVNull without any transgene insertion. We have previously shown that AdV transfected DCs expressed inflammatory cytokines such as IL-1β and IL-12 [27]. In this study, we found that AdVNull-transfected DCOVA/Null secreted IL-6 (0.4 ng/mL), indicating that AdV transfection also induces DCs to express inflammatory cytokine IL-6. Furthermore, AdVIL-6-transfected DCOVA/IL-6 secreted much more IL-6 (1.95 ng/mL), indicating that DCOVA/IL-6 cells also secrete transgene-encoded IL-6. In addition, we also demonstrated that both AdVIL-6- and AdVNull-transfected DCOVA/IL-6 and DCOVA/Null up-regulated CD54, CD80 and Iab, indicating that AdV-mediated transfection enhances DC maturation, which is consistent with several previous reports [27–29]. The AdV-induced DC maturation has been shown to be linked to NF-κB-dependent [30] and PI3 kinase-mediated TNF-α induction pathway [31].
In this study, we demonstrated that DCOVA/IL-6 vaccine stimulates potent effector CTL responses and immunity against OVA-expressing B16 melanoma. The polyclonal naïve CD4+25+Foxp3+ Tr cells develop in the thymus and then enter peripheral tissues where they suppress the activation of other self-reactive T cells [32]. The transcription factor Foxp3 controls regulatory T cell development [33,34]. The activation and transgene expression of Foxp3 have been reported to induce immune suppressive effects of T cells, DCs and macrophages [35,36]. It has also been shown that an elevated number of Tr cells was detected in tumors [37,38], which suppressed the antitumor immune responses by inhibition of T cell proliferation and effector function [39–41] as well as DC maturation [42]. Therefore, the question of how to combat immune tolerance becomes a critical challenge in cancer vaccine development [43]. In this study, we demonstrate that DCOVA/IL-6 vaccine counteracts CD4+25+Foxp3+ Tr-mediated immunosuppression in mice with transfer of purified naïve CD4+25+Foxp3+ Tr cells. In addition, for the first time, we demonstrate that the counteraction of CD4+25+Foxp3+ Tr suppression by DCOVA/IL-6 vaccine is derived from transgene-encoded IL-6 signaling and possible via IL-6-induced Foxp3 down-regulation. OVA protein is a well-established model antigen to study anti-tumor immunity [44]. Many previous studies also have used OVA as a model antigen for tumor immunotherapeutic studies [45–47]. However, future study using less immunogenic tumor antigen will be interesting. Overall, our results suggest that IL-6 may overcome Tr-mediated suppression of antigen-specific T cell responses in tumor microenvironments. It has also been demonstrated that IL-6 activates in vivo T cell responses [12] and exerts anti-apoptotic activity on a wild variety of cells, including the naïve and activated T cells [48–50]. Therefore, the potent CTL responses and antitumor immunity induced by DCOVA/IL-6 vaccine may be derived from a combination of the above transgene-encoded IL-6-mediated stimulatory effects.
3. Experimental Section
3.1. Reagents, Cell Lines and Animals
The biotin-labeled antibodies (Abs) specific for CD11c, CD40, CD54, CD80, Iab, and fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled Abs specific for CD4, CD8, and CD44 were obtained from PharMingen Canada Inc. (Mississauga, ON, Canada). The anti-ovalbumin (OVA) Ab was obtained from Sigma (Oakville, ON, Canada). The PE-labeled H-2Kb/OVA257–264 tetramer was obtained from Beckman Coulter (San Diego, CA, USA). The PE-Cy5-conjugated anti mouse Foxp3 antibody was obtained from eBioscience Inc. (San Diego, CA, USA). The highly lung metastatic OVA-expressing B16 melanoma cell line BL6-10OVA was generated in our laboratory [51]. Naïve C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). All animal experiments were carried out in accordance with the Canadian Council for Animal Care guidelines.
3.2. Recombinant Adenovirus Construction
The construction of recombinant adenovirus (AdV) expressing IL-6 (AdVIL-6) was performed by insertion of mouse IL-6 gene cloned from ConA-stimulated T cells into pShuttle vector (Stratagene Inc., La Jolla, CA, USA) using the cloned IL-6 cDNA to form pLpAIL-6 expressing IL-6 gene [11]. The PmeI-digested shuttle vector was then co-transformed into BJ5183 E. coli cells already containing the backbone vector for homologous recombination to form the recombinant vector AdVIL-6 as described previously (Figure 1A) [52,53]. The control AdVNull without any transgene insert was previously constructed in our laboratory (Figure 1A) [52,53]. The recombinant AdVIL-6 vector was then linearized by PacI digestion, and then transfected into 293 cells using lipofectamine (Gibco/BRL, Burlington, ON, Canada) to generate recombinant adenovirus AdVIL-6 as described previously [52,53]. All recombinant AdVs were amplified in 293 cells and purified by cesium chloride ultracentrifugation gradients [52,53]. To assess transgene IL-6 expression, we performed RT-PCR using RNA purified from AdVIL-6-transfected 293 cells as described previously [52,53].
3.3. Preparation of Dendritic Cells
C57BL/6 mouse bone marrow (BM)-derived dendritic cells (DCs) were prepared as described previously [1]. Briefly, BM cells from femora and tibia of naïve C57BL/6 mice were depleted of red blood cells with 0.84% Tris-ammonium chloride, and plated in DC culture medium (Dulbecco’s Modified Eagle Medium (DMEM) plus 10% fetal calf serum (FCS), granulocyte macrophage colony-stimulation factor (GM-CSF) (20 ng/mL) and IL-4 (20 ng/mL)). On day three, the non-adherent granulocytes, T and B cells were gently removed and fresh media was added. After two days, the loosely adherent proliferating DC aggregates were dislodged and re-plated. On day six, DCs displaying typical morphologic features (that is, numerous dendritic processes) were further pulsed with ovalbumin (OVA) (0.5 mg/mL) for overnight and termed DCOVA.
3.4. Preparation of CD4+25+Foxp3+ Regulatory T (Tr) Cells
Mouse splenocytes were first depleted of red cells with 0.84% Tris-ammonium chloride. T cells were purified by passing splenocytes through nylon wool-columns as described previously [1]. Naïve CD4+ T cells were purified by using Dynal CD8 microbeads (Dynal Inc., Lake Success, NY, USA), and CD4+25+Foxp3+ Tr cells were then further purified from naïve CD4+ T cell population using biotin-anti-CD25 antibody and anti-biotin MACS beads (Miltenyi Biotech, Auburn, CA, USA), as previously described [54].
3.5. Adenovirus (AdV) Transfection of DCs
DCOVA were transfected with AdVIL-6 expressing the transgene IL-6 and control AdVNull without any transgene insert at a multiplicity of infection (MOI) of 150 to form DCOVA/IL-6 and DCOVA/Null vaccines as previously described [52,53]. The transfected cells were then harvested for phenotypic analysis by flow cytometry. Moreover, the supernatants of DCOVA/IL-6 and DCOVA/Null were assessed for the secretion of IL-6 using the IL-6 enzyme-linked immunosorbent assay (ELISA) kit (BD Bioscience, Mississauga, ON, Canada).
3.6. Flow Cytometric Analysis
For phenotypic analysis, DCOVA, DCOVA/IL-6, and DCOVA/Null were stained with biotin-conjugated anti-mouse antibodies (2 mg/mL) specific for major histocompatibility complex (MHC) class II (Iab), CD40, CD54 or CD80 and the cells were analyzed by flow cytometry. For tetramer analysis, peripheral blood of immunized C57BL/6 mice [DCOVA, DCOVA/IL-6, and DCOVA/Null (1 × 106 cells/mouse)] were stained with PE-labeled H-2Kb/OVA257–264 tetramer and FITC-labeled anti-CD8 antibody on day six after immunization followed by flow cytometric analysis. In another set of experiments, mice were first i.v. transferred with CD4+25+Foxp3+ Tr cells (1 × 106 cells/mouse). One day after Tr cell transfer, mice were i.v. immunized with DCOVA/IL-6, DCOVA/Null with or without i.v. treatment of anti-IL-6 antibody (0.5 mg/mL), and the CTL responses were analyzed six days after immunization by flow cytometry. To assess Foxp3 expression, CD4+25+Foxp3+ Tr cells were incubated in culture medium with IL-2 (40 units/mL) in the presence or absence of IL-6 (40 ng/mL) for overnight. The cells were permeabilized with cytofix/cytoperm solution (BD Biosciences, San Diego, CA, USA) and then stained with PE-Cy5-conjugated anti-Foxp3 antibody followed by flow cytometric analysis.
3.7. In Vivo Cytotoxicity Assay
The in vivo cytotoxicity assay was performed as described previously [51]. Briefly splenocytes derived from naïve C57BL/6 mice were incubated with high (3.0 μM, CFSEhigh) or low (0.6 μM, CFSElow) concentrations of CFSE. CFSEhigh cells were further pulsed with OVAI (OVA257–264) peptide (SIINFEKL), and washed extensively to remove free peptide. However, the CFSElow cells were pulsed with the control Mut peptide (FEQNTAQP) to become the internal controls. CFSEhigh and CFSElow target cells were co-injected i.v. at a ratio of 1:1 into the above immunized mice six days after immunization. Sixteen hours after injection, spleens were removed from the immunized mice to analyze the residual OVA-specific CFSEhigh and irrelevant control CFSElow target cells remaining in recipients’ spleens by flow cytometry.
3.8. Animal Studies
For evaluation of therapeutic antitumor immunity, C57BL/6 mice were first challenged by i.v. injection with 1 × 106 OVA-expressing BL6-10OVA tumor cells. Six days after tumor cell injection, mice were vaccinated i.v. with 1 × 106 engineered DCOVA/IL-6, DCOVA/Null, and PBS, respectively. Three weeks after tumor cell challenge, mice were sacrificed, and numbers of lung metastatic tumor colonies were counted. The metastasis on freshly isolated lungs appeared as discrete black pigmented foci that can be easily distinguishable from normal lung tissues and further confirmed by histopathological examination. Tumor metastatic foci too numerous to count were assigned an arbitrary value of >300.
3.9. Statistical Analyses
Statistical analyses were performed using Student’s t-test or Mann-Whitney U test to compare variables from different groups [54]. A value of p < 0.05 is considered significant.
4. Conclusions
The results of our study demonstrate that AdV-mediated IL-6 transgene-engineered DC vaccine stimulates potent CTL responses and antitumor immunity by counteracting CD4+25+ Tr immunosuppression via IL-6-induced Foxp3 down-regulation. Thus, IL-6 may be a good candidate for engineering DCs for effective cancer immunotherapy.
Acknowledgments
This research work was supported by research grants from Canadian Breast Cancer Foundation (CBCF) and Saskatchewan Cancer Agency. Kalpana Kalyanasundaram Bhanumathy was supported by the Terry Fox Postdoctoral Fellowship of Saskatchewan Health Research Foundation (SHRF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ye Z., Tang C., Xu S., Zhang B., Zhang X., Moyana T., Yang J., Xiang J. Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell. Mol. Immunol. 2007;4:277–285. [PubMed] [Google Scholar]
- 2.Klebanoff C.A., Acquavella N., Yu Z., Restifo N.P. Therapeutic cancer vaccines: Are we there yet? Immunol. Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palucka K., Ueno H., Banchereau J. Recent developments in cancer vaccines. J. Immunol. 2011;186:1325–1331. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L., Senovilla L., Vacchelli E., Eggermont A., Fridman W.H., Galon J., Sautès-Fridman C., Tartour E., Zitvogel L., Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melief C.J. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluns K.S., Lefrancois L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama Y., Watanabe M., Maruyama K., Ruscetti F.W., Wiltrout R.H., Yamaguchi K. Enhancement of antitumor immunity against B16 melanoma tumor using genetically modified dendritic cells to produce cytokines. Gene Ther. 2000;7:2113–2121. doi: 10.1038/sj.gt.3301353. [DOI] [PubMed] [Google Scholar]
- 9.Ojima T., Iwahashi M., Nakamura M., Matsuda K., Naka T., Nakamori M., Ueda K., Ishida K., Yamaue H. The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen. Int. J. Oncol. 2006;28:947–953. [PubMed] [Google Scholar]
- 10.Tourkova I.L., Yurkovetsky Z.R., Gambotto A., Makarenkova V.P., Perez L., Balkir L., Robbins P.D., Shurin M.R., Shurin G.V. Increased function and survival of IL-15-transduced human dendritic cells are mediated by up-regulation of IL-15Ralpha and Bcl-2. J. Leukoc. Biol. 2002;72:1037–1045. [PubMed] [Google Scholar]
- 11.Zhang W., Chen Z., Li F., Kamencic H., Juurlink B., Gordon J.R., Xiang J. Tumour necrosis factor-alpha (TNF-alpha) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and anti-tumour immunity than DCs generated in recombinant TNF-alpha. Immunology. 2003;108:177–188. doi: 10.1046/j.1365-2567.2003.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasare C., Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Schuler G., Steinman R.M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;17:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strioga M.M., Felzmann T., Powell D.J., Jr., Ostapenko V., Dobrovolskiene N.T., Matuskova M., Michalek J., Schijns V.E. Therapeutic dendritic cell-based cancer vaccines: The state of the art. Crit. Rev. Immunol. 2013;33:489–547. doi: 10.1615/critrevimmunol.2013008033. [DOI] [PubMed] [Google Scholar]
- 19.Huang C., Ramakrishnan R., Trkulja M., Ren X., Gabrilovich D.I. Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines. Cancer Immunol. Immunother. 2012;61:573–579. doi: 10.1007/s00262-011-1198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt A., Sievers E., Lukacs-Kornek V., Decker G., Raskopf E., Meumann N., Büning H., Sauerbruch T., Strassburg C.P., Schmidt-Wolf I.G., et al. Improving immunotherapy of hepatocellular carcinoma (HCC) using dendritic cells (DC) engineered to express IL-12 in vivo. Liver Int. 2013 doi: 10.1111/liv.12284. [DOI] [PubMed] [Google Scholar]
- 21.Qu Y., Taylor J.L., Bose A., Storkus W.J. Therapeutic effectiveness of intratumorally delivered dendritic cells engineered to express the pro-inflammatory cytokine, interleukin (IL)-32. Cancer Gene Ther. 2011;18:663–673. doi: 10.1038/cgt.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taga T., Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 23.Naugler W.E., Karin M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 25.Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 26.Ankathatti Munegowda M., Xu S., Freywald A., Xiang J. CD4+ Th2 cells function alike effector Tr1 and Th1 cells through the deletion of a single cytokine IL-6 and IL-10 gene. Mol. Immunol. 2012;51:143–149. doi: 10.1016/j.molimm.2012.02.120. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Zhang X., Zhang W., Chen Z., Chan T., Ali K., Jia Z., Xiang J. Adenovirus-mediated CD40 ligand gene-engineered dendritic cells elicit enhanced CD8(+) cytotoxic T-cell activation and antitumor immunity. Cancer Gene Ther. 2002;9:202–208. doi: 10.1038/sj.cgt.7700429. [DOI] [PubMed] [Google Scholar]
- 28.Hirschowitz E.A., Weaver J.D., Hidalgo G.E., Doherty D.E. Murine dendritic cells infected with adenovirus vectors show signs of activation. Gene Ther. 2000;7:1112–1120. doi: 10.1038/sj.gt.3301210. [DOI] [PubMed] [Google Scholar]
- 29.Rouard H., Leon A., Klonjkowski B., Marquet J., Tenneze L., Plonquet A., Agrawal S.G., Abastado J.P., Eloit M., Farcet J.P., et al. Adenoviral transduction of human “clinical grade” immature dendritic cells enhances costimulatory molecule expression and T-cell stimulatory capacity. J. Immunol. Methods. 2000;241:69–81. doi: 10.1016/s0022-1759(00)00214-3. [DOI] [PubMed] [Google Scholar]
- 30.Morelli A.E., Larregina A.T., Ganster R.W., Zahorchak A.F., Plowey J.M., Takayama T., Logar A.J., Robbins P.D., Falo L.D., Thomson A.W. Recombinant adenovirus induces maturation of dendritic cells via an NF-κB-dependent pathway. J. Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philpott N.J., Nociari M., Elkon K.B., Falck-Pedersen E. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA. 2004;101:6200–6205. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluestone J.A., Abbas A.K. Natural vs. adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 33.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 34.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 35.Hong J., Li N., Zhang X., Zheng B., Zhang J.Z. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc. Natl. Acad. Sci. USA. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipscomb M.W., Taylor J.L., Goldbach C.J., Watkins S.C., Wesa A.K., Storkus W.J. DC expressing transgene Foxp3 are regulatory APC. Eur. J. Immunol. 2010;40:480–493. doi: 10.1002/eji.200939667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liyanage U.K., Moore T.T., Joo H.G., Tanaka Y., Herrmann V., Doherty G., Drebin J.A., Strasberg S.M., Eberlein T.J., Goedegebuure P.S., et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 38.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 39.Levings M.K., Sangregorio R., Roncarolo M.G. Human CD25(+)CD4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin B., Banz A., Bienvenu B., Cordier C., Dautigny N., Bécourt C., Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J. Immunol. 2004;172:3391–3398. doi: 10.4049/jimmunol.172.6.3391. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa H., Jäger E., Ritter G., Old L.J., Gnjatic S. CD4+CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 42.Misra N., Bayry J., Lacroix-Desmazes S., Kazatchkine M.D., Kaveri S.V. Cutting edge: Human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J. Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 43.Pardoll D.M. Cancer vaccines. Nat. Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed K.A., Wang L., Munegowda M.A., Mulligan S.J., Gordon J.R., Griebel P., Xiang J. Direct in vivo evidence of CD4+ T cell requirement for CTL response and memory via pMHC-I targeting and CD40L signaling. J. Leukoc. Biol. 2012;92:289–300. doi: 10.1189/jlb.1211631. [DOI] [PubMed] [Google Scholar]
- 45.Moulin V., Morgan M.E., Eleveld-Trancikova D., Haanen J.B., Wielders E., Looman M.W., Janssen R.A., Figdor C.G., Jansen B.J., Adema G.J. Targeting dendritic cells with antigen via dendritic cell-associated promoters. Cancer Gene Ther. 2012;19:303–311. doi: 10.1038/cgt.2012.2. [DOI] [PubMed] [Google Scholar]
- 46.Yewdall A.W., Drutman S.B., Jinwala F., Bahjat K.S., Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato M., Chamoto K., Nishimura T. A novel tumor-vaccine cell therapy using bone marrow-derived dendritic cell type 1 and antigen-specific Th1 cells. Int. Immunol. 2003;15:837–843. doi: 10.1093/intimm/dxg081. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Sasson S.Z., Makedonski K., Hu-Li J., Paul W.E. Survival and cytokine polarization of naive CD4(+) T cells in vitro is largely dependent on exogenous cytokines. Eur. J. Immunol. 2000;30:1308–1317. doi: 10.1002/(SICI)1521-4141(200005)30:5<1308::AID-IMMU1308>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 49.Curnow S.J., Scheel-Toellner D., Jenkinson W., Raza K., Durrani O.M., Faint J.M., Rauz S., Wloka K., Pilling D., Rose-John S., et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J. Immunol. 2004;173:5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 50.Teague T.K., Schaefer B.C., Hildeman D., Bender J., Mitchell T., Kappler J.W., Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J. Exp. Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia D., Hao S., Xiang J. CD8+ cytotoxic T-APC stimulate central memory CD8+ T cell responses via acquired peptide-MHC class I complexes and CD80 costimulation, and IL-2 secretion. J. Immunol. 2006;177:2976–2984. doi: 10.4049/jimmunol.177.5.2976. [DOI] [PubMed] [Google Scholar]
- 52.Chan T., Sami A., El-Gayed A., Guo X., Xiang J. HER-2/neu-gene engineered dendritic cell vaccine stimulates stronger HER-2/neu-specific immune responses compared to DNA vaccination. Gene Ther. 2006;13:1391–1402. doi: 10.1038/sj.gt.3302797. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Xie Y., Chan T., Sami A., Ahmed S., Liu Q., Xiang J. Adjuvant effect of HER-2/neu-specific adenoviral vector stimulating CD8+ T and natural killer cell responses on anti-HER-2/neu antibody therapy for well-established breast tumors in HER-2/neu transgenic mice. Cancer Gene Ther. 2011;18:489–499. doi: 10.1038/cgt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y., Zhang X., Zhao T., Li W., Xiang J. Natural CD8+25+ regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyteYewdall-mediated immunity against B16 melanoma. Biochem. Biophys. Res. Commun. 2013;438:152–155. doi: 10.1016/j.bbrc.2013.07.044. [DOI] [PubMed] [Google Scholar]