Abstract
This study was designed to evaluate the effect of low level of Aflatoxin B1 (AFB1) on oxidative stress, immune reaction and inflammation response and the possible ameliorating effects of dietary alpha-lipoic acid (α-LA) in broilers. Birds were randomly allocated into three groups and assigned to receive different diets: basal diet, diet containing 74 μg/kg AFB1, and 300 mg/kg α-LA supplementation in diet containing 74 μg/kg AFB1 for three weeks. The results showed that the serum levels of malondialdehyde, tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) in the AFB1-treated group were significantly increased than the control group. In addition, the increased expressions of interleukin 6 (IL6), TNFα and IFNγ were observed in birds exposed to the AFB1-contaminated diet. These degenerative changes were inhibited by α-LA-supplement. The activities of total superoxide dismutase and glutathione peroxidase, the levels of humoral immunity, and the expressions of nuclear factor-κB p65 and heme oxygenase-1, however, were not affected by AFB1. The results suggest that α-LA alleviates AFB1 induced oxidative stress and immune changes and modulates the inflammatory response at least partly through changes in the expression of proinflammatory cytokines of spleen such as IL6 and TNFα in broiler chickens.
Keywords: lipoic acid, aflatoxin, alleviate, inflammation, chickens
1. Introduction
Aflatoxins (AFs), produced mainly by Aspergillus. flavus and A. parasiticus [1,2] and usually found in various agricultural commodities [3], are known to be very dangerous mycotoxins. Aflatoxin B1 (AFB1), is one of the most commonly found metabolites and exhibits the highest toxigenic effects [4], which can induce reactive oxygen species (ROS) generation which causes oxidative stress, leading to impairment of DNA, RNA, proteins, lipids, and so on [5]. Meanwhile, AFB1 has immunosuppressive properties, and mainly exerts its effects on cell-mediated immunity while inducing an inflammatory response [3]. It is also increasingly recognized in long term consumption of low levels of AFB1, naturally occurring under some field conditions and in some food/feed, and can be harmful to animal and human health [6]. Furthermore, natural toxins probably pose greater threat to human and animal health than synthetic toxins [7]. However, information on the immunosuppressive effects of low dose AFB1 from naturally contaminated feed is limited, particularly with respect to the effects of low doses of AFB1 on the expression of inflammation-related genes.
Several studies have described alpha-lipoic acid (α-LA) and its reduced form dihydrolipoic acid (DHLA), as an “ideal” antioxidant couple, due to their very high protection ability against oxidative stress through multiple pathways [8,9]. Subsequent studies have shown that α-LA has been used in the prevention or treatment of several pathological conditions that are mediated via oxidative stress. Interestingly, it has been shown that α-LA can inhibit the release of various cytokines, including tumor necrosis factor alpha (TNFα) and interleukin 6 (IL6) [10]. Sola et al. also found that α-LA had an effect on reducing body inflammation response in the areas with metabolic syndrome [11].
Although many data on the ability of α-LA to improve antioxidant defenses and immune function and inhibit inflammatory response are available, it is not clear whether α-LA supplementation could also reverse the inflammatory status induced by AFB1 in broiler chickens. In this study, we investigated the effects of AFB1 contaminated diets on oxidative status, immunity, and the expression of inflammation-related genes of spleen in broiler chickens and whether the supplementation of α-LA is able to counteract its negative effects.
2. Results
2.1. Effect on Serum Oxidant and Antioxidant Status
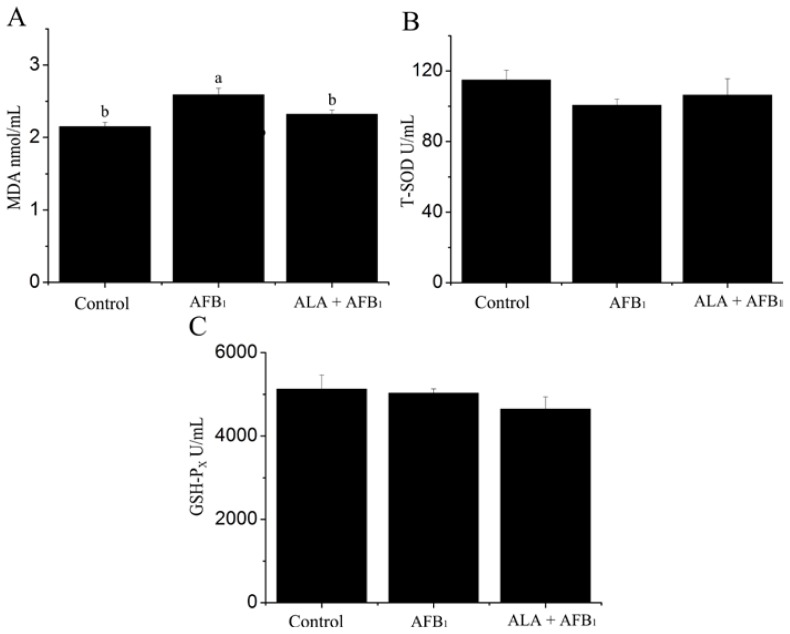
Serum oxidant and antioxidant parameters of birds fed dietary treatments are summarized in Figure 1. The administration of AFB1 resulted in a significant increase in serum malondialdehyde (MDA) content when compared to untreated control group (p < 0.05; Figure 1A). Activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX), however, were not affected by AFB1 treatment (p > 0.05) (Figure 1B,C). Supplementation of α-LA inhibited the elevating MDA levels upon AFB1 administration (Figure 1A). These results indicated that the free radicals in serum were effectively scavenged when treated with α-LA.
Figure 1.
Effect of alpha-lipoic acid (α-LA) on serum antioxidant parameters in broilers fed a diet containing aflatoxin B1 (AFB1). Lipid peroxidation marker: (A) MDA (malondialdehyde); Antioxidant enzymes: (B) T-SOD (total superoxide dismutase) and (C) GSH-PX (glutathione peroxidase). (n = 6). a,b Means with different letters are significantly different, p < 0.05.
2.2. Effect on Serum Immune Response
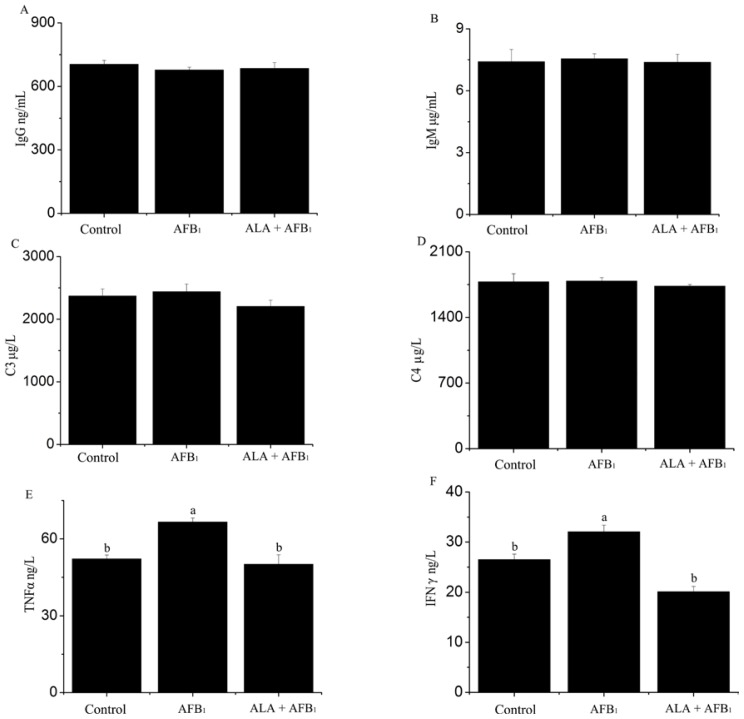
Serum immune parameters of the experimental groups are summarized in Figure 2. There was a significant increase in TNFα and interferon gamma (IFNγ) in AFB1-treated chickens (p < 0.05; Figure 2E,F). However, this increase was prevented in AFB1-fed birds supplemented with α-LA, suggesting that α-LA supplementation can down-regulate the inflammatory processes occurring in serum of birds by AFB1. In addition, Figure 2A–D show that neither immunoglobulin G (IgG), immunoglobulin M (IgM), nor complement 3 (C3), complement 4 (C4) serum concentrations were affected by AFB1 (p > 0.05), suggesting that lower dosage AFB1 maybe not affect humoral immune response.
Figure 2.
Effect of alpha-lipoic acid on serum immune response in broilers fed a diet containing aflatoxin B1 (AFB1). Humoral immunity: (A) IgG (immunoglobulin G); (B) IgM (immunoglobulin M); (C) C3 (complement 3); and (D) C4 (complement 4); Cytokines: (E) TNFα (tumor necrosis factor alpha) and (F) IFNγ (interferon gamma). (n = 6). a,b Means with different letters are significantly different, p <0.05.
2.3. Effect on Gene mRNA Expression
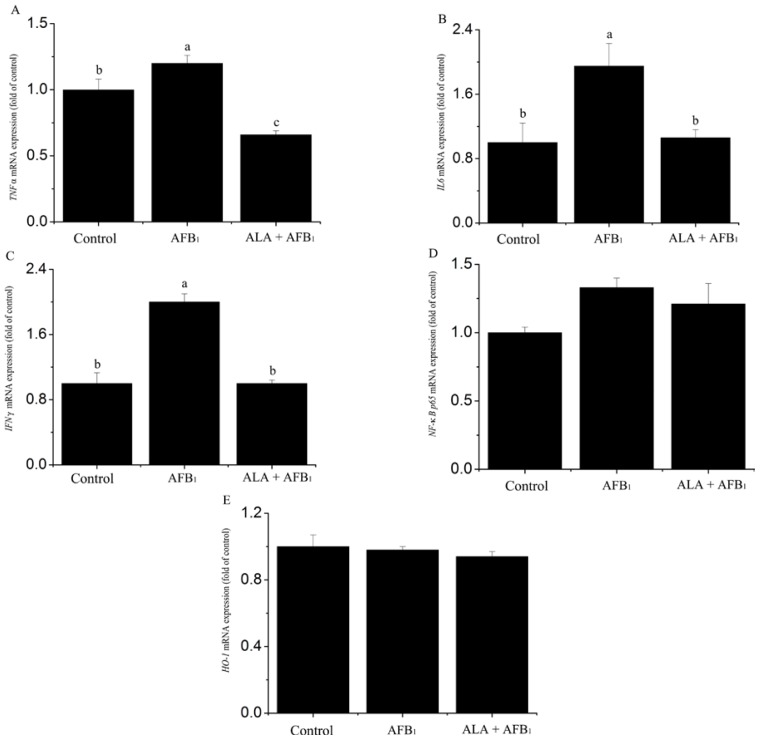
The mRNA expression of inflammation-related genes was measured by real-time polymerase chain reaction (PCR) in spleen samples collected at the end of the experiment (Figure 3). In birds exposed to the AFB1-contaminated diet, the mRNA expression of IL6, TNFα and IFNγ was up-regulated (p < 0.05; Figure 3A–C). However, neither the gene expression of nuclear factor-κB p65 (NF-κB p65) nor that of heme oxygenase-1 (HO-1) was altered by AFB1 (p > 0.05; Figure 3D,E). Increased expression of IL6, TNFα and IFNγ genes due to AFB1 was prevented (p < 0.05) by the addition of α-LA to the AFB1 diet (Figure 3A–C).
Figure 3.
Effect of alpha-lipoic acid on inflammation-related genes of spleen in broilers fed a diet containing aflatoxin B1 (AFB1). (A) TNFα (tumor necrosis factor alpha); (B) IL6 (interleukin 6); (C) IFNγ (interferon gamma); (D) NF-κB p65 (nuclear factor-κB p65); and (E) HO-1 (Heme oxygenase-1). (n = 6). a,b,c Means with different letters are significantly different, p < 0.05.
3. Discussion
Previous studies have shown that acute aflatoxicosis can pose a considerable threat to productivity, tissue development and biochemical parameters [12]. Moreover, chronic AF treatment in animals can lead to poor growth performance [13], alter immune health and systemic inflammation, and cause tissue damage [14]. In this study, the effects of AFB1 of 74 μg/kg on productivity parameters (body weight (BW) gain, feed consumption, and feed conversion ratio) and the relative weights of spleens did not show significant changes compared to control group (p > 0.05; data not shown). This might be due to the low dosage of AFB1 used in the diet for broiler chickens. Similar results for chronic exposure to AFB1 have also been reported in previous studies [15,16]. It is likely that these parameters were not sufficiently sensitive to the chronic effects of low dose of AFB1.
One of the aims of the current study was to investigate the effects of AFB1 contaminated diets and the α-LA addition of feed on the serum oxidative stress. Our results showed that dietary exposure to AFB1 increased the serum MDA levels. The MDA level, is widely used as an indicator of lipid peroxidation (LPO), and the increase in LPO levels is associated with oxidative stress [17], which may result in pathological conditions and diseases [18]. In this study, low dose of AFB1 (74 μg/kg) might have resulted in overall oxidative stress in broiler chickens. It was previously reported that AFB1 increased the production of lipid peroxidation in broiler chickens [19] and hens [20]. The induction of oxidative stress is commonly related with an imbalance between oxidants system and antioxidants system [21]. It is of surprise that exposed AFB1 diets had no effect on the indicators of the enzymatic antioxidant systems of serum in chickens (p > 0.05). These results suggest that oxidative damage induced by AFB1 mainly through increasing the lipid peroxidation of serum in this model using chickens exposed to AFB1 contamination at a low dose of 74 μg/kg.
It is well-known that the immune system is very sensitive to AFs [22,23]. The immune system mainly involves the humoral immunity and cell-mediated immunity. In addition, there are different effects of AFB1 on the humoral immunity according to different species studies, but the effects are less sensitive than those on the cell-mediated immunity. It has been found that AFs can affect humoral immune [24,25]. However, in the present study, the results showed no significant changes in the serum IgG, IgA, IgM, C3 and C4 (Figure 2) in the consumption of AFB1 contaminated diets chickens were contradictory to the report showing that the AFB1-exposed diets had significant effect on the IgA and IgG titer serum concentrations of mice [26]. Similarly, some studies emphasize that AFB1 did not change the humoral immunity [3,27]. However, some other studies showed an increase in plasma globulin titers (IgM and IgG) in pigs exposed to dietary AFB1 [28]. The effects of humoral immune to AFB1 addition maybe depend on the type, the dose, the duration of exposure, the susceptibility of each species (pigs, rats, chicken) [29,30], or other experimental conditions. Moreover, immune response may also vary according to animal health and management status. These results confirmed that low dose AFB1 does not cause a significant modulation of the humoral immune response in broiler chickens.
With regard to the serum production of cytokines, which were used as further understanding of immune system status, our study found an increase of TNFα and IFNγ in broiler chickens consumed the AFB1 contamination diet compared to the control group. Pro-inflammatory cytokines such as TNFα and IFNγ are important in tissue immune response and inflammation. Contrary to our data, suppressive effects of inflammatory cytokines were observed in rats/mice during respiratory aflatoxicosis [31] or after oral intoxication [32]. In addition, Marin et al. [24] also reported that AFs could decrease proinflammatory (IL1, TNF) and increase anti-inflammatory (IL10) mRNA expression in phytohemagglutinin-stimulated blood cells. In agree with our study, Chaytor et al. [14] reported an increased serum TNFα level of broiler chickens fed 180 μg of aflatoxin (AF)/kg and 900 μg of deoxynivalenol (DON)/kg, which maybe due to an acute-phase response to inflammation induced by the toxic effects of AFs and DON. Thus, this issue is still controversial and need further investigation. These results indicate that low level AFB1 could lead to inflammation and alter immune response. These changes may be associated with oxidative stress by AFB1, which was supported by Shay et al. [9].
As mentioned before, part of the initial aim for the current study was to investigate the in vivo expression of inflammation-related genes (mainly proinflammation cytokines such as TNFα, IL6, IFNγ and transcription factors) in broiler chickens consumed AFB1 contaminated feed. Given that some cytokines (TNFα, IL6, and IFNγ) and transcription factors (NF-κB, HO-1) are critical for both inflammation response and tissue function, it has been observed that AF preforms part of its immunosuppressive effects through some cytokines [33]. Meanwhile, many researchers have reported that AFB1 can lead to the alteration of cytokine expression and production in vitro or ex vivo [32,34,35]. However, no data is available concerning the in vivo modulation of AFB1 on inflammation-related gene of lymphoid organs, except only one study using pigs exposed to diet contaminated with 385, 867 or 1807 μg pure AFB1 per kg feed. To our best knowledge, this is the first study that investigates the expression of inflammation-related genes of spleen during AFB1 intoxication in chickens. In the current study, the mRNA level of TNFα, IL6, IFNγ, NF-κB p65 and HO-1 in spleen were measured by quantitative PCR. Spleen was chosen because this secondary lymphoid organ is a site for both innate and adaptive immune response [3,36]. The increase of IFNγ, IL6 and TNFα mRNA (proinflammatory cytokines) was observed in chickens exposed to AFB1 (Figure 3). Our data indicate that low dose of AFB1 also resulted in the inflammation response in spleen, and the inflammatory status was also observed in serum of chickens. This is in agreement with results obtained in pigs that received with 385, 867 or 1807 μg AFB1/kg feed [3], which finding is a significant up-regulation of cytokines mRNA by the highest dose of AFB1. Modification of inflammation-related gene expression by AFB1 has been confirmed in the liver of rats [37]. By using rats exposed to a chronic intermittent dosing AFB1 (1600 μg/kg feed), Hinton et al. [34] demonstrated that the induction of an inflammatory response (the increase in the production of IL1 and IL6) was associated with liver injury. In a parallel research, we also observed liver lesions of aflatoxicosis including oxidative damage and an inflammatory infiltrate in chickens exposed to AFB1 (74 μg/kg feed) (unpublished). So the expression of the splenic inflammatory cytokine, herein, is consistent with our observations of liver inflammation response. Moreover it supports the hypothesis that the inflammatory reaction at partly is responsible for the liver cell injury induced by AF.
It has been reported that α-LA has been used as a nutritive supplement, a pharmacotherapy and antioxidant in foods [9]. Indeed, the supplement of α-LA can protect against oxidative stress induced either by certain drugs or under various physiological and pathophysiological conditions [38,39]. In addition, previous studies undertaken in our lab have shown that supplementation of α-LA into AFs free-diets could enhance the antioxidant capability of broilers [40]. We showed that α-LA supplementation to AFB1-treated chickens significantly decreased the levels of MDA compared with those treated with AFB1 alone. This may be attributed to the ability that α-LA can inhibit lipid peroxidation by directly scavenging free radicals and chelating metals that play an important role in increased production of free radicals [8,41]. Furthermore, there is evidence that α-LA has an anti-inflammatory effect [42] by blocking the production and expression of cytokines and membrane co-stimulatory molecules. In our current study, α-LA was shown to inhibit the production of TNFα and IFNγ and the expression of the IFNγ, IL6 and TNFα genes of spleens in AFB1-treated chickens. In accordance with these observations obtained herein, Zhang et al. [43], and Kang et al. [44], have demonstrated various anti-inflammatory action of α-LA. All these results also support the anti-inflammination effects of α-LA, which may be responsible for its effect of potent antioxidant.
Previous studies showed that α-LA decreased the production/expression of proinflammatory cytokines and protected from the tissue damage caused by LPS in associated with alterations of NF-κB and HO-1 activity [45,46]. It is surprise of that no change in the expression of NF-κB and HO-1 gene was observed in different diet, suggesting that the suppression of α-LA on the spleen inflammation induced by low dose AFB1 might be not through modulating the transcriptional level of NF-κB and HO-1. Further studies are needed to confirm the underling mechanisms of α-LA alleviating the spleen inflammatory response caused by chronic low dose of AFB1.
4. Experimental Section
4.1. Collection of Feed Ingredients Contaminated with Aflatoxin B1
A total of 100 feed ingredients were sampled in the scope of the nation, and the contents of mycotoxins including AFB1, deoxynivalenol, zearalenone and ochratoxin A were tested and determined using high performance liquid chromatography (HPLC) according to the method of Trucksess et al. [47]. Two samples of AFB-free peanut meal and peanut meal seriously contaminated with AFB1 (330 μg/kg) were selected and incorporated into the basal diet by proportion.
4.2. Animals
One-day-old male broiler chickens (Ross 308) were obtained from a commercial hatchery (Chia Tai Co., Ltd., Baoding, Hebei, China). The brooding temperature was maintained at 35 °C (65% relative humidity (RH)) for the first 2 days, and then decreased gradually to 21 °C (45% RH) until 28 days and maintained as such until the end of the experiment. The light regime was 23l:1d. All chicks were provided ad libitum to water and a commercial diet during the rearing period. The animal care protocol in this experiment was according to commercial management practice, and approved by the Animal Welfare Committee of China Agricultural University.
4.3. Experimental Design
After a 10 days adaptation period to the diet and surrounding, a total of 120 eleven-day-old birds with similar body weights (BW) were randomly assigned to 3 groups with 4 replicates pens of 10 birds per pen. Three treatment groups included: fed the basal diet with 21% normal peanut meal (without any mycotoxin) (control); fed the diet containing 74 μg/kg AFB1 (21% moldy peanut meal naturally contaminated with 330 μg/kg AFB1 substituted for normal peanut meal by the same proportion in basal diet); fed the diet supplemented with 300 mg/kg dl-α-lipoic acid (Sigma Chemical, St. Louis, MO, USA) and AFB1 (determined 74 μg/kg AFB1 without other mycotoxins). All essential nutrients in the basal diet met or were slightly lower than the nutrient requirements of National Research Council [48]. The feeding trial period lasted for 3 weeks.
At the end of the experiment, six chicks with body weights close to the average were selected from per treatment. Blood sample was drawn from a wing vein with a 5 mL syringe within 30 s and then transferred to iced tubes. Serum was obtained from the blood by centrifugation at 3000× g for 10 min and was stored at –20 °C for further biochemical analysis. All chickens were sacrificed for tissue collection after blood sample collection [49]. A portion of the spleen was collected from the animals, flash-frozen in liquid nitrogen and stored at –70 °C until processed for inflammation-related genes mRNA measurements.
4.4. Parameter Analysis
Lipid peroxidation was assessed on the basic of serum MDA content and antioxidant enzyme levels in serum were estimated by measuring SOD and GSH-PX activities. The analyses were performed using MDA, SOD and GSH-PX Assay Kits, which were obtained from the Nanjing Jian-cheng Bioengineering Institute (Nanjing, Jiangsu, China). Serum MDA content was measured using the thiobarbituric acid method [50], reading the absorbance at 532 nm with the spectrometer and were expressed in nmol/mL. Serum SOD activity was assayed by the xanthine oxidase method [51], which monitor the degree of inhibition of nitroblue tetrazolium reduction by O2-generated by xanthine and xanthine oxidase, the absorbance was read at 550 nm using a spectrophotometer. Serum GSH-PX activity was detected by determination of the reduction of glutathione (GSH), the GSH react with 5,5-dithiobis (2-nitrobenzoic acid), produce yellow colored compounds which were detected at 412 nm using a spectrophotometer [52].
In this present study, immune response status in serum was estimated by measuring the levels of IgG, IgM, C3, C4, TNFα and IFNγ. These indices were analyzed using ELISA method. The details of all determination procedures followed the manufacturer’s instructions for the commercial kits (R&D System, Inc., Minneapolis, MN, USA).
4.5. Gene Expression Analyses
The mRNA concentrations of spleens for broiler chickens TNFα, IL6, IFNγ, NF-κB p65, and HO-1 were quantified by quantitative real time PCR. β-actin was used as a house-keeping gene to normalize the gene expression data. The primer information for all the genes is listed in Table 1.
Table 1.
Gene-specific primer of related genes.
| Gene | Genebank number | Primers position | Primers sequences(5′→3′) | Product size |
|---|---|---|---|---|
| β-actin | AW05994 | Forward | tgcgtgacatcaaggagaag | 300 bp |
| Reverse | tgccagggtacattgtggta | |||
| TNFα # | AY765397.1 | Forward | tgtgtatgtgcagcaacccgtagt | 229 bp |
| Reverse | ggcattgcaatttggacagaagt | |||
| IFNγ | NM_205149.1 | Forward | tgagccagattgtttcgatg | 246 bp |
| Reverse | tccttttgaaactcggagga | |||
| IL6 | NM_204628.1 | Forward | agatgtgcaagaagttcacc | 286 bp |
| Reverse | accacttcatcgggatttat | |||
| NF-κB p65 | D13719.1 | Forward | ttgctgctggagttgatgtc | 167 bp |
| Reverse | tgctatgtgaagaggcgttg | |||
| HO-1 * | NM_205344.1 | Forward | ggtcccgaatgaatgcccttg | 137 bp |
| Reverse | accgttctcctggctcttgg |
Total RNAs were extracted from the spleens by TRIZOL Reagent Kit (Invitrogen, San Diego, CA, USA). Reverse transcription (RT) was carried out using an RT reactions (10 μL) consisted of 500 ng total RNA, 5 mmol/L MgCl2, 1 μL RT buffer, 1 mmol/L dNTP, 2.5 U AMV, 0.7 nmol/L oligo d(T) and 10 U ribonuclease inhibitor (TaKaRa, Dalian, China). The cDNA was amplified in a 20 μL PCR reaction containing 0.2 μmol/L of each specific primer (Sangon, Shanghai, China) and SYBR green master mix (TaKaRa, Dalian, China). Each cycle consisted of denaturation at 95 °C for 10 s, annealing at 95 °C for 5 s, and extension at 60 °C for 34 s. Each sample was measured in duplicate analysis. If the difference between two duplications was greater than 15%, the sample was analyzed again. The PCR products were verified by electrophoresis on a 0.8% agarose-gel and by DNA sequencing. Standard curves were generated using pooled cDNA from the samples being assayed, and the comparative cycle threshold (Ct) method (2−ΔΔCt) was used to quantitate mRNA expression according to Livak and Schmittgen [55].
4.6. Statistical Analyses
The variability of results was expressed as the mean ± standard error (X ± SE). The significance of differences between mean values was determined using one-way ANOVA. Means were considered significantly different at p < 0.05.
5. Conclusions
Based on our results and the aforementioned discussion, we showed that the production of cytokines and spleen inflammation response caused by consuming diets containing AFB1 at concentrations as low as 74 μg/kg. The supplement of α-LA alleviates oxidative stress and immune changes induced by AFB1, and modulates the inflammatory response at least partly through changes in the expression of proinflammatory cytokines of spleen in broiler chickens. Thus, α-LA may be the possible potential application by feed to counteract some of the negative effects of AFB1 in poultry. Moreover, this study data may also provide some new insights for prevention and treatment of poison/toxicity in human and animal.
Acknowledgments
We appreciate the support of Beijing Municipal Natural Science Foundation (Grant No. 6102014); the National Basic Research Program of China (Grant No. 2012CB124704); Key Projects in the National Science & Technology Pillar Program (Grant No. 2012BAD39B00); and National Science and Technology Program for the Rural Development in China (Grant No. 2011BAD26B00).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Author Contributions
Y.L., Q.-G.M. and C.J. designed the experiments. Y.L., L.-H.Z. and G.-X.D. performed the experiments. Y.L. analyzed the data. Y.L., L.-H.Z., G.-X.D., Q.-G.M. and C.J. contributed reagents/materials/analysis tools. Y.L., H.W. and C.J. wrote the paper.
References
- 1.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wogan G.N. Aflatoxin carcinogenesis. In: Bush H., editor. Methods in Cancer Research. Academic Press; New York, NY, USA: 1973. pp. 309–344. [Google Scholar]
- 3.Meissonnier G.M., Pinton P., Laffitte J., Cossalter A.-M., Gong Y.Y., Wild C.P., Bertin G., Galtier P., Oswald I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008;231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Richard J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Mary V.S., Theumer M.G., Arias S.L., Rubinstein H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin b1 in rat spleen mononuclear cells. Toxicology. 2012;302:299–307. doi: 10.1016/j.tox.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Wagacha J., Muthomi J. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008;124:1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Ames B.N. Dietary carcinogens and anticarcinogens oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 8.Packer L., Witt E.H., Tritschler H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 9.Shay K.P., Moreau R.F., Smith E.J., Smith A.R., Hagen T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong A., Dukic-Stefanovic S., Gasic-Milenkovic J., Schinzel R., Wiesinger H., Riederer P., Münch G. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur. J. Neurosci. 2001;14:1961–1967. doi: 10.1046/j.0953-816x.2001.01820.x. [DOI] [PubMed] [Google Scholar]
- 11.Sola S., Mir M.Q., Cheema F.A., Khan-Merchant N., Menon R.G., Parthasarathy S., Khan B.V. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome results of the irbesartan and lipoic acid in endothelial dysfunction (island) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 12.Miazzo R., Peralta M.F., Magnoli C., Salvano M., Ferrero S., Chiacchiera S.M., Carvalho E.C., Rosa C.A., Dalcero A. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult. Sci. 2005;84:1–8. doi: 10.1093/ps/84.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Modirsanei M., Mansoori B., Khosravi A.R., Kiaei M.M., Khazraeinia P., Farkhoy M., Masoumi Z. Effect of diatomaceous earth on the performance and blood variables of broiler chicks during experimental aflatoxicosis. J. Sci. Food Agric. 2008;88:626–632. [Google Scholar]
- 14.Chaytor A.C., See M.T., Hansen J.A., de Souza A.L., Middleton T.F., Kim S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011;89:124–135. doi: 10.2527/jas.2010-3005. [DOI] [PubMed] [Google Scholar]
- 15.Oğuz H., Kurtoglu V., Coskun B. Preventive efficacy of clinoptilolite in broilers during chronic aflatoxin (50 and 100 ppb) exposure. Res. Vet. Sci. 2000;69:197–201. doi: 10.1053/rvsc.2000.0417. [DOI] [PubMed] [Google Scholar]
- 16.Magnoli A.P., Monge M.P., Miazzo R.D., Cavaglieri L.R., Magnoli C.E., Merkis C.I., Cristofolini A.L., Dalcero A.M., Chiacchiera S.M. Effect of low levels of aflatoxin B1 on performance, biochemical parameters, and aflatoxin B1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 2011;90:48–58. doi: 10.3382/ps.2010-00971. [DOI] [PubMed] [Google Scholar]
- 17.Ozen H., Karaman M., Cifremio Y., Tuzcu M., Ozcan K., Erdaf D. Effectiveness of melatonin on aflatoxicosis in chicks. Res. Vet. Sci. 2009;86:485–489. doi: 10.1016/j.rvsc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Niki E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Eraslan G., Akdogan M., Yarsan E., Sahindokuyucu F., Essiz D., Altintas L. The effects of aflatoxins on oxidative stress in broiler chickens. Turk. J. Vet. Anim. Sci. 2005;29:701–707. [Google Scholar]
- 20.Matur E., Ergul E., Akyazi I., Eraslan E., Inal G., Bilgic S., Demircan H. Effects of saccharomyces cerevisiae extract on haematological parameters, immune function and the antioxidant defence system in breeder hens fed aflatoxin contaminated diets. Br. Poult. Sci. 2011;52:541–550. doi: 10.1080/00071668.2011.617726. [DOI] [PubMed] [Google Scholar]
- 21.Sies H. Physiological society symposium: Impaired endothelial and smooth muscle cell function in oxidative stress. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 22.Oswald I., Comera C. Immunotoxicity of mycotoxins. Rev. Med. Vet. 1998;149:585–590. [Google Scholar]
- 23.Bondy G.S., Pestka J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- 24.Marin D., Taranu I., Bunaciu R., Pascale F., Tudor D., Avram N., Sarca M., Cureu I., Criste R., Suta V. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 2002;80:1250–1257. doi: 10.2527/2002.8051250x. [DOI] [PubMed] [Google Scholar]
- 25.Oguz H., Hadimli H., Kurtoglu V., Erganis O. Evaluation of humoral immunity of broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Rev. Med. Vet. 2003;154:483–486. [Google Scholar]
- 26.Choi K.-C., Lee B.-S., Chung W.-T., Choi M.-S., Lee J.-C. Protective effects of apigenin and quercetin on aflatoxin B1-induced immunotoxicity in mice. Food Sci. Biotechnol. 2010;19:987–992. [Google Scholar]
- 27.Van Heugten E., Spears J., Coffey M., Kegley E., Qureshi M. The effect of methionine and aflatoxin on immune function in weanling pigs. J. Anim. Sci. 1994;72:658–664. doi: 10.2527/1994.723658x. [DOI] [PubMed] [Google Scholar]
- 28.Panangala V., Giambrone J., Diener U., Davis N., Hoerr F., Mitra A., Schultz R., Wilt G. Effects of aflatoxin on the growth performance and immune responses of weanling swine. Am. J. Vet. Res. 1986;47:2062–2067. [PubMed] [Google Scholar]
- 29.Dwivedi N., Flora G., Kushwaha P., Flora S.J. Alpha-lipoic acid protects oxidative stress, changes in cholinergic system and tissue histopathology during co-exposure to arsenic-dichlorvos in rats. Environ. Toxicol. Pharmacol. 2013;37:7–23. doi: 10.1016/j.etap.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Meissonnier G., Marin D., Galtier P., Bertin G., Taranu I., Oswald I., Mengheri E. Modulation of the immune response by a group of fungal food contaminant, the aflatoxins. In: Mengheri E., Roselli M., Britti M.S., Finamore A., editors. Nutrition and Immunity. Research Signpost; Kerala, India: 2006. pp. 147–166. [Google Scholar]
- 31.Jakab G.J., Hmieleski R.R., Zarba A., Hemenway D.R., Groopman J.D. Respiratory aflatoxicosis: Suppression of pulmonary and systemic host defenses in rats and mice. Toxicol. Appl. Pharmacol. 1994;125:198–205. doi: 10.1006/taap.1994.1065. [DOI] [PubMed] [Google Scholar]
- 32.Dugyala R.R., Sharma R.P. The effect of aflatoxin B1 on cytokine mrna and corresponding protein levels in peritoneal macrophages and splenic lymphocytes. Int. J. Immunopharmacol. 1996;18:599–608. doi: 10.1016/s0192-0561(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 33.Yarru L.P., Settivari R.S., Gowda N.K., Antoniou E., Ledoux D.R., Rottinghaus G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009;88:2620–2627. doi: 10.3382/ps.2009-00204. [DOI] [PubMed] [Google Scholar]
- 34.Hinton D.M., Myers M.J., Raybourne R.A., Francke-Carroll S., Sotomayor R.E., Shaddock J., Warbritton A., Chou M.W. Immunotoxicity of aflatoxin B1 in rats: Effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol. Sci. 2003;73:362–377. doi: 10.1093/toxsci/kfg074. [DOI] [PubMed] [Google Scholar]
- 35.Moon E.-Y., Rhee D.-K., Pyo S. In vitro suppressive effect of aflatoxin B1 on murine peritoneal macrophage functions. Toxicology. 1999;133:171–179. doi: 10.1016/s0300-483x(99)00023-2. [DOI] [PubMed] [Google Scholar]
- 36.OLÁH I., VERVELDE L. Structure of the avian lymphoid system. In: Davison F., Kaspers B., Schat K.A., editors. Avian Immunology. Academic Press; London, UK: 2008. pp. 13–50. [Google Scholar]
- 37.Ellinger-Ziegelbauer H., Stuart B., Wahle B., Bomann W., Ahr H.-J. Characteristic expression profiles induced by genotoxic carcinogens in rat liver. Toxicol. Sci. 2004;77:19–34. doi: 10.1093/toxsci/kfh016. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Zaher A.O., Abdel-Hady R.H., Mahmoud M.M., Farrag M.M.Y. The potential protective role of α-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243:261–270. doi: 10.1016/j.tox.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Zalejska-Fiolka J., Wielkoszyński T., Kasperczyk S., Kasperczyk A., Birkner E. Effects of oxidized cooking oil and α-lipoic acid on blood antioxidants: Enzyme activities and lipid peroxidation in rats fed a high-fat diet. Biol. Trace Elem. Res. 2012;145:217–221. doi: 10.1007/s12011-011-9186-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen P., Ma Q.-G., Ji C., Zhang J.-Y., Zhao L.-H., Zhang Y., Jie Y.-Z. Dietary lipoic acid influences antioxidant capability and oxidative status of broilers. Int. J. Mol. Sci. 2011;12:8476–8488. doi: 10.3390/ijms12128476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Şehirli Ö., Tatlıdede E., Yüksel M., Erzik C., Çetinel S., Yeğen B.Ç., Şener G. Antioxidant effect of α-lipoic acid against ethanol-induced gastric mucosal erosion in rats. Pharmacology. 2007;81:173–180. doi: 10.1159/000111145. [DOI] [PubMed] [Google Scholar]
- 42.Maczurek A., Hager K., Kenklies M., Sharman M., Martins R., Engel J., Carlson D.A., Munch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008;60:1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Han P., Wu N., He B., Lu Y., Li S., Liu Y., Zhao S., Liu L., Li Y. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity. 2011;19:1647–1653. doi: 10.1038/oby.2011.121. [DOI] [PubMed] [Google Scholar]
- 44.Kang K.P., Kim D.H., Jung Y.J., Lee A.S., Lee S., Lee S.Y., Jang K.Y., Sung M.J., Park S.K., Kim W. α-Lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol. Dial. Transplant. 2009;24:3012–3020. doi: 10.1093/ndt/gfp242. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W.-J., Wei H., Hagen T., Frei B. α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou T.-C. The protective effect of α-lipoic acid in lipopolysaccharide-induced acute lung injury is mediated by heme oxygenase-1. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/590363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trucksess M.W., Stack M.E., Nesheim S., Albert R.H., Romer T.R. Multifunctional column coupled with liquid chromatography for determination of aflatoxins B1, B2, G1, and G2 in corn, almonds, brazil nuts, peanuts, and pistachio nuts: Collaborative study. J. AOAC Int. 1994;77:1512–1521. [PubMed] [Google Scholar]
- 48.National Research Council. Nutrient Requirements of Poultry. 9th rev. ed. National Academies Press; Washington, DC, USA: 1994. [Google Scholar]
- 49.Cai Y., Song Z., Zhang X., Wang X., Jiao H., Lin H. Increased de novo lipogenesis in liver contributes to the augmented fat deposition in dexamethasone exposed broiler chickens (Gallus gallus domesticus) Comp. Biochem. Physiol. 2009;150:164–169. doi: 10.1016/j.cbpc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 51.Winterbourn C.C., Hawkins R., Brian M., Carrell R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975;85:337–341. [PubMed] [Google Scholar]
- 52.Lawrence R.A., Burk R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 53.Hong Y.H., Lillehoj H.S., Hyen Lee S., Woon Park D., Lillehoj E.P. Molecular cloning and characterization of chicken lipopolysaccharide-induced TNF-α factor (LITAF) Dev. Comp. Immunol. 2006;30:919–929. doi: 10.1016/j.dci.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Druyan S., Cahaner A., Ashwell C. The expression patterns of hypoxia-inducing factor subunit α-1, heme oxygenase, hypoxia upregulated protein 1, and cardiac troponin T during development of the chicken heart. Poult. Sci. 2007;86:2384–2389. doi: 10.3382/ps.2007-00152. [DOI] [PubMed] [Google Scholar]
- 55.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]