Abstract
Daidzein and genistein are two major components of soy isoflavones. They exist abundantly in plants and possess multiple bioactivities. In contrast, ortho-hydroxydaidzein (OHD) and ortho-hydroxygenistein (OHG), including 6-hydroxydaidzein (6-OHD), 8-hydroxydaidzein (8-OHD), 3′-hydroxydaidzein (3′-OHD), 6-hydroxygenistein (6-OHG), 8-hydroxygenistein (8-OHG), and 3′-hydroxygenistein (3′-OHG), are rarely found in plants. Instead, they are usually isolated from fermented soybean foods or microbial fermentation broth feeding with soybean meal. Accordingly, the bioactivity of OHD and OHG has been investigated less compared to that of soy isoflavones. Recently, OHD and OHG were produced by genetically engineering microorganisms through gene cloning of cytochrome P450 (CYP) enzyme systems. This success opens up bioactivity investigation and industrial applications of OHD and OHG in the future. This article reviews isolation of OHD and OHG from non-synthetic sources and production of the compounds by genetically modified microorganisms. Several bioactivities, such as anticancer and antimelanogenesis-related activities, of OHD and OHG, are also discussed.
Keywords: soy isoflavones, daidzein, genistein, hydroxylation, ortho-hydroxydaidzein, ortho-hydroxygenistein, bioactivity, isolation, production, cancer, melanogenesis
1. Introduction
Isoflavones are naturally occurring dietary phytoestrogens distributed in the leaves, seeds, bark, and flowers of some plants, specifically in legumes, such as soybeans [1]. In plants, these compounds provide protection against UV radiation, pathogens and herbivores [2]. Two major isoflavones found in soybeans are daidzin and genistin, which are the glycoside conjugates of daidzein and genistein, respectively. They account for more than 0.1% (w/w) of the dry weight of soybeans. In the past few decades, isoflavones have been intensively investigated due to their possible role in preventing certain hormone-dependent and other diseases including breast and prostate cancers, osteoporosis, and cardiovascular diseases [3]. In structure-activity relationships, the number and positions of functional groups in the chemical structures of isoflavones dramatically affect the functions of the isoflavones [4]. Therefore, many scientists focused on studies of isolation and bioactivity characterization of different derivatives of daidzein and genistein based on the multifunctional activities of soy isoflavones. Among various soy isoflavone derivatives, ortho-hydroxydaidzein (OHD) and ortho-hydroxygenistein (OHG) are an important group because of the ortho-dihydroxyl groups in their structures, which may enhance bioactivity compared with those of the precursors daidzein and genistein. Based on the difference in the hydroxylation position, OHD and OHG divide into 6-hydroxydaidzein (6-OHD), 8-hydroxydaidzein (8-OHD), 3′-hydroxydaidzein (3′-OHD), 6-hydroxygenistein (6-OHG), 8-hydroxygenistein (8-OHG), and 3′-hydroxygenistein (3′-OHG). The chemical structures of OHD and OHG together with daidzein and genistein are shown in Figure 1.
Figure 1.
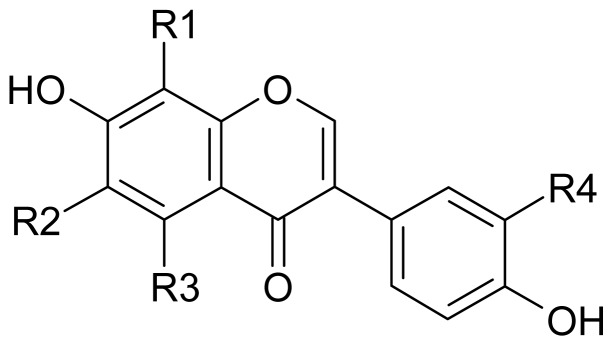
Structures of ortho-hydroxydaidzein (OHD) and ortho-hydroxygenistein (OHG). Daidzein, R1 = R2 = R3 = R4 = H; 6-Hydroxydaidzein (6-OHD), R1 = R3 = R4 = H, R2 = OH; 8-Hydroxydaidzein (8-OHD), R1 = OH, R2 = R3 = R4 = H; 3′-Hydroxydaidzein (3′-OHD), R1 = R2 = R3 = H, R4 = OH; Genistein, R1 = R2 = R4 = H, R3 = OH; 6-Hydroxygenistein (6-OHG), R1 = R4 = H, R2 = R3 = OH; 8-Hydroxygenistein (8-OHG), R1 = R3 = OH, R2 = R4 = H; 3′-Hydroxydaidzein (3′-OHG), R1 = R2 = H, R3 = R4 = OH.
Although several thousands of different isoflavones have been purified and identified from plants, OHD and OHG have not been isolated from plants with the exception of 3′-OHD and 3′-OHG. Why hydroxylation at either the C-6 or C-8 carbon position of the isoflavone skeleton is not favored during isoflavone biosynthesis in plants is unknown. Most OHD and OHG are isolated from fermented soybean foods or microbial fermentation broth feeding with soybean meal. During fermentation, the microorganism possessing a unique cytochrome P450 enzyme system catalyzes ortho-hydroxylation of either daidzein or genistein into OHD and OHG, respectively. Different OHD and OHG can be isolated from different fermented soybean foods used by different microorganisms (which is discussed in detail below). To overcome the problem of the rarity of OHD and OHG in nature, in recent years, scientists successfully used genetically recombinant microorganisms harboring the cytochrome P450 (CYP) gene to produce OHD and OHG. This breakthrough will attract more scientists studying the bioactivity of OHD and OHG in the future because it is easier to produce OHD and OHG. Moreover, production of OHD and OHG by recombinant microorganisms can be easily scaled up, and OHD and OHG can be used for industrial applications. In this review, we surveyed reports on OHD and OHG from the past few decades. This review focuses on the isolation, bioactivity, and production of OHD and OHG, and is divided into those sections accordingly.
In addition, the absorption and metabolism of soy isoflavones daidzein and genistein by humans are also an important field of study. Many reports have demonstrated that OHD and OHG are intermediate metabolites of daidzein and genistein in humans and rats in vitro [5,6] or in vivo [7]. These reports have been thoroughly reviewed in other articles [8,9], and thus are not discussed in this review.
2. Isolation of OHD and OHG
The non-synthetic sources for isolating OHD and OHG can be divided into three groups: plants, fermented soybean foods, and microbial fermentation broth feeding with soybean meal. Only 3′-OHD and 3′-OHG have been found in plants, while other OHD and OHG were isolated from fermented soybean foods or microbial fermentation broth. In Asian countries, many traditional fermented products use soybeans as the fermented substrate, such as natto, soy sauces, and miso (Japanese fermented soybean paste), doenjang (Korean fermented soybean paste), douchi (Chinese fermented soybeans), and tempeh (Indonesian fermented soybean cake). Fungi are usually the major microorganisms involved in the preparation of these products. For examples, tempeh is produced mainly by Rhizopus sp. and others by Aspergillus sp. [10]. In addition, diverse bacteria are also involved in the preparation of Indonesian tempeh and Korean doenjang. Many studies have been conducted on the metabolism of soy isoflavones by the fungi in these fermented soybean foods [11,12]. The results showed that each isoflavone glycoside was hydrolyzed to the respective free isoflavone aglycone by fungal beta-glucosidase during soybean fermentation. Furthermore, during fermentation, free isoflavone daidzein and genistein are biotransformed into OHD and OHG, respectively, by enzymes from the microorganisms [13,14]. The catalyzing enzyme has been identified as CYPs [15,16]. CYPs are heme-containing monooxygenases wildly distributed in nature, including microorganisms, plants, and animals [17]. CYPs from humans [18,19], murine [20], and microorganisms [16,21] can biotransform daidzein or genistein into OHD and OHG, respectively. However, CYPs from plants cannot catalyze ortho-hydroxylation of daidzein or genistein. The phenomenon reasonably explains the fact that rare OHD and OHG were isolated from plants; nevertheless, the detailed mechanism is unknown. In addition to fermented soybean products, OHD and OHG can also be purified from the fermentation broth of microorganisms containing CYPs to catalyze ortho-hydroxylation of daidzein or genistein, when soybean meal is used as a substrate. Furthermore, OHD and OHG were recently produced by using genetically modified microorganisms harboring CYPs through the biotransformation of daidzein or genistein. This will be discussed later in this review. The isolation of OHD and OHG from three non-synthetic sources is shown in Table 1.
Table 1.
Isolation of OHD and OHG. Products that are underlined were isolated from non-synthetic sources for the first time.
| Sources | Name | Microorganisms | Products | Ref. |
|---|---|---|---|---|
| Plants | Orobus tuberuosus | --- a | 3′-OHG | [22] |
| Calophyllum polyanthum | --- | 3′-OHG | [23] | |
| Erycibe expansa | --- | 3′-OHG | [24] | |
| Eclipta prostrata | --- | 3′-OHG | [25] | |
| Sophora japonica | --- | 3′-OHG | [26] | |
| Machaerium villosum | --- | 3′-OHD | [29] | |
| Dalbergia odorifera | --- | 3′-OHD | [30] | |
| Styphnolobium japponicum | --- | 3′-OHD | [31] | |
|
| ||||
| Soybean Foods | Indonesian Tempeh | Rhizopus and other bacteria | 6-OHD, 8-OHD, 3′-OHG | [27,32] |
| Japanese Soybean koji | A. saitoi | 8-OHD, 8-OHG, 6-OHD | [33,34] | |
| Japanese Miso | A. oryzae | 8-OHD, 8-OHG, 6-OHD, 3′-OHD | [35–38] | |
| Chinese Douchi | A. oryzae | 6-OHD, 8-OHD, 3′-OHD, 8-OHG | [39] | |
| Korean Doenjang | diverse fungi and bacteria | 6-OHD, 8-OHD, 3′-OHD | [40] | |
|
| ||||
| Microbial Fermentation Broth | --- | A. niger | 8-OHG, 3′-OHG | [28] |
| --- | Streptomyces sp. | 8-OHD, 3′-OHD | [43,44] | |
| --- | Micrococcus sp. or Arthrobacter sp. | 6-OHG | [45] | |
| --- | P. productus | 6-OHG | [46] | |
not involved.
Among various OHD and OHG, 3′-OHG (orobol) from Orobus tuberuosus was the first to be purified in nature, in 1939 [22]. Then, 3′-OHG was isolated from several other plants, including the seeds of Calophyllum polyanthum [23], the stems of Erycibe expansa [24], whole plants of Eclipta prostrate [25], and the flowers of Sophora japonica (SophoraeFlos) [26]. In addition to plants, 3′-OHG was also isolated in tempeh [27] and the fermentation broth of Aspergillus niger [28]. In contrast, 3′-OHD was initially isolated from the heartwood of Machaerium villosum in 1968 [29] and then from the heartwood of Dalbergia odorifera [30] and identified as the single major flavonoid in fruits of Styphnolobium japonicum [31]. In addition, 3′-OHD exists in most fermented soybean products.
6-OHD, until now isolated only from fermented soybeans, was purified from tempeh for the first time in 1964 [32] and then isolated from every fermented soybean product, including Japanese soybean koji [33,34], Japanese miso [35–38], Chinese douchi [39], and Korean doenjang [40]. For fermented soybean foods prepared by using the fungal Aspergillus species, such as miso and douchi, OHD and OHG were produced by CYP57B3 from the microorganism [15]. However, for tempeh, OHD and OHG are not produced by the fungal Rhizopus sp. in the tempeh but by bacteria isolated from tempeh [41,42]. The enzyme of the bacteria for the biotransformation of OHD and OHG from daidzein and genistein remains unknown. In addition, the microorganism in Korean doenjang that produces OHD has not been identified.
8-OHD and 8-OHG were initially isolated from microbial fermentation broth: 8-OHG from the cultivation of A. niger in 1975 [28] and 8-OHD from the cultivation of Streptomyces sp. in 1989 [43,44]. Similar to 3′-OHD and 6-OHD, 8-OHD and 8-OHG have been isolated from almost every fermented soybean product.
Among all OHD and OHG, 6-OHG is rarely discovered in non-synthetic sources. Klus and Barz identified 6-OHG as the metabolite of genistein with tempeh-derived bacterial Micrococcus or Arthrobacter in 1998 [45]. However, the catalyzing enzyme in the bacteria that produces 6-OHG has not been identified. Until now, there has been no report of production of 6-OHG by genetically modified microorganisms harboring CYPs. It seems that natural CYPs do not favor catalyzing 6-hydroxylation of genistein. The mechanism must be studied in the future. For the non-monooxylation catalyzing reaction, however, Tsuchihashi et al. isolated 6-OHG from the metabolites of human intestinal bacterial Peptostreptococcus productus by feeding the main isoflavone tectoridin (4′,5-dihydro-6-methoxy-7-(o-glucoside)isoflavone) from the flowers of Pueraria thomsonii [46]. The microbial enzyme from the P. productus strain caused O-demethylation at the C-6 methoxy group accompanied by hydrolysis of the glycosidic linkage substituted at the C-7 hydroxyl group. However, the production of 6-OHG from tectoridin by the P. productus strain is rarely applied in biotechnological use due to the difficulty growing the bacteria and rarity of the precursor in nature. Currently, the quantitatively pronounced conversions of genistein yielding 6-OHG by the two strains from tempeh are recommended for biotechnological production of the difficult-to-synthesize polyhydroxylated isoflavones.
3. Bioactivity of OHD and OHG
OHD and OHG possess excellent antioxidant and free radical-scavenging activities due to the ortho-dihydroxyl groups in their structures. The structure-activity relationships for antioxidant activity have been thoroughly discussed elsewhere [47]. In this section, other bioactivities, such as anticancer and antimelanogenesis-related activities, of OHD and OHG are listed in Table 2 and discussed below.
Table 2.
Bioactivity of OHD and OHG.
| Classification | Compound | Bioactivity | Ref. |
|---|---|---|---|
| Anticancer-Related Activity | 3′-OHG | Antiproliferative activity toward T47D tumorigenic breast epithelial cells; | [52,53] |
|
| |||
| 8-OHD | Increases life span against S180 bearing mice; | [43] | |
| Suppression of MDR in Caco-2 colon adenocarcinoma cells; | [58] | ||
|
| |||
| 8-OHG | Antimutagenesis activity; | [36] | |
| Antiproliferative activity toward HL-60 promyelocytic leukemia cells; | [35] | ||
|
| |||
| 6-OHD | Suppression of HCT-116 colon cancer cell proliferation in vitro and in vivo; | [54] | |
|
| |||
| 3′-OHD | Suppression of EGF receptor-positive skin cancer cell proliferation in vitro and in vivo; | [55,56] | |
| Suppression of MDR transporters; | [57] | ||
|
| |||
| Antimelanogenesis- Related Activity | 6-OHD | Competitive tyrosinase inhibitor; | [60] |
|
| |||
| 8-OHD | Irreversible tyrosinase inhibitor; | [61,62] | |
| Antimelanogenesis activity in human volunteers; | [63] | ||
|
| |||
| 8-OHG | Irreversible tyrosinase inhibitor; | [61,62] | |
|
| |||
| 3′-OHD | Antimelanogenesis activity in human skin equivalents; | [64,65] | |
| Potent autophage inducer; | [67] | ||
|
| |||
| Other Bioactivities | 3′-OHG | HIV-1 integrase inhibitor; | [25] |
| Antiinflammatory activity; | [68] | ||
| Hepatoprotective activity; | [24] | ||
|
| |||
| 6-OHG | Hepatoprotective activity; | [46] | |
|
| |||
| 8-OHD | Aldose reductase inhibitor; | [69] | |
|
| |||
| 6-OHD | Suppression on adipogenesis of 3T3-L1 preadipocytes; | [70] | |
| Promoting differentiation of 3T3-L1 preadipocytes; | [71] | ||
| Antitrypanosomal activity | [72] | ||
|
| |||
| 3′-OHD | Antitrypanosomal activity; | [72,73] | |
| Improving atopic dermatitis symptoms; | [74] | ||
3.1. Anticancer-Related Bioactivity
Numerous in vitro and in vivo studies have shown that soy isoflavones exhibit antiproliferative activity against malignancies of the breast, colon, skin, and prostate [48–51]. In particular, genistein has received attention as a potential anticarcinogenic compound. Although it is generally thought that genistein and daidzein might play an important role in preventing these types of cancers, OHD and OHG might inhibit cancer growth rather than genistein and daidzein. For example, Spencer et al. found that genistein was selectively taken up into T47D tumorigenic breast epithelial cells and was subject to metabolism by CYP enzymes leading to the formation of 3′-OHG, which induced G2-M cell cycle arrest in T47D cells [52]. The authors found that the antiproliferative actions of 3′-OHG may be mediated by initial oxidative DNA damage, activation of ataxia telangiectasia and Rad3-related kinase (ATR), and downstream regulation of the p53 pathway leading to cell cycle arrest in G2-M [53]. The authors suggested this was one mechanism by which genistein exerts its anticancer activity in vivo.
Some literature reported the anticancer-related bioactivity of OHD and OHG together with the isolation of OHD and OHG from fermented soybean foods or fermentation broth. When Funayama et al. first isolated 8-OHD from the fermentation broth of Streptomyces sp., they reported that 8-OHD increased the life span of S180 mouse sarcoma-bearing mice [43]. Hirota et al. isolated various OHD and OHG from miso, and found that 8-OHG showed the most potent antimutagenic activity [36] and antiproliferative activity toward human promyelocytic leukemia cells [35].
Recently, Dong et al. reported several study results showing the anticancer-related activity of 6-OHD and 3′-OHD. They demonstrated that 6-OHD suppressed anchorage-dependent and -independent growth and induced cell cycle arrest at the S and G2/M phases in HCT-116 human colon cancer cells [54]. In a xenograft mouse model, the authors found that 6-OHD significantly decreased tumor growth and the volume and weight of HCT-116 xenografts by suppressing cyclin-dependent kinase 1 and 2 (CDK1 and CDK2) activity in tumors. The results suggest that CDK1 and CDK2 are potential molecular targets of 6-OHD to suppress HCT-116 cell proliferation in vitro and in vivo. For 3′-OHD, the authors showed that the molecule triggered cell cycle arrest at the G1 phase through attenuating the expression of CDK1 and displayed an antiproliferative effect against epidermal growth factor (EGF) receptor-positive skin cancer [55]. 3′-OHD also significantly inhibited UVB-induced cyclooxygenase 2 (COX-2) expression by suppressing NF-κB transcription activity in mouse skin epidermal JB6 P+ cells [56]. Furthermore, topical application of 3′-OHD clearly suppressed the incidence and multiplicity of UVB-induced tumors in hairless mouse skin. Taken together, these results provide insight into the anticancer activity of 3′-OHD as a potential skin cancer chemopreventive agent.
The development of multidrug resistance (MDR) to conventional chemoradiation therapy usually leads to failure in treating cancer. Accordingly, suppressing MDR takes advantage of decreased chemotherapy dosage and adverse effects, as well as improved efficacy in cancer therapy. Lo et al. demonstrated that 3′-OHD causes cell death in human cervical cancer cells through the ROS-dependent suppression of MDR transporters [57]. This effect of 3′-OHD significantly increased the cytotoxicity of epirubicin, an anticancer drug in human cervical cancer HeLa cells. In another study, researchers showed that 8-OHD effectively circumvents MDR in human colon adenocarcinoma Caco-2 cells through the ROS-dependent inhibition of efflux transporters [58]. These findings, together with those described above, revealed multiple functions of OHD and OHG in anticancer activity and high potency for development as cancer chemopreventive agents.
3.2. Antimelanogenesis-Related Bioactivity
Melanogenesis is a biosynthetic pathway for the formation of the pigment melanin in human skin. A key enzyme, tyrosinase, catalyzes the first and only rate-limiting steps in melanogenesis, and the down-regulation of enzyme activity guarantees the inhibition of melanogenesis. Because of the cosmetically important issue of hyperpigmentation, there is a big demand for tyrosinase inhibitors. This encourages researchers to seek potent tyrosinase inhibitors for cosmetic use [59].
We isolated several isoflavone derivatives from soygerm koji fermented with A. oryzae and demonstrated 6-OHD [60], 8-OHD, and 8-OHG [61] could be potent tyrosinase inhibitors. Among them, 6-OHD acted competitively 6-fold more than kojic acid on the l-tyrosine binding site of the enzyme while 8-OHD and 8-OHG irreversibly inactivated the enzyme and belong to the suicide substrates of tyrosinase with low partition ratios, low Michaelis constants, and high maximal inactivation rate constants [62]. To our knowledge, 8-OHD and 8-OHG are currently the most potent suicide substrates of mushroom tyrosinase and have high potential in application as a skin-whitening agent. 8-OHD was creamed in the appropriate condition and showed higher depigmenting activity than that of ascorbic acid 2-glucoside (AA2G) in an in vivo assay conducted with 45 volunteers by our laboratory [63].
In addition to our findings, Korean scientists have also conducted successful experiments in this field. Park et al. isolated several OHDs from Korean fermented soybean product [40], and confirmed the antimelanogenesis activity of 8-OHD and 3′-OHD [64]. Na et al. evaluated antimelanogenesis activity by using reconstituted human skin equivalents and demonstrated that the two OHDs can reduce pigmentation in African-American human skin equivalents more effectively than the standard skin-whitening drug, kojic acid [65]. In the report, they observed that antimelanogenesis activity of 8-OHD and 3′-OHD was exhibited through repression of microphthalmia-association transcription factor (MITF), the primary regulator for the expression of melanogenesis-related proteins [66]. Recently, Kim et al. identified 3′-OHD as a potent autophage inducer and confirmed that melanogenesis inhibition of 3′-OHD mediated activation of melanosome autophagy in melanocytes [67]. These study results together with our previous findings emphasize the application of 8-OHD and 3′-OHD in skin-whitening cosmetics; thus, mass production of OHD for industrial use should be established in the future. This issue will be discussed later in this review.
3.3. Other Bioactivity
In addition to anticancer and antimelanogenesis-related activities, reports on OHD and OHG with other bioactivities are also collected in Table 2. Among them, some assaying OHD and OHG were isolated from the three types of non-synthetic sources in Table 1. Tewtrakul et al. isolated 3′-OHG (orobol) from E. prostrate and identified potent HIV-1 integrase inhibitory activity, which supports the use of E. prostrate in AIDS treatment [25]. They also found that the compound down-regulated iNOS and COX-2 mRNA expression, which supports the traditional use of the plant for treating inflammatory-related disease [68]. Matsuda et al. isolated 3′-OHG from E. expansa and identified its hepatoprotective activity [24]. Tsuchihashi et al. also found that 6-OHG from the metabolite of human intestinal bacterial strain feeding with isoflavone tectoridin from Pueraria flowers has extremely potent hepatoprotective activity [46]. 8-OHD from Japanese soybean products was identified as an aldose reductase inhibitor, which is an attractive pharmacological target for treating diabetic complications [69].
Some studies focused on the bioactivity of 6-OHD and 3′-OHD, which were from organic synthesis. Recently, Seo et al. reported that 6-OHD at high concentrations (40 to 80 μM) suppressed adipogenesis in 3T3-L1 preadipocytes by directing targeting phosphatidylinositol 3-kinase (PI3K) [70]. In contrast, Chen et al. recently reported that 6-OHD at low concentrations (10 to 20 μM) significantly promoted 3T3-L1 preadipocytes differentiation [71]. The potential application of 6-OHD in either obesity or diabetes remains unclear. In the future, an in vivo study should be evaluated to clarify the effect of 6-OHD on adipocytes. In addition, Tasdemir et al. demonstrated that 6-OHD showed strong inhibition of the protozoa Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense, which cause human African trypanosomiasis, also known as sleeping sickness, and severe mortality and morbidity in sub-Saharan Africa [72]. In the same report, 3′-OHD also showed comparable antitrypanosomal activity. This finding was consistent with that by Salem and Werbovetz, who demonstrated that 3′-OHD displayed potent and selective toxicity against Trypanosoma brucei brucei [73]. In another study on atopic dermatitis (AD), a chronically relapsing skin disorder presenting with severe itching and inflammation, 3′-OHD improved AD symptoms, including visual clinical features, and reduced ear thickening and scratching behavior in vivo. Furthermore, orally administered 3′-OHD in the mouse model significantly inhibited epidermal thickening and infiltration of mast cells. The improved statuses were similar to those in the untreated control group [74]. In addition to this OHD and OHG bioactivity, more thorough research on the bioactivity of OHD and OHG will be reported in the future along with easier and increased production of OHD and OHG by genetically modified microorganisms.
4. Production of OHD and OHG
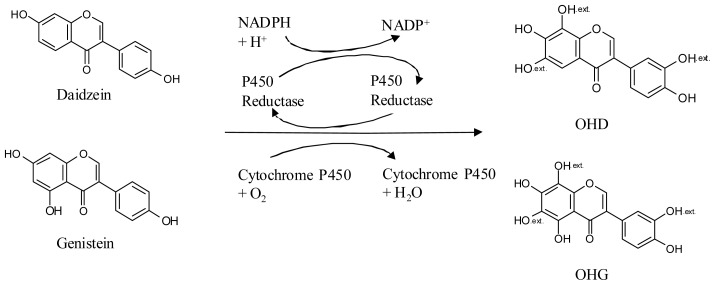
In contrast to daidzein and genistein, which exist abundantly in plants, especially in soybeans, most OHD and OHG are biotransformed products from daidzein and genistein by microorganisms, where the CYP enzyme is the key enzyme. The biotransformation process is shown in Figure 2, where an electron transport chain is established from NADPH, P450 reductase, CYP, finally to a substrate. The ortho-hydroxylation of the precursors by CYP might occur at the C-6, C-8, or C-3′ position based on the regioselectivity of the enzyme.
Figure 2.
Production of OHD and OHG by the cytochrome P450 enzyme system. The functional groups (OH.ext.) in the structures of the reaction products indicate possible hydroxylation positions.
Recently, some CYPs with the capacity to catalyze the ortho-hydroxylation of daidzein and genistein were identified. Many excellent studies have been performed by Kim et al. [21,75–84]. They subcloned every CYP in the genome of Streptomyces avermitilis MA-4680, Nocardia farcinica IFM10152, Bacillus subtilis 168, B. megaterium, B. cereus, and B. licheniformis and co-expressed the CYP with redox partners camA and camB from Pseudomonas putida in Escherichia coli. Then, the researchers screened the recombinant cells for the ability to catalyze ortho-hydroxylation of flavonoids [21]. Dozens of positive CYPs were identified; CYPs with the capacity to catalyze the ortho-hydroxylation of daidzein and genistein are listed in Table 3. CYP107H1 from B. subtilis catalyzed 3′-hydroxylation of daidzein; however, the production yield was very low [75]. The nfa33880 gene encoding CYP from N. farcinica catalyzed 6- and 8-hydroxylation of daidzein with low production yields [76]. Another CYP from the same microorganism (CYP154) showed distinct O-dealkylation and subsequent hydroxylation of formononetin (7-hydroxy-4′-methoxyisoflavone) to produce 3′-OHD [77]. Although the production yield was still low, this type of serial reaction mechanism is unusual among bacterial CYPs. In addition to the positive-screened CYP, Kim et al. also altered the substrate specificity of non-OHD-producing CYP (CYP102D1) from a long chain fatty acid to an aromatic compound such as daidzein through site-directed mutation on the active site of the enzyme. The mutating CYP102D1 (F96V/M246I) catalyzed 6- and 8-hydroxylation of daidzein [78]. The resulting production yield of 8-OHD (2.42 mg/L) in the study is the highest value in the literature.
Table 3.
Production of OHD and OHG by recombinant microorganisms harboring heterogeneous monooxygenase. Products that are underlined have the highest reported values.
| Monooxygenase name | Monooxygenase sources | Recombinant hosts | Products | Yield (mg/L) | Ref. |
|---|---|---|---|---|---|
| CYP107H1 a | B. subtilis | E. coli | 3′-OHD | <0.1 | [75] |
| Nfa33880 a | N. farcinica | E. coli | 8-OHD 6-OHD |
0.76 0.56 |
[76] |
| CYP154 a | N. farcinica | E. coli | 3′-OHD | 0.99 | [77] |
| CYP102D1 b | S. avermitilis | E. coli |
8-OHD 6-OHD |
2.42 1.18 |
[78] |
| CYP105D7 c | S. avermitilis | S. avermitilis | 3′-OHD | 9.3 | [79] |
| CYP105D7 a | S. avermitilis | S. avermitilis | 3′-OHD | 37.5 | [80] |
| CYP107Y1 a | S. avermitilis | S. avermitilis | 3′-OHG | 6.75 | [21] |
| Sam5 | S. espanaensis | E. coli | 3′-OHD | 75 | [82] |
| CYP57B3 a | A. oryzae | S. cerevisiae | 3′-OHG | N.D. d | [16] |
| CYP57B3 c | A. oryzae | P. pastoris | 6-OHD | 9.1 | [87] |
Co-expressed with P450 reductase (camA/camB);
Mutated CYP;
Engineered fusion with P450 reductase;
Not determined.
Due to the low production yields of OHD and OHG, Kim et al. used two strategies to improve the yields [79,80]. First, the researchers changed the host to Steptomyces. Second, they genetically fused the CYP gene with the redox partner gene. During the catalysis process, CYP must be associated with electron donor partner proteins (CPRs) to transfer two electrons from NADPH to the CYP heme domain. However, the electron transfer between CPR and CYP usually limits the reaction rate of CYPs. One self-sufficient type of CYP is encoded in a single polypeptide that possesses a CPR domain fused to the heme domain [85]. Self-sufficient CYPs usually exhibit catalytic activity due to the high efficiency of the electron transfer from CPR to its fused P450 partner. Thus, genetic fusion of non-self-sufficient P450 with CPR mimics the natural self-sufficient CYPs and improves catalytic activity [86]. After performing the two strategies, Kim et al. improved 3′-OHD production by CYP105D7 from S. avermitilis higher than that of the wild-type strain and reached a production yield of 9.3 mg/L by using whole cell biotransformation [79] and 37.5 mg/L by using fermentation in a 7-L jar fermentor [80]. In addition to OHD, the only CYP discovered by Kim et al. that catalyzes ortho-hydroxylation of genistein was CYP107Y1 from S. avermitilis with a 3′-OHG production yield of 6.75 mg/L [21]. No study on the production of 8-OHG or 6-OHG by recombinant microorganisms has been reported. Screening new CYP from other microorganisms or genetically modifying CYP to alter its regioselection are two possible approaches for producing 8-OHG and 6-OHG in the future. In either approach, the high throughput screening (HTS) assay method is essential to obtain good results. The Kim research group recently developed a solid agar plate-based HTS assay method for screening ortho-specific hydroxylation of daidzein by sensing formaldehyde generated from the O-dealkylation reaction. Through the technique, they successfully screened one mutant G1 (A273H/G274E/T277G) from CYP102D1 with a 4-fold increase production yield of 3′-OHD (14.3 mg/L) compared with that of CYP102D1 (F96V/M246I) [81]. The fascinating technique would be an ideal system for primary HTS assay for the CYP reaction.
In addition to heme-containing monooxygenase (CYP), the Kim research group recently used recombinant E. coli harboring a non-heme, flavin-dependent monooxygenase (Sam5) from Saccharothrix espanaensis to produce 3′-OHD with a production yield of 75 mg/L [82]. The recombinant strain catalyzed multiple flavonoids to produce corresponding ortho-hydroxyflavonoids, including 3′-OHG, 3′,8-dihydroxygenistein, 3′-hydroxyapigenin, 3′,8-dihydroxyapigenin and 3′-hydroxynaringenin. The regioselective hydroxylation of diverse flavonoids shown by the recombinant strain has not been shown by other bacterial monooxygenase. Hence, the Sam5 system has highly potency for production of bioactive hydroxylated flavonoids. Because the production yield of OHD by the Sam5 system was greatly higher than those by bacterial CYP system, this finding opens further research on non-heme monooxygenase for production of OHD and OHG in the future.
As described in Section 2 of this review, A. oryzae is the most commonly used microorganism in fermented soybean products. The microorganism transforms soy isoflavones into their corresponding ortho-hydroxyl derivatives. A. oryzae contains 155 putative P450 genes in its genome, from which 142 P450 proteins are expressed [15]. Among them, CYP57B3 was recently shown to catalyze 3′-hydroxylation of genistein in cooperation with a CPR from Saccharomyces cerevisiae [16]. However, the authors did not measure the production yield of 3′-OHG in their study. To improve production, we recently fused CYP57B3 with the reductase domain of a self-sufficient CYP102A1 gene from B. megaterium to form an artificial, self-sufficient P450. Through measuring the production of ortho-hydroxydaidzein derivatives from daidzein with recombinant P. pastoris harboring the fusion gene, 6-OHD was produced as high as 9.1 mg/L, the highest production yield in the literature [87]. In addition, recombinant P. pastoris also produced a large amount of 3′-OHG [88].
CYP57B3 is the only CYP from eukaryotic cells that can produce OHD and OHG; others are from bacteria. Using the eukaryotic CYP expressed in yeast to produce OHD or OHG has several advantages compared to using bacterial CYP expressed in E. coli or Streptomyces. First, all bacterial CYPs are soluble and distributed in cytoplasm, while eukaryotic CYPs are membrane-anchored and mainly distributed on the endoplasmic reticulum membrane. For the insoluble substrate daidzein and genistein, which are transported via the endomembrane system in cells, membrane-anchored CYPs can access the substrate more easily than soluble bacterial CYPs. In fact, the production yields of OHD and OHG using bacterial CYP expressed in E. coli are usually very low [75–78]; Second, to improve the production of OHD and OHG in the bacterial system, the host could be changed to Streptomyces strains, which produce moderate amounts of OHD and OHG and are more suitable hosts than E. coli. However, Streptomyces contains not only CYP catalyzing ortho-hydroxylation of daidzein and genistein but also several dioxygenases that break down the precursors daidzein and genistein and the products OHD and OHG. Thus, OHD and OHG products in recombinant Streptomyces are not stable [83]. In contrast, the precursors and products are stable in yeast [89]. Although the enzyme catalyzing degradations of hydroxyisoflavones in Streptomyces was recently identified as tyrosinase, however, ortho-hydroxylation of daidzein in a tyrosinase gene-knock out mutant (ΔmelC2) appeared to show little daidzein hydroxylation activity due to a low expression level of the CYP gene [84]. The reason may be related to the function of the tyrosinase with sporulation and cell stress environment. The drawback of using Streptomyces as a host to produce OHD and OHG needs to be resolved in the future; Third, CYP57B3 has been shown to catalyze the conversion of the isoflavone genistein, the flavanone naringenine [16], and the isoflavone daidzein [87]. However, the CYPs from bacteria catalyzed only one type of flavonoid as a substrate for each CYP [21]. These results reveal that CYP57B3 has greater flexibility in substrates binding than those of the CYPs from bacteria. The board substrate spectrum of the eukaryotic CYP57B3 provides CYP more application opportunities.
5. Conclusions
Small amounts of OHD and OHG are usually isolated from natural sources, if they exist. The rare isolation of OHD and OHG has limited the investigation of the bioactivities of the compounds. The collective reports describing bioactivity in this review show that more studies evaluating the bioactivity of OHD and OHG were completed in the last five years (2009–2013) compared with the previous decade. The trend should continue because of the easier availability of OHD and OHG from microbial production via genetic engineering. Daidzein and genistein are inexpensive and available in kilogram-grade with 99% purity. After OHD and OHG are produced from daidzein and genistein by recombinant microorganisms harboring necessary CYPs, industrial-scale production can be achieved by scaling up the fermentation process. Moreover, bioconversion of daidzein and genistein into OHD and OHG, respectively, by genetically modified microorganisms could be improved through multiple approaches, such as searching for new CYPs with higher catalyzing activity, improving the catalyzing capacity of CYPs by various mutation and HTS methods, and optimizing the fermentation control on a larger scale. Overall, more bioactivity evaluations and larger-scale production promise real applications of OHD and OHG in the future.
Acknowledgments
This research was financially supported by grants from the National Scientific Council of Taiwan (project no. NSC 101-2221-E-024-014-MY2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Franke A.A., Custer L.J., Cerna C.M., Narala K.K. Quantitation of phytoestrogens in legumes by HPLC. J. Agric. Food Chem. 1994;42:1905–1913. [Google Scholar]
- 2.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 3.Vitale D.C., Piazza C., Melilli B., Drago F., Salomone S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013;38:15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 4.Scotti L., Bezerra Mendonça F.J., Jr., Magalhaes Moreira D.R., da Silva M.S., Pitta I.R., Scotti M.T. SAR, QSAR and docking of anticancer flavonoids and variants: A review. Curr. Top. Med. Chem. 2012;12:2785–2809. doi: 10.2174/1568026611212240007. [DOI] [PubMed] [Google Scholar]
- 5.Roberts-Kirchhoff E.S., Crowley J.R., Hollenberg P.F., Kim H. Metabolism of genistein by rat and human cytochrome P450s. Chem. Res. Toxicol. 1999;12:610–616. doi: 10.1021/tx9802320. [DOI] [PubMed] [Google Scholar]
- 6.Kulling S.E., Honig D.M., Simat T.J., Metzler M. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J. Agric. Food Chem. 2000;48:4963–4972. doi: 10.1021/jf000524i. [DOI] [PubMed] [Google Scholar]
- 7.Kulling S.E., Honig D.M., Metzler M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J. Agric. Food Chem. 2001;49:3024–3033. doi: 10.1021/jf0012695. [DOI] [PubMed] [Google Scholar]
- 8.Xiao J., Högger P. Metabolism of dietary flavonoids in liver microsomes. Curr. Drug Metab. 2013;14:381–391. doi: 10.2174/1389200211314040003. [DOI] [PubMed] [Google Scholar]
- 9.Barnes S., Prasain J., D’Alessandro T., Arabshahi A., Botting N., Lila M.A., Jackson G., Janle E.M., Weaver C.M. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011;2:235–244. doi: 10.1039/c1fo10025d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K.I., Erh M.H., Su N.W., Liu W.H., Chou C.C., Cheng K.C. Soyfoods and soybean products: From traditional use to modern applications. Appl. Microbiol. Biotechnol. 2012;96:9–22. doi: 10.1007/s00253-012-4330-7. [DOI] [PubMed] [Google Scholar]
- 11.Naim M., Gestetner B., Zilkah S., Birk Y., Bondi A. Soybean isoflavones, characterization, and antifungal activity. J. Agric. Food Chem. 1974;22:806–810. doi: 10.1021/jf60195a031. [DOI] [PubMed] [Google Scholar]
- 12.Kudou S., Fleury Y., Welti D., Magnolato D., Uchida T., Kitamura K., Okubo K. Malonyl isoflavone glycosides in soybean seeds (Glycine max Merril) Agric. Biol. Chem. 1991;55:2227–2233. [Google Scholar]
- 13.Esaki H., Watanabe R., Onozaki H., Kawakishi S., Osawa T. Formation mechanism for potent antioxidative o-dihydroxyisoflavones in soybeans fermented withAspergillus saitoi. Biosci. Biotechnol. Biochem. 1999;63:851–858. doi: 10.1271/bbb.63.851. [DOI] [PubMed] [Google Scholar]
- 14.Chang T.S., Ding H.Y., Tai S.S.K., Wu C.Y. Metabolism of the soy isoflavone daidzein and genistein by the fungi used for the preparation of various fermented soybean foods. Biosci. Biotechnol. Biochem. 2007;71:1330–1333. doi: 10.1271/bbb.60573. [DOI] [PubMed] [Google Scholar]
- 15.Nazir K., Ichinose H., Wariishi H. Molecular characterization and isolation of cytochrome P450 genes from the filamentous fungusAspergillus oryzae. Arch. Microbiol. 2010;192:395–408. doi: 10.1007/s00203-010-0562-z. [DOI] [PubMed] [Google Scholar]
- 16.Nazir N.H., Ichinose H., Wariishi H. Construction and application of a functional library of cytochrome P450 monooxygenases from the filamentous fungusAspergillus oryzae. Appl. Environ. Microbiol. 2011;77:3147–3150. doi: 10.1128/AEM.02491-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernhardt R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006;124:128–145. doi: 10.1016/j.jbiotec.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Peng W.X., Wang L.S., Li H.D., Abd El-Aty A.M., Chen G.L., Zhou H.H. Evidence for the involvement of human liver microsomes CYP1A2 in the mono-hydroxylation of daidzein. Clin. Chim. Acta. 2003;334:77–85. doi: 10.1016/s0009-8981(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 19.Hu M., Krausz K., Chen J., Ge X., Li J., Gelboin H.L., Gonzalez F.J. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab. Dispos. 2003;31:924–931. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 20.Breinholt V.M., Rasmussen S.E., Brøsen K., Friedberg T.H. In vitro metabolism of genistein and tangeretin by human and murine cytochrome P450s. Pharmacol. Toxicol. 2003;93:14–22. doi: 10.1034/j.1600-0773.2003.930102.x. [DOI] [PubMed] [Google Scholar]
- 21.Pandey B.P., Lee N., Choi K.Y., Jung E., Jeong D.H., Kim B.G. Screening of bacterial cytochrome P450s responsible for regiospecific hydroxylation of (iso)flavonoids. Enzym. Microbial. Technol. 2011;48:386–392. doi: 10.1016/j.enzmictec.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Charaux C., Rabate J. Constitution chimique de I’orobol. Bull. Soc. Chim. Biol. 1939;21:1330–1333. [Google Scholar]
- 23.Ma C.H., Chen B., Qi H.Y., Li B.G., Zhang G.L. Two pyranocoumarins from the seeds ofCalophyllum polyanthum. J. Nat. Prod. 2004;67:1598–1600. doi: 10.1021/np0499158. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda H., Morikawa T., Xu F., Ninomiya K., Yoshikawa M. New isoflavones and pterocarpane with hepatoprotective activity from the stems ofErycibe expansa. Planta Med. 2004;70:1201–1209. doi: 10.1055/s-2004-835852. [DOI] [PubMed] [Google Scholar]
- 25.Tewtrakul S., Subhadhirasakul S., Cheenpracha S., Karalai C. HIV-1 protease and HIV-1 integrase inhibitory substances fromEclipta prostrata. Phytother. Res. 2007;21:1092–1095. doi: 10.1002/ptr.2252. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q.C., Zhang W.Y., Jin W., Lee I.S., Min B.S., Jung H.J., Na M., Lee S., Bae K. Flavonoids and isoflavonoids from Sophorae Flos improve glucose uptake in vitro. Planta Med. 2010;76:79–81. doi: 10.1055/s-0029-1185944. [DOI] [PubMed] [Google Scholar]
- 27.Kiriakidis S., Högemeier O., Starcke S., Dombrowski F., Hahne J.C., Pepper M., Jha H.C., Wernert N. Novel tempeh (fermented soyabean) isoflavones inhibit in vivo angiogenesis in the chicken chorioallantoic membrane assay. Br. J. Nutr. 2005;93:317–323. doi: 10.1079/bjn20041330. [DOI] [PubMed] [Google Scholar]
- 28.Umezawa H., Tobe H., Shibamoto N., Nakamura F., Nakamura K., Matsuzaki M., Takeuchi T. Isolation of isoflavones inhibiting dopa decarboxylase from fungi andStreptomyces. J. Antibiot. 1975;28:947–952. doi: 10.7164/antibiotics.28.947. [DOI] [PubMed] [Google Scholar]
- 29.De Oliveira A.B., Gottlieb O.R., Ollis W.D. A quimica de leguminosas Brasileiras. XVII. Constituents do Machaerium villosum. Anais Acad. Brasil. Cienc. 1968;40:147–150. [Google Scholar]
- 30.Chan S.C., Chang Y.S., Wang J.P., Chen S.C., Kuo S.C. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood ofDalbergia odorifera. Planta Med. 1998;64:153–158. doi: 10.1055/s-2006-957394. [DOI] [PubMed] [Google Scholar]
- 31.Kite G.K., Veitch N.C., Boalch M.E., Lewis G.P., Leon C.J., Simmonds M.S.J. Flavonol tetraglycosides from fruits of Styphnolobium japonicum (Leguminosae) and the authentication of Fructus Sophorae and Flos Sophorae. Phytochemistry. 2009;70:785–794. doi: 10.1016/j.phytochem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Gyoergy P., Murata K., Ikehata H. Antioxidants isolated from fermented soybeans (tempeh) Nature. 1964;203:870–872. doi: 10.1038/203870a0. [DOI] [PubMed] [Google Scholar]
- 33.Esaki H., Onozaki H., Morimitsu Y. Potent antioxidative isoflavones isolated from soybean fermented withAspergillus saitoi. Biosci. Biotechnol. Biochem. 1998;62:740–746. doi: 10.1271/bbb.62.740. [DOI] [PubMed] [Google Scholar]
- 34.Esaki H., Kawakishi S., Morimitsu Y., Osawa T. New potent antioxidative o-dihydroxyisoflavones in fermented Japanese soybean products. Biosci. Biotechnol. Biochem. 1999;63:1637–1639. doi: 10.1271/bbb.63.1637. [DOI] [PubMed] [Google Scholar]
- 35.Hirota A., Taki S., Kawaii S., Yano M., Abe N. 1,1-Diphenyl-2-picrylhydrazyl radical-scavenging compounds from soybean miso and antiproliferative activity of isoflavones from soybean miso toward the cancer cell lines. Biosci. Biotechnol. Biochem. 2000;64:1038–1040. doi: 10.1271/bbb.64.1038. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.C., Inaba M., Abe N., Hirota A. Antimutagenic activity of 8-hydroxyisoflavones and 6-hydroxydaidzein from soybean miso. Biosci. Biotechnol. Biochem. 2003;67:903–906. doi: 10.1271/bbb.67.903. [DOI] [PubMed] [Google Scholar]
- 37.Hirota A., Inaba M., Chen Y.C., Abe N., Taki S., Yano M., Kawaii S. Isolation of 8-hydroxyglycitein and 6-hydroxydaidzein from soybean miso. Biosci. Biotechnol. Biochem. 2004;68:1372–1374. doi: 10.1271/bbb.68.1372. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama Y., Sakurai Y., Hirota A. Isolation of 2,4,4′-trihydroxydeoxybenzoin and 3′-hydroxydaidzein from soybean miso. Biosci. Biotechnol. Biochem. 2010;74:1293–1294. doi: 10.1271/bbb.100073. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y.C., Sugiyama Y., Abe N., Kuruto-Niwa R., Nozawa R., Hirota A. DPPH radical-scavenging compounds from dou-chi, a soybean fermented food. Biosci. Biotechnol. Biochem. 2005;69:999–1006. doi: 10.1271/bbb.69.999. [DOI] [PubMed] [Google Scholar]
- 40.Park J.S., Park H.Y., Kim D.H., Kim D.H., Kim H.K. ortho-Dihydroxyisoflavone derivatives from aged Doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorg. Med. Chem. Lett. 2008;18:5006–5009. doi: 10.1016/j.bmcl.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Klus K., Borger-Papendorf G., Barz W. Formation of 6,7,4′-trihydroxyisoflavone (factor 2) from soybean seed isoflavones by bacteria isolated from tempe. Phytochemistry. 1993;34:979–981. [Google Scholar]
- 42.Klus K., Barz W. Formation of polyhydroxylated isoflavones from the soybean seed isoflavones daidzein and glycitein by bacteria isolated from tempe. Arch. Microbiol. 1995;164:428–434. doi: 10.1007/BF02529741. [DOI] [PubMed] [Google Scholar]
- 43.Funayama S., Anraku Y., Mita A., Komiyama K., Omura S. Structure study of isoflavonoids possessing antioxidant activity from the fermentation broth of Streptomyces sp. J. Antibiot. 1989;42:1350–1355. doi: 10.7164/antibiotics.42.1350. [DOI] [PubMed] [Google Scholar]
- 44.Komiyama K., Funayama S., Anraku Y., Mita A., Takahashi Y., Omura S. Isolation of isoflavonoids possessing antioxidant activity from the fermentation broth of Streptomyces sp. J. Antibiot. 1989;42:1344–1349. doi: 10.7164/antibiotics.42.1344. [DOI] [PubMed] [Google Scholar]
- 45.Klus K., Barz W. Formation of polyhydroxylated isoflavones from the isoflavones genistein and biochanin A by bacteria isolated from tempe. Phytochemistry. 1998;47:1045–1048. doi: 10.1007/BF02529741. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchihashi R., Kodera M., Sakamoto S., Nakajima Y., Yamazaki T., Niiho Y., Nohara T., Kinjo J. Microbial transformation and bioconversion of isoflavones from Pueraria flowers by human intestinal bacterial strains. J. Nat. Med. 2009;63:254–260. doi: 10.1007/s11418-009-0322-z. [DOI] [PubMed] [Google Scholar]
- 47.Goto H., Terao Y., Akai S. Synthesis of various kinds of isoflavones, isoflavanes, and biphenyl-ketones and their 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activities. Chem. Pharm. Bull. 2009;57:346–360. doi: 10.1248/cpb.57.346. [DOI] [PubMed] [Google Scholar]
- 48.Bennink M.R. Dietary soy reduces colon carcinogenesis in human and rats. Soy and colon cancer. Adv. Exp. Med. Biol. 2001;492:11–17. doi: 10.1007/978-1-4615-1283-7_2. [DOI] [PubMed] [Google Scholar]
- 49.Farina H.G., Pomies M., Alonso D.F., Gomez D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006;16:885–891. [PubMed] [Google Scholar]
- 50.Hewitt A.L., Singletary K.W. Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model. Cancer Lett. 2003;192:133–143. doi: 10.1016/s0304-3835(02)00712-7. [DOI] [PubMed] [Google Scholar]
- 51.Jian L. Soy, isoflavones, and prostate cancer. Mol. Nutr. Food Res. 2009;53:217–226. doi: 10.1002/mnfr.200800167. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen D.T., Hernandez-Montes E., Vauzour D., Schonthal A.H., Rice-Evans C., Cadenas E., Spencer J.P.E. The intracellular genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone mediates G2-M cell cycle arrest in cancer cells via modulation of the p38 signaling pathway. Free Radic. Biol. Med. 2006;41:1225–1239. doi: 10.1016/j.freeradbiomed.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 53.Vauzour D., Vafeiadou K., Rice-Evans C., Cadenas E., Spencer J.P.E. Inhibition of cellular proliferation by the genistein metabolite 5,7,30,40-tetrahydroxyisoflavone is mediated by DNA damage and activation of the ATR signalling pathway. Arch. Biochem. Biophy. 2007;468:159–166. doi: 10.1016/j.abb.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Lee D.E., Lee K.W., Jung S.K., Lee E.J., Hwang J.A., Lim T.G., Kim B.Y., Bode A.M., Lee H.J., Dong Z. 6,7,4′-Trihydroxyisoflavone inhibits HCT-116 human colon cancer cell proliferation by targeting CDK1 and CDK2. Carcinogenesis. 2011;32:629–635. doi: 10.1093/carcin/bgr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D.E., Lee K.W., Byun S., Jung S.K., Song N., Lim S.H., Heo Y.S., Kim J.E., Kang N.J., Kim B.Y., et al. 7,3′,4′-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4. J. Biol. Chem. 2011;286:14246–14256. doi: 10.1074/jbc.M110.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee D.E., Lee K.W., Song N.R., Seo S.K., Heo Y.S., Kang N.J., Bode A.M., Lee H.J., Dong Z. 7,3′,4′-Trihydroxyisoflavone inhibits epidermal growth factor-induced proliferation and transformation of JB6 P+ mouse epidermal cells by suppressing cyclin-dependent kinases and phosphatidylinositol 3-kinase. J. Biol. Chem. 2010;285:21458–21466. doi: 10.1074/jbc.M109.094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo Y.L., Wang W., Ho C.T. 7,3′,4′-Trihydroxyisoflavone modulates multidrug resistance transporters and induces apoptosis via production of reactive oxygen species. Toxicology. 2012;302:221–232. doi: 10.1016/j.tox.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Lo Y.L. A potential daidzein derivative enhances cytotoxicity of epirubicin on human colon adenocarcinoma caco-2 cells. Int. J. Mol. Sci. 2012;14:158–176. doi: 10.3390/ijms14010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang T.S., Ding H.Y., Lin H.C. Identifying 6,7,4′-trihydroxyisoflavone as a potent tyrosinase inhibitor. Biosci. Biotechnol. Biochem. 2005;69:1999–2001. doi: 10.1271/bbb.69.1999. [DOI] [PubMed] [Google Scholar]
- 61.Chang T.S., Ding H.Y., Tai S.S.K., Wu C.Y. Tyrosinase inhibitors isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem. 2007;105:1430–1438. [Google Scholar]
- 62.Chang T.S. Two potent suicide substrates of mushroom tyrosinase: 7,8,4′-Trihydroxyisoflavone and 5,7,8,4′-tetrahydroxyisoflavone. J. Agric. Food Chem. 2007;55:2010–2015. doi: 10.1021/jf063095i. [DOI] [PubMed] [Google Scholar]
- 63.Tai S.S.K., Lin C.G., Wu M.H., Chang T.S. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int. J. Mol. Sci. 2009;10:4257–4266. doi: 10.3390/ijms10104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park J.S., Kim D.H., Lee J.K., Lee J.Y., Kim D.H., Kim H.K., Lee H.J., Kim H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg. Med. Chem. Lett. 2010;20:1162–1164. doi: 10.1016/j.bmcl.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 65.Goh M.J., Park J.S., Bae J.H., Kim D.H., Kim H.K., Na Y.J. Effects of ortho-dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesis in B16 melanoma cells and human skin equivalents. Phytother. Res. 2012;26:1107–1112. doi: 10.1002/ptr.3682. [DOI] [PubMed] [Google Scholar]
- 66.Chang T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials. 2012;5:1661–1685. [Google Scholar]
- 67.Kim E.S., Shin J.H., Seok S.H., Kim J.B., Chang H., Park S.J., Jo Y.K., Choi E.S., Park J.S., Yeom M.H., et al. Autophagy mediates anti-melanogenic activity of 30-ODI in B16F1 melanoma cells. Biochem. Biophy. Res. Commun. 2013;442:165–170. doi: 10.1016/j.bbrc.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 68.Tewtrakul S., Subhadhirasakul S., Tansakul P., Cheenpracha S., Karalai C. Antiinflammatory constituents from Eclipta prostrata using RAW264.7 macrophage cells. Phytother. Res. 2011;25:1313–1316. doi: 10.1002/ptr.3383. [DOI] [PubMed] [Google Scholar]
- 69.Fujita T., Funako T., Hayashi H. 8-Hydroxydaidzein, an aldose reductase inhibitor from okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004;68:1588–1590. doi: 10.1271/bbb.68.1588. [DOI] [PubMed] [Google Scholar]
- 70.Seo S.G., Yang H., Shin S.H., Min S., Kim Y.A., Yu J.G., Lee D.E., Chung M.Y., Heo Y.S., Kwon J.Y., et al. A metabolite of daidzein, 6,7,4′-trihydroxyisoflavone, suppresses adipogenesis in 3T3-L1 preadipocytes via ATP-competitive inhibition of PI3K. Mol. Nutr. Food Res. 2013;57:1446–1455. doi: 10.1002/mnfr.201200593. [DOI] [PubMed] [Google Scholar]
- 71.Chen L., Li Q.Y., Shi X.J., Mao S.L., Du Y.L. 6-Hydroxydaidzein enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. J. Agric. Food Chem. 2013;61:10714–10719. doi: 10.1021/jf402694m. [DOI] [PubMed] [Google Scholar]
- 72.Tasdemir D., Kaiser M., Brun R., Yardley V., Schmidt T.J., Tosun F., Ruedi P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006;50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salem M.M., Werbovetz K.A. Isoflavonoids and other compounds from Psorothamnus arborescens with antiprotozoal activities. J. Nat. Prod. 2006;69:43–49. doi: 10.1021/np0502600. [DOI] [PubMed] [Google Scholar]
- 74.Kim B.B., Kim J.R., Kim J.H., Kim Y.A., Park J.S., Yeom M.H., Lee H., Lee K.W., Kang N.J. 7,3′,4′-Trihydroxyisoflavone ameliorates the development of dermatophagoides farinae-induced atopic dermatitis in NC/Nga Mice. Evid. Based Complement. Alternat. Med. 2013;2013:636597. doi: 10.1155/2013/636597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roh C., Choi K.Y., Pandey B.P., Kim B.G. Hydroxylation of daidzein by CYP107H1 from Bacillus subtilis 168. J. Mol. Catal. B Enzym. 2009;59:248–253. [Google Scholar]
- 76.Choi K.Y., Kim T.J., Koh S.K., Roh C.H., Pandey B.P., Lee N., Kim B.G. A-ring ortho-specific monohydroxylation of daidzein by cytochrome P450s of Nocardia farcinica IFM10152. Biotechnol. J. 2009;4:1586–1595. doi: 10.1002/biot.200900157. [DOI] [PubMed] [Google Scholar]
- 77.Choi K.Y., Park H.Y., Kim B.G. Characterization of bi-functional CYP154 from Nocardia farcinica IFM10152 in the O-deakylation and ortho-hydroxylation of formononetin. Enzym. Microb. Technol. 2010;47:327–334. [Google Scholar]
- 78.Choi K.Y., Jung E.O., Jung D.H., Pandey B.P., Yun H., Park H.Y., Kazlauskas R.J., Kim B.G. Cloning, expression and characterization of CYP102D1, a self-sufficient P450 monooxygenase fromStreptomyces avermitilis. FEBS J. 2012;279:1650–1662. doi: 10.1111/j.1742-4658.2011.08462.x. [DOI] [PubMed] [Google Scholar]
- 79.Choi K.Y., Jung E.O., Jung D.H., An B.R., Pandey B.P., Yun H., Sung C., Park H.Y., Kim B.G. Engineering of daidzein 3′-hydroxylase P450 enzyme into catalytically self-sufficient cytochrome P450. Microb. Cell Fact. 2012;11:81. doi: 10.1186/1475-2859-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pandey B.P., Roh C., Choi K.Y. Regioselective hydroxylation of daidzein using P450 (CYP105D7) from Streptomyces avermitilis MA4680. Biotechnol. Bioeng. 2010;105:697–704. doi: 10.1002/bit.22582. [DOI] [PubMed] [Google Scholar]
- 81.Choi K.Y., Jung E.O., Kazlauskas R., Kim B.G. Development of colorimetric HTS assay of cytochrome P450 for ortho-specific hydroxylation and engineering of CYP102D1 with enhanced catalytic activity and regioselectivity. Chembiochem. 2013;14:1231–1238. doi: 10.1002/cbic.201300212. [DOI] [PubMed] [Google Scholar]
- 82.Lee H., Kim B.G., Ahn J.H. Production of bioactive hydroxyflavones by using monooxygenase fromSaccharothrix espanaensis. J. Biotechnol. 2014;176:11–17. doi: 10.1016/j.jbiotec.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Kim H.J., Choi K.Y., Jung D.H., Jung J.Y., Jung E.O., Yang Y.H., Kim B.G., Oh M.K. Transcriptomic study for screening genes involved in the oxidative bioconversions ofStreptomyces avermitilis. Bioprocess Biosyst. Eng. 2013;36:1621–1630. doi: 10.1007/s00449-013-0935-1. [DOI] [PubMed] [Google Scholar]
- 84.Pandey B.P., Lee N., Choi K.Y., Kim J.N., Kim E.J., Kim B.G. Identification of the specific electron transfer proteins, ferredoxin, and ferredoxin reductase, for CYP105D7 in Streptomyces avermitilis MA4680. Appl. Microbiol. Biotechnol. 2014 doi: 10.1007/s00253-014-5525-x. [DOI] [PubMed] [Google Scholar]
- 85.Jung S.T., Lauchli R., Arnold F.H. Cytochrome P450: Taming a wild type enzyme. Curr. Opin. Biotechnol. 2011;22:1–9. doi: 10.1016/j.copbio.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hlavica P. Assembly of non-natural electron transfer conduits in the cytochrome P450 system: A critical assessment and update of artificial redox constructs amenable to exploitation in biotechnological areas. Biotechnol. Adv. 2009;27:103–121. doi: 10.1016/j.biotechadv.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Chang T.S., Chao S.Y., Chen Y.C. Production of ortho-hydroxydaidzein derivatives by a recombinant strain of Pichia pastoris harboring a cytochrome P450 fusion gene. Process Biochem. 2013;48:426–429. [Google Scholar]
- 88.Chang T.S. Department of Biological Science and Technology. National University of Tainan; 2014. Unpublished observation. [Google Scholar]
- 89.Chang T.S. Department of Biological Science and Technology. National University of Tainan; 2014. Unpublished observation. [Google Scholar]