Abstract
In this retrospective pilot study, the expression of the prostate-specific membrane antigen (PSMA), the epithelial cell adhesion molecule (EpCAM), the vascular endothelial growth factor (VEGF) and the gastrin-releasing peptide receptor (GRPR) in locally recurrent prostate cancer after brachytherapy or external beam radiotherapy (EBRT) was investigated, and their adequacy for targeted imaging was analyzed. Prostate cancer specimens were collected of 17 patients who underwent salvage prostatectomy because of locally recurrent prostate cancer after brachytherapy or EBRT. Immunohistochemistry was performed. A pathologist scored the immunoreactivity in prostate cancer and stroma. Staining for PSMA was seen in 100% (17/17), EpCAM in 82.3% (14/17), VEGF in 82.3% (14/17) and GRPR in 100% (17/17) of prostate cancer specimens. Staining for PSMA, EpCAM and VEGF was seen in 0% (0/17) and for GRPR in 100% (17/17) of the specimens’ stromal compartments. In 11.8% (2/17) of cases, the GRPR staining intensity of prostate cancer was higher than stroma, while in 88.2% (15/17), the staining was equal. Based on the absence of stromal staining, PSMA, EpCAM and VEGF show high tumor distinctiveness. Therefore, PSMA, EpCAM and VEGF can be used as targets for the bioimaging of recurrent prostate cancer after EBRT to exclude metastatic disease and/or to plan local salvage therapy.
Keywords: prostate cancer, immunohistochemistry, PSMA, EpCAM, VEGF, GRPR
1. Introduction
Prostate cancer is the most commonly diagnosed cancer among men, and its incidence rates remain as the highest in many regions of the world [1]. About 18% of patients with localized disease develop a prostate-specific antigen (PSA) recurrence within five years after brachytherapy and external beam radiotherapy (EBRT) with doses higher than 72 Gy. This is in contrast to 49% of patients who were treated with doses less than 72 Gy [2]. At present, there is no imaging modality that can accurately discriminate between locally and distant recurrent prostate cancer after treatment with curative intent. Selecting patients for salvage cryotherapy of the prostate or salvage prostatectomy by excluding distant metastases proves a diagnostic challenge.
New diagnostic techniques are needed to improve the imaging of recurrent prostate cancer. Furthermore, although early-stage and locally recurrent prostate cancer can be cured, treatment of metastasized disease is currently only palliative, making it important for new therapeutic applications to be devised [3].
Significant expression of certain antigens in prostate cancer as compared to normal tissue could be used for antigen-targeted imaging or therapy. Numerous pre-clinical and clinical trials focused on this topic have already shown promising results [4–8].
Among the antigens used for diagnostic applications, the prostate-specific membrane antigen (PSMA) was demonstrated to be a useful target [9–13]. PSMA is a Type II integral membrane glycoprotein, which is overexpressed in prostate cancer in comparison to benign prostate tissue [11,14,15]. Next to expression in prostate, PSMA was found to be expressed in the neovasculature of solid tumors in comparison to normal vasculature [16,17]. The biological role of PSMA is not completely understood [10,18,19].
Ross et al. demonstrated a significant correlation between PSMA expression in prostate cancer and the Gleason score, pathological stage and biochemical recurrence [11]. Indium-111 capromab pendetide (ProstaScint®) is a radiolabelled antibody directed against PSMA. Correlation of scan results with pathological specimens suggests that ProstaScint is able to detect soft tissue metastases [20–23]. However, for routine use in clinical practice, the sensitivity of ProstaScint is not high enough, because the antibody targets the intracellular epitope of PSMA, thereby probably targeting only damaged or necrotic/apoptotic cells. Furthermore, the role of ProstaScint in the diagnosis of recurrent disease has to be elucidated [24].
Another antigen that can be used as an imaging target is the epithelial cell adhesion molecule (EpCAM). EpCAM is a transmembrane glycoprotein, which is highly expressed in rapidly proliferating tumors of epithelial origin [25–27]. This protein is found to be strongly expressed in several carcinomas [28–31]. In normal epithelium, there is a lower expression of EpCAM [32]. EpCAM mediates epithelial-specific intercellular cell-adhesion. Next, it is suggested that EpCAM is involved in cell migration, signaling, proliferation and differentiation [33]. The expression of EpCAM is inversely related to prognosis in several carcinomas [33]. For prostate cancer, this relation is controversial [32,34].
Signal protein vascular endothelial growth factor (VEGF) and its receptors are involved in (tumor-related) angiogenesis [35,36]. VEGF is overexpressed in a variety of tumors, including gliomas, breast, renal cell and hepatocellular cancer [37]. VEGF is a potential target, as its expression has also been demonstrated in prostate cancer [38,39]. The expression of VEGF in normal prostate, benign prostate hyperplasia and prostate cancer in relation to tumor grade is inconsistent in the current literature [7,40–49]. As for EpCAM, the prognostic value of VEGF expression is controversial [50–53].
The gastrin-releasing peptide receptor (GRPR) can be a promising imaging target. GRPR is a glycosylated seven-transmembrane G-protein coupled receptor, which is expressed in numerous cancers, such as those of the lung, colon and prostate [54–59]. GRPR seems to be overexpressed in prostate cancer in comparison to sparse expression in normal prostate tissue [60–62]. Binding of GRPR stimulates the growth of prostate cancer cells in vitro and in vivo [63,64]. A significant inverse correlation was found between GRPR expression and an increasing Gleason score [60].
Currently, there is no knowledge about the expression of PSMA, EpCAM, VEGF and GRPR in locally recurrent prostate cancer after brachytherapy or external beam radiotherapy. Therefore, the aim of this pilot study was to investigate the expression of these antigens using immunohistochemistry and to analyze their potency for new diagnostic applications in locally recurrent prostate cancer.
2. Results and Discussion
2.1. Results
In Table 1, the results of the immunohistochemical staining of different antibodies in prostate cancer specimens are presented.
Table 1.
Gleason sum scores, therapy characteristics and staining intensities of the antibodies in the salvage prostatectomy specimens.
| Patient No. | Radiotherapy (Dose in Gy) | Hormonal Status Prior to Salvage Prostatectomy | Interval between Radiotherapy and Salvage Prostatectomy (Months) | Stage | Gleason | PSMA | EpCAM | VEGF | GRPR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | EBRT (Dose unknown) | LHRH + AA | 51 | pT3b | 7 | ++ | + | ++ | +++ |
| 2 | Brachytherapy (HDR) | None | 45 | pT2c | 8 | + | ++ | ++ | +++ |
| 3 | EBRT (70) | LHRH + AA | 58 | pT3b | 8 | + | − | + | +++ |
| 4 | EBRT (70) | None | 24 | pT3a | 7 | + | + | − | ++ |
| 5 | EBRT (Dose unknown) | None | 80 | pT2c | 7 | ++ | + | − | + |
| 6 | Brachytherapy (LDR) | None | 47 | pT2c | cnd | + | + | − | +++ |
| 7 | EBRT (70) | LHRH + AA | 120 | pT3a | 7 | +++ | − | + | + |
| 8 | EBRT (66) | None | 31 | pT3b | 8 | + | − | + | + |
| 9 | EBRT (66) | None | 78 | pT4 | 7 | +++ | +++ | + | +++ |
| 10 | EBRT (66) | None | 48 | pT3b | 8 | +++ | ++ | ++ | +++ |
| 11 | EBRT (Dose unknown) | Unknown | 63 | pT3b | 7 | +++ | +++ | +++ | ++ |
| 12 | Brachytherapy (LDR) | None | 41 | pT4 | 7 | +++ | +++ | + | ++ |
| 13 | EBRT (70) | None | 49 | pT3a | 8 | + | ++ | ++ | ++ |
| 14 | EBRT (68) | LHRH | 58 | pT3a | 6 | +++ | +++ | ++ | +++ |
| 15 | Brachytherapy (LDR) | AA | 88 | pT3b | 8 | +++ | ++ | ++ | +++ |
| 16 | EBRT (68) | None | 13 | pT3b | 6 | + | ++ | + | ++ |
| 17 | EBRT (70) | LHRH | 34 | pT3b | 10 | +++ | ++ | + | ++ |
cnd, could not be determined; EBRT, external beam radiotherapy; HDR, high dose rate; LDR, low dose rate; LHRH, luteinizing-hormone-releasing hormone agonist; AA, androgen receptor antagonist.
Overall, staining for PSMA was seen in 100% (17/17), EpCAM in 82.3% (14/17), VEGF in 82.3% (14/17) and GRPR in 100% (17/17) of prostate cancer specimens. Staining for PSMA, EpCAM and VEGF was seen in 0% (0/17) and for GRPR in 100% (17/17) of the specimens’ stromal compartments. Immunohistochemical staining intensity frequency, number and percent are shown in Table 2.
Table 2.
Immunohistochemical staining intensity of prostate cancer and stroma.
| Staining Intensity | PSMA Prostate Cancer | PSMA Stroma | EpCAM Prostate Cancer | EpCAM Stroma | VEGF Prostate Cancer | VEGF Stroma | GRPR Prostate Cancer | GRPR Stroma |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 (0%) | 17 (100%) | 3 (17.7%) | 17 (100%) | 3 (17.7%) | 17 (100%) | 0 (0%) | 0 (0%) |
| 1+ | 7 (41.2%) | - | 4 (23.5%) | - | 7 (41.2%) | - | 3 (17.7%) | 3 (17.7%) |
| 2+ | 2 (11.8%) | - | 6 (35.3%) | - | 6 (35.3%) | - | 6 (35.3%) | 8 (47.0%) |
| 3+ | 8 (47.0%) | - | 4 (23.5%) | - | 1 (5.8%) | - | 8 (47.0%) | 6 (35.3%) |
| Overall+ | 17/17 (100%) | 0/17 (0%) | 14/17 (82.3%) | 0/17 (0%) | 14/17 (82.3%) | 0/17 (0%) | 17/17 (100%) | 17/17 (100%) |
In 11.8% (2/17) of cases, the GRPR staining intensity of prostate cancer was higher than that of stroma. In 88.2% (15/17) of cases, the GRPR staining intensity of prostate cancer was equal to the staining intensity of stroma. Tumor distinctiveness is shown in Table 3.
Table 3.
Tumor distinctiveness.
| Tumor distinctiveness | PSMA | EpCAM | VEGF | GRPR |
|---|---|---|---|---|
| 0 | - | 3 (17.7%) | 3 (17.7%) | 15 (88.2%) |
| 1 | 7 (41.2%) | 4 (23.5%) | 7 (41.2%) | 2 (11.8%) |
| 2 | 2 (11.8%) | 6 (35.3%) | 6 (35.3%) | - |
| 3 | 8 (47.0%) | 4 (23.5%) | 1 (5.8%) | - |
Tumor distinctiveness = staining intensity tumor – staining intensity stroma.
2.2. Staining Pattern
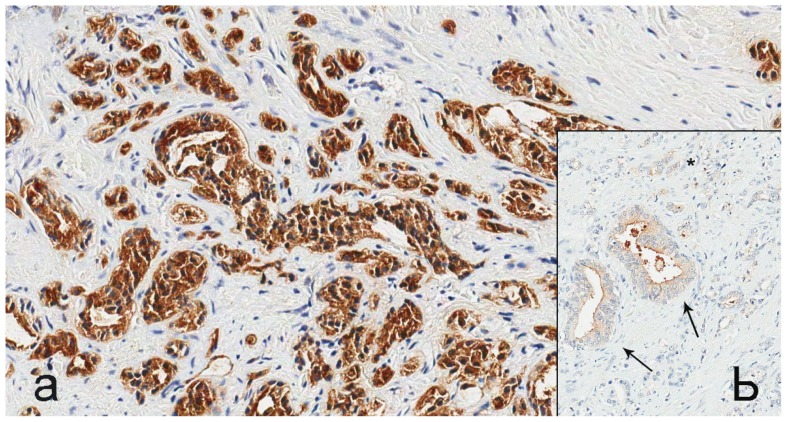
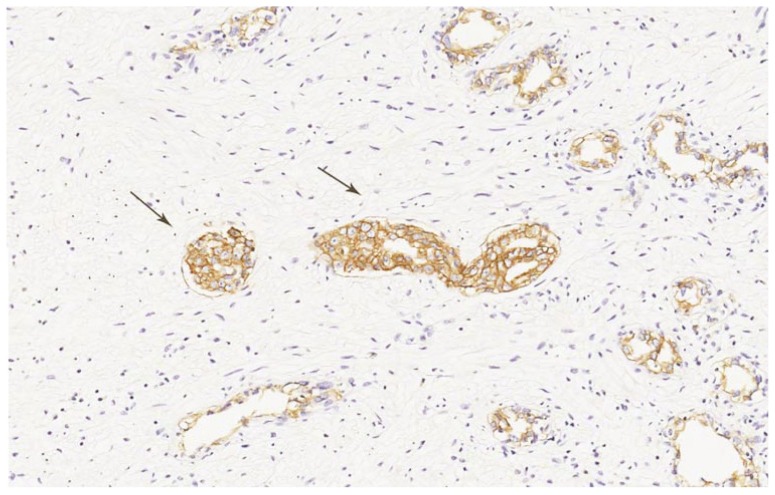
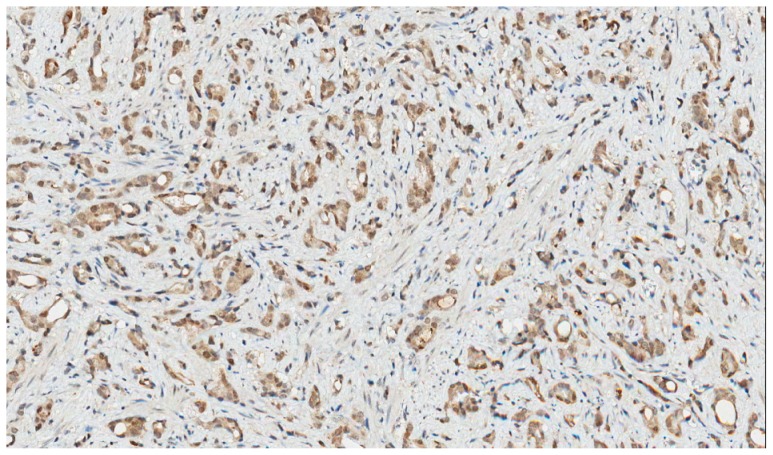
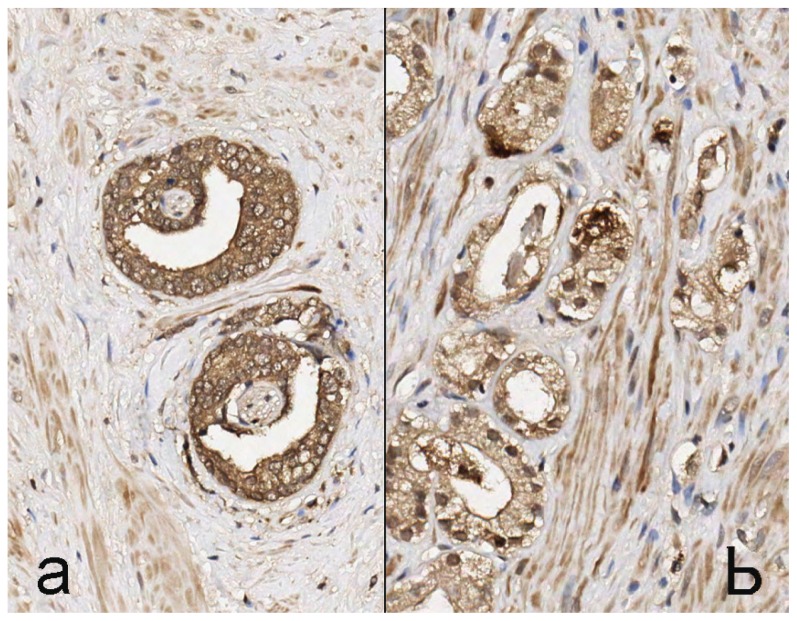
PSMA staining in prostate cancer specimens was membranous and cytoplasmic (Figure 1). Six cases with focal uptake amidst negative cancer tissue were observed (Patient No. 2–4, 6, 8, 13) (Figure 1). For PSMA, there was minimal staining of prostatic intraepithelial neoplasia (PIN) and normal prostate epithelium. EpCAM stained cytoplasm and at the basal membrane (Figure 2). VEGF and GRPR staining in prostate cancer tissue was cytoplasmic and diffuse (Figures 3 and 4). Staining of striated muscle was observed for GRPR.
Figure 1.
PSMA staining in prostate cancer tissue. (a) Membranous and cytoplasmic brown staining for PSMA in prostate cancer cells, 400× magnification; (b) focal brown staining for PSMA in prostate cancer cells (arrows) amidst negative cancer tissue (*), 200× magnification.
Figure 2.
EpCAM staining in prostate cancer tissue. Strong brown cytoplasmic staining for EpCAM in prostate cancer cells (arrows), 400× magnification.
Figure 3.
VEGF staining in prostate cancer tissue. Strong brown cytoplasmic staining for VEGF in prostate cancer cells, 200× magnification.
Figure 4.
GRPR staining in prostate cancer tissue. (a) Strong brown cytoplasmic staining for GRPR in prostate cancer cells and weak background staining of prostate stromal cells; (b) equal staining intensity of prostate cancer and stroma, 200× magnification.
2.3. Discussion
This study has shown that PSMA, EpCAM, VEGF and GRPR are expressed in locally recurrent prostate cancer after brachytherapy and external beam radiotherapy. Staining for PSMA and GRPR was observed in all prostate cancer specimens (17/17), while EpCAM and VEGF staining was observed in 82.3% (14/17) of cases (Tables 1 and 2). Staining for PSMA, EpCAM and VEGF was absent (0/17) in specimens’ stromal compartments, while GRPR staining in stroma was observed in 100% (17/17) of cases. In 88.2% (15/17) of cases, the GRPR staining intensity of prostate cancer was equal to the staining intensity of stroma. Staining of striated muscle was observed for GRPR, which is in agreement with the findings of other groups [60–62].
The high expression of PSMA, EpCAM, VEGF and GRPR in locally recurrent prostate cancer after ionizing therapy could have several reasons, which will be postulated next. First, high expression of either antigen is correlated with a tendency for recurrence. This holds true for PSMA, as its expression in primary prostate cancer is positively correlated with factors, like a high Gleason grade, a higher tumor stage and, most important in this case, biochemical recurrence [11,18]. The same goes for EpCAM, as a recent study by Benko et al. showed that higher EpCAM expression correlated with a higher Gleason score and a shorter disease-free survival [34]. However, other research groups found conflicting results [26,30,65]. Expression of VEGF in prostate cancer positively correlated with high a Gleason score, lymph node metastases and the progression of disease [66]. Although there is no information about GRPR expression and recurrence, the process might be comparable.
Second is the upregulation of antigens due to radiotherapy. It has been demonstrated that VEGF is upregulated after radiotherapy for rectal cancer [67]. For EpCAM, PSMA and GRPR, this is currently unknown. Third, recurrent cancer could have the tendency for upregulation of certain antigens. In ovarian cancer, EpCAM upregulation was seen in recurrent cancer when compared to primary cancer in matched samples [68], while VEGF levels were significantly higher in recurrent acute lymphoblastic leukemia compared with newly diagnosed cases [69]. For all the postulations, we have no direct evidence, as we did not have prostate cancer biopsy samples before and after radiation therapy that could have been compared to the salvage prostatectomy samples.
The absence of non-specific background staining of normal tissue or prostate stroma for the anti-PSMA, EpCAM and VEGF antibodies proves the specificity for cancer tissue. Background staining for GRPR was evident. Although different protocols were used and different blocking agents were tested, staining of non-cancer tissue remained. Staining of normal prostate and muscle with the anti-GRPR antibody has been described in the literature by other authors [60–62], and at first glance, this might pose a problem for the future use of GRPR as a target for new therapeutic or diagnostic modalities. However, pre-clinical and clinical studies have shown very low uptake of GRPR-targeted bombesin-like radiopharmaceuticals in muscle with high tumor-to-muscle ratios [70].
The current study has several limitations. First, we did not have matched samples of prostate cancer tissue. In an ideal situation, we would have compared prostate biopsy samples at initial diagnosis before radiation therapy, the biopsy samples in which local recurrence was confirmed after radiation therapy and the salvage prostatectomy samples; Second, staining intensity was assessed qualitatively on a scale from zero to three. Although strong staining suggests a higher antigen density than weak staining does, it is impossible to make a comparison between the different antibodies used in this study, as different protocols were used and as there is most likely different antigen sensitivity for each antibody. A quantification of antigen-densities has to be performed to be able to make a comparison between antigens; Third, due to the low number of patients (n = 17), the power of the statistics would be too weak, and therefore, we were unable to correlate staining intensity with clinicopathological parameters.
Finally, we did not have the follow-up data to compare antigen expression with outcome.
There is not much knowledge about the expression of antigens in recurrent prostate cancer. Locally recurrent prostate cancer is, to our knowledge, an unexplored field. Therefore, this is the first study to show the data about the expression of PSMA, VEGF, EpCAM and GRPR in locally recurrent prostate cancer after ionizing therapy.
Antigen-based targeted imaging of the prostate could be useful in several scenarios: if salvage local therapy was planned without a biopsy or with repeated negative biopsies, but with a strong suspicion of local recurrence. Other applications for these imaging strategies would be to rule out micrometastatic disease prior to local salvage therapy or the application of salvage focal therapy only to areas of uptake on imaging in order to minimize side-effects in irradiated tissue. The unique overexpression on prostate cancer cells makes PSMA the most attractive target for the delivery of imaging agents [10]. Several clinical studies with PSMA as the target have been reported with encouraging results [71,72]. Bombesin-like radiopharmaceuticals, which are natural ligands of GRPR, can be relatively easy synthesized in large quantities and have shown promising results in several clinical studies [70]. However, due to its background staining, more careful selection of the protocol may be required for optimal targeting. Only a few studies consider VEGF and EpCAM-based diagnostics for targeted cancer imaging [73–75], but based on the results reported in this study, both antigens can be used for the detection of locally recurrent prostate cancer.
3. Experimental Section
3.1. Materials
Patients in our retrospective study were diagnosed with locally recurrent prostate cancer based on PSA relapse and transrectal ultrasound-guided prostate biopsies. Prostate cancer specimens were collected of 17 patients who underwent salvage prostatectomy (The Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital Amsterdam (NKI/AVL)), because of locally recurrent prostate cancer after brachytherapy (4 patients) or external beam radiotherapy (13 patients). All tissue specimens were anonymous and encoded with a unique code. According to Dutch law, no further Institutional Review Board approval was required (http://www.federa.org). Pretreatment biopsies were not available.
3.2. Immunohistochemistry
Formalin-fixed, paraffin-embedded blocks of prostate tissue were cut into 4-mm-thick sections and mounted on Starfrost microscope slides. Hematoxylin and eosin stained sections were graded by an experienced pathologist (Stefano Rosati), based on the criteria of the Gleason grading system. Remaining sections were processed for immunohistochemistry.
3.2.1. PSMA
After deparaffinization, antigen retrieval was performed by heating microwave (700 W) for 20 min in a 10 mM citrate buffer at pH 6.0, with a cool down period of 20 min afterwards. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for 20 min. Slides were than incubated with the primary anti-human-PSMA mouse monoclonal antibody, YPSMA-1 (Abcam, Cambridge, UK), diluted at 1:400 in 1% bovine serum albumin/phosphate-buffered saline (1% BSA/PBS) for 1 h at room temperature. The secondary step consisted of incubation with rabbit anti-mouse antibody conjugated to polymer-horseradish peroxidase (DAKO, Glostrup, Denmark), diluted at 1:100 in 1% BSA/PBS with 1% AB serum. For the tertiary step, goat anti-rabbit antibody conjugated to polymer-horseradish peroxidase (DAKO, Glostrup, Denmark) was used, diluted at 1:100 in 1% BSA/PBS with 1% AB serum. Both the secondary and tertiary step required incubation for 30 min at room temperature. Next, the slides were immersed for 10 min in a solution of 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA) and 0.03% hydrogen peroxide in PBS for the visualization of the signal as brown staining. After washing with demineralized water, the slides were slightly counterstained with hematoxylin, dehydrated and mounted with Eukitt mounting medium (Sigma-Aldrich, Steinheim, Germany).
3.2.2. GRPR
After deparaffinization, antigen retrieval was performed by heating microwave (700 W) for 20 min in 0.1 M Tris/HCl buffer at pH 9.0, with a cool down period of 20 min afterwards. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in Tris-buffered saline (TBS) for 20 min. Slides were then incubated with a normal goat serum diluted at 1:10 in TBS for 30 min at room temperature. Afterwards, the slides were incubated with the primary anti-human-GRPR rabbit polyclonal antibody, ab39963 (Abcam, Cambridge, UK), diluted at 1:250 in 1% BSA/TBS overnight at 4 °C. Only a secondary step with goat anti-rabbit antibody conjugated to polymer-horseradish peroxidase (DAKO, Glostrup, Denmark) was applied, diluted at 1:100 in 1% BSA/TBS with 1% AB serum for 60 min at room temperature. Next, the slides were immersed for 10 min in a solution of 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich, Steinheim, Germany) and 0.03% hydrogen peroxide in PBS for the visualization of the signal as brown staining. After washing with demineralized water, the slides were slightly counterstained with hematoxylin, dehydrated and mounted with Eukitt mounting medium (Sigma-Aldrich, Steinheim, Germany).
3.2.3. EpCAM
After deparaffinization, antigen retrieval was performed by incubation with 0.1% protease for 30 min at room temperature. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in PBS for 20 min. Slides were than incubated with the primary mouse monoclonal anti-EpCAM antibody (Clone VU-1D9, Leica Biosystems, Newcastle, UK) diluted at 1:100 in 1% BSA/PBS for 1 h at room temperature. The secondary step consisted of incubation with rabbit anti-mouse antibody conjugated to polymer-horseradish peroxidase (DAKO, Glostrup, Denmark), diluted at 1:100 in 1% BSA/PBS with 1% AB serum. For the tertiary step, goat anti-rabbit antibody conjugated to polymer-horseradish peroxidase (DAKO, Glostrup, Denmark) was used, diluted at 1:100 in 1% BSA/PBS with 1% AB serum. Both the secondary and tertiary step required incubation for 30 min at room temperature. Next, the slides were immersed for 10 min in a solution of 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich, Steinheim, Germany) and 0.03% hydrogen peroxide in PBS for visualization of the signal as brown staining. After washing with demineralized water, the slides were slightly counterstained with hematoxylin, dehydrated and mounted with Eukitt mounting medium (Sigma-Aldrich, Steinheim, Germany).
3.2.4. VEGF
After deparaffinization, microwave antigen retrieval (700 W) was performed for 20 min in 10 mM Tris/1 mM EDTA buffer at pH 9.0, with a cool down period of 20 min afterwards. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in PBS for 20 min. Slides were incubated with a normal goat serum diluted at 1:10 in PBS for 30 min at room temperature. The primary step consisted of incubation with rabbit anti-human antibody VEGF A-20 sc-152, (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted at 1:200 in 1% BSA/PBS for 1 h at room temperature. Only a secondary step with goat anti-rabbit antibody conjugated to polymer-horseradish peroxidase (DAKO, Glostrup, Denmark) was applied, diluted at 1:100 in 1% BSA/TBS with 1% AB serum for 30 min at room temperature. Next, the slides were immersed for 10 min in a solution of 0.05% 3,3′-diaminobenzidine (Sigma-Aldrich, Steinheim, Germany) and 0.03% hydrogen peroxide in PBS for the visualization of the signal as brown staining. After washing with demineralized water, the slides were slightly counterstained with hematoxylin, dehydrated and mounted with Eukitt mounting medium (Sigma-Aldrich, Steinheim, Germany).
3.3. Assessment of Staining Patterns
The assessment of staining patterns was performed as described before [8]. For each antigen, a pathologist (Stefano Rosati) blinded to clinical and pathological data, scored the staining intensity (0 = no staining; 1+ = weak staining; 2+ = moderate staining; 3+ = strong staining) of tumor areas for all the specimens. Specimens in which one or more tumor areas with different staining intensities were present were scored for the most prevalent intensity. Specimens with focal uptake amidst negative cancer tissue scored 1+. Furthermore, different patterns of immunoreactivity were observed and documented. To evaluate background staining, all specimens were evaluated in a field that contained both prostate cancer and stroma. Tumor distinctiveness was assessed for each antigen by subtracting the staining intensity of stroma from the staining intensity of prostate cancer.
4. Conclusions
The current study is the first to present data on the expression of PSMA, EpCAM, VEGF and GRPR in locally recurrent prostate cancer after brachytherapy or external beam radiotherapy. Based on the absence of stromal staining, PSMA, EpCAM and VEGF show high tumor distinctiveness. GRPR has a very low tumor distinctiveness. Therefore, PSMA, EpCAM and VEGF can be used as targets for the bioimaging of recurrent prostate cancer after EBRT to exclude metastatic disease and/or to plan local salvage therapy.
Acknowledgments
The Center for Translational Molecular Medicine, Project Prostate Cancer Molecular Medicine (03O-203), has financially contributed to the current research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O., Bray F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Kupelian P.A., Potters L., Khuntia D., Ciezki J.P., Reddy C.A., Reuther A.M., Carlson T.P., Klein E.A. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:25–33. doi: 10.1016/s0360-3016(03)00784-3. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J., Attard G., Bahl A., Huland H., Klotz L., Kuban D., Oudard S., Watson W. Advances in the management of high-risk localised and metastatic prostate cancer. BJU Int. 2012;109:8–13. doi: 10.1111/j.1464-410X.2011.10871.x. [DOI] [PubMed] [Google Scholar]
- 4.Hillier S.M., Maresca K.P., Femia F.J., Marquis J.C., Foss C.A., Nguyen N., Zimmerman C.N., Barrett J.A., Eckelman W.C., Pomper M.G., et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–6940. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kularatne S.A., Wang K., Santhapuram H.K., Low P.S. Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol. Pharm. 2009;6:780–789. doi: 10.1021/mp900069d. [DOI] [PubMed] [Google Scholar]
- 6.Entwistle J., Brown J.G., Chooniedass S., Cizeau J., MacDonald G.C. Preclinical evaluation of VB6-845: An anti-EpCAM immunotoxin with reduced immunogenic potential. Cancer Biother. Radiopharm. 2012;27:582–592. doi: 10.1089/cbr.2012.1200.271. [DOI] [PubMed] [Google Scholar]
- 7.Köllermann J., Helpap B. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1 in benign, premalignant, and malignant prostate tissue. Am. J. Clin. Pathol. 2001;116:115–121. doi: 10.1309/1LBM-6X32-JH6W-ENUD. [DOI] [PubMed] [Google Scholar]
- 8.Ananias H.J., van den Heuvel M.C., Helfrich W., de Jong I.J. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101–1108. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 9.Bouchelouche K., Choyke P.L., Capala J. Prostate specific membrane antigen—A target for imaging and therapy with radionuclides. Discov. Med. 2010;9:55–61. [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh A., Heston W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 2004;91:528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 11.Ross J.S., Sheehan C.E., Fisher H.A., Kaufman R.P., Jr., Kaur P., Gray K., Webb I., Gray G.S., Mosher R., Kallakury B.V. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 12.Elsasser-Beile U., Buhler P., Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr. Drug Targets. 2009;10:118–125. doi: 10.2174/138945009787354601. [DOI] [PubMed] [Google Scholar]
- 13.Bouchelouche K., Capala J. Image and treat—An individualized approach to urological tumors. Curr. Opin. Oncol. 2010;22:274–280. doi: 10.1097/CCO.0b013e3283373d5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostwick D.G., Pacelli A., Blute M., Roche P., Murphy G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Lapidus R.G., Tiffany C.W., Isaacs J.T., Slusher B.S. Prostate-specific membrane antigen (PSMA) enzyme activity is elevated in prostate cancer cells. Prostate. 2000;45:350–354. doi: 10.1002/1097-0045(20001201)45:4<350::aid-pros10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Liu H., Moy P., Kim S., Xia Y., Rajasekaran A., Navarro V., Knudsen B., Bander N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–3634. [PubMed] [Google Scholar]
- 17.Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 18.Minner S., Wittmer C., Graefen M., Salomon G., Steuber T., Haese A., Huland H., Bokemeyer C., Yekebas E., Dierlamm J., et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–288. doi: 10.1002/pros.21241. [DOI] [PubMed] [Google Scholar]
- 19.Rajasekaran A.K., Anilkumar G., Christiansen J.J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 20.Bermejo C.E., Coursey J., Basler J., Austenfeld M., Thompson I. Histologic confirmation of lesions identified by Prostascint scan following definitive treatment. Urol. Oncol. 2003;21:349–352. doi: 10.1016/s1078-1439(02)00253-3. discussion 353. [DOI] [PubMed] [Google Scholar]
- 21.Lau H.Y., Kindrachuk G., Carter M., Prestage K., Webber D., Stauffer E., Haseman M. Surgical confirmation of ProstaScint abnormalities in two patients with high risk prostate cancer. Can. J. Urol. 2001;8:1199–1202. [PubMed] [Google Scholar]
- 22.Hinkle G.H., Burgers J.K., Olsen J.O., Williams B.S., Lamatrice R.A., Barth R.F., Rogers B., Maguire R.T. Prostate cancer abdominal metastases detected with indium-111 capromab pendetide. J. Nucl. Med. 1998;39:650–652. [PubMed] [Google Scholar]
- 23.Manyak M.J., Hinkle G.H., Olsen J.O., Chiaccherini R.P., Partin A.W., Piantadosi S., Burgers J.K., Texter J.H., Neal C.E., Libertino J.A., et al. Immunoscintigraphy with indium-111-capromab pendetide: Evaluation before definitive therapy in patients with prostate cancer. Urology. 1999;54:1058–1063. doi: 10.1016/s0090-4295(99)00314-3. [DOI] [PubMed] [Google Scholar]
- 24.Taneja S.S. ProstaScint® Scan: Contemporary use in clinical practice. Rev. Urol. 2004;6:S19–S28. [PMC free article] [PubMed] [Google Scholar]
- 25.Trzpis M., McLaughlin P.M., de Leij L.M., Harmsen M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Went P., Vasei M., Bubendorf L., Terracciano L., Tornillo L., Riede U., Kononen J., Simon R., Sauter G., Baeuerle P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate, and lung cancers. Br. J. Cancer. 2006;94:128–135. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Gun B.T., Melchers L.J., Ruiters M.H., de Leij L.F., McLaughlin P.M., Rots M.G. EpCAM in carcinogenesis: The good, the bad, or the ugly. Carcinogenesis. 2010;31:1913–1921. doi: 10.1093/carcin/bgq187. [DOI] [PubMed] [Google Scholar]
- 28.Stoecklein N.H., Siegmund A., Scheunemann P., Luebke A.M., Erbersdobler A., Verde P.E., Eisenberger C.F., Peiper M., Rehders A., Esch J.S., et al. Ep-CAM expression in squamous cell carcinoma of the esophagus: A potential therapeutic target and prognostic marker. BMC Cancer. 2006;6:165. doi: 10.1186/1471-2407-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Went P.T., Lugli A., Meier S., Bundi M., Mirlacher M., Sauter G., Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum. Pathol. 2004;35:122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Poczatek R.B., Myers R.B., Manne U., Oelschlager D.K., Weiss H.L., Bostwick D.G., Grizzle W.E. Ep-Cam levels in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. J. Urol. 1999;162:1462–1466. [PubMed] [Google Scholar]
- 31.Varga M., Obrist P., Schneeberger S., Mühlmann G., Felgel-Farnholz C., Fong D., Zitt M., Brunhuber T., Schäfer G., Gastl G., et al. Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin. Cancer Res. 2004;10:3131–3136. doi: 10.1158/1078-0432.ccr-03-0528. [DOI] [PubMed] [Google Scholar]
- 32.Ni J., Cozzi P.J., Duan W., Shigdar S., Graham P.H., John K.H., Li Y. Role of the EpCAM (CD326) in prostate cancer metastasis and progression. Cancer Metastasis Rev. 2012;31:779–791. doi: 10.1007/s10555-012-9389-1. [DOI] [PubMed] [Google Scholar]
- 33.Patriarca C., Macchi R.M., Marschner A.K., Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012;38:68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Benko G., Spajić B., Krušlin B., Tomas D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol. Oncol. 2013;31:468–474. doi: 10.1016/j.urolonc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 38.Kaushal V., Mukunyadzi P., Dennis R.A., Siegel E.R., Johnson D.E., Kohli M. Stage-specific characterization of the vascular endothelial growth factor axis in prostate cancer: Expression of lymphangiogenic markers is associated with advanced-stage disease. Clin. Cancer Res. 2005;11:584–593. [PubMed] [Google Scholar]
- 39.Peyromaure M., Badoual C., Camparo P., Grabar S., Goulvestre C., Fulla Y., Vieillefond A., Mao K., Dinh-Xuan A.T. Plasma levels and expression of vascular endothelial growth factor-A in human localized prostate cancer. Oncol. Rep. 2007;18:145–149. [PubMed] [Google Scholar]
- 40.Borre M., Nerstrøm B., Overgaard J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin. Cancer Res. 2000;6:1882–1890. [PubMed] [Google Scholar]
- 41.Jackson M.W., Bentel J.M., Tilley W.D. Vascular endothelial growth factor (VEGF) expression in prostate cancer and benign prostatic hyperplasia. J. Urol. 1997;157:2323–2328. [PubMed] [Google Scholar]
- 42.Ferrer F.A., Miller L.J., Andrawis R.I., Kurtzman S.H., Albertsen P.C., Laudone V.P., Kreutzer D.L. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: In situ and in vitro expression of VEGF by human prostate cancer cells. J. Urol. 1997;157:2329–2333. [PubMed] [Google Scholar]
- 43.El-Gohary Y.M., Silverman J.F., Olson P.R., Liu Y.L., Cohen J.K., Miller R., Saad R.S. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am. J. Clin. Pathol. 2007;127:572–579. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 44.Pasquali D., Rossi V., Staibano S., de Rosa G., Chieffi P., Prezioso D., Mirone V., Mascolo M., Tramontano D., Bellastella A., et al. The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: Up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinology. 2006;147:4245–4251. doi: 10.1210/en.2006-0614. [DOI] [PubMed] [Google Scholar]
- 45.Pallares J., Rojo F., Iriarte J., Morote J., Armadans L.I., de Torres I. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol. Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 46.Walsh K., Sriprasad S., Hopster D., Codd J., Mulvin D. Distribution of vascular endothelial growth factor (VEGF) in prostate disease. Prostate Cancer Prostatic Dis. 2002;5:119–122. doi: 10.1038/sj.pcan.4500575. [DOI] [PubMed] [Google Scholar]
- 47.Kwak C., Jin R.J., Lee C., Park M.S., Lee S.E. Thrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int. 2002;89:303–309. doi: 10.1046/j.1464-4096.2001.01417.x. [DOI] [PubMed] [Google Scholar]
- 48.Strohmeyer D., Rössing C., Bauerfeind A., Kaufmann O., Schlechte H., Bartsch G., Loening S. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45:216–224. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 49.Mazzucchelli R., Montironi R., Santinelli A., Lucarini G., Pugnaloni A., Biagini G. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–79. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Mao K., Badoual C., Camparo P., Delongchamps N.B., Vieillefond A., Dinh-Xuan A.T., Peyromaure M. The prognostic value of vascular endothelial growth factor (VEGF)-A and its receptor in clinically localized prostate cancer: A prospective evaluation in 100 patients undergoing radical prostatectomy. Can. J. Urol. 2008;15:4257–4262. [PubMed] [Google Scholar]
- 51.Boddy J.L., Fox S.B., Han C., Campo L., Turley H., Kanga S., Malone P.R., Harris A.L. The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF1a, HIF2a, and the prolyl hydroxylases in human prostate cancer. Clin. Cancer Res. 2005;11:7658–7663. doi: 10.1158/1078-0432.CCR-05-0460. [DOI] [PubMed] [Google Scholar]
- 52.Jans J., van Dijk J.H., van Schelven S., van der Groep P., Willems S.H., Jonges T.N., van Diest P.J., Bosch J.L. Expression and localization of hypoxia proteins in prostate cancer: Prognostic implications after radical prostatectomy. Urology. 2010;75:786–792. doi: 10.1016/j.urology.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Mao K., Camparo P., Badoual C., Peyromaure M., Delongchamps N.B., Vieillefond A., Dinh-Xuan A.T. The association of vascular endothelial growth factor receptor-1 with the risk of cancer progression following radical prostatectomy. Oncol. Rep. 2008;19:171–175. [PubMed] [Google Scholar]
- 54.Aprikian A.G., Cordon-Cardo C., Fair W.R., Reuter V.E. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer. 1993;71:3952–3965. doi: 10.1002/1097-0142(19930615)71:12<3952::aid-cncr2820711226>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 55.Cutz E., Chan W., Track N.S. Bombesin, calcitonin and leuenkephalin immunoreactivity in endocrine cells of human lung. Experientia. 1981;37:765–767. doi: 10.1007/BF01967969. [DOI] [PubMed] [Google Scholar]
- 56.Price J., Penman E., Wass J.A., Rees L.H. Bombesin-like immunoreactivity inhumangastrointestinal tract. Regul. Pept. 1984;9:1–10. doi: 10.1016/0167-0115(84)90002-8. [DOI] [PubMed] [Google Scholar]
- 57.Spindel E.R., Chin W.W., Price J., Rees L.H., Besser G.M., Habener J.F. Cloning and characterization of cDNAs encoding human gastrin-releasing peptide. Proc. Natl. Acad. Sci. USA. 1984;81:5699–5703. doi: 10.1073/pnas.81.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Track N.S., Cutz E. Bombesin-like immunoreactivity in developing human lung. Life Sci. 1982;30:1553–1556. doi: 10.1016/0024-3205(82)90243-0. [DOI] [PubMed] [Google Scholar]
- 59.Xiao D., Wang J., Hampton L.L., Weber H.C. The human gastrinreleasing peptide receptor gene structure, its tissue expression and promoter. Gene. 2001;264:95–103. doi: 10.1016/s0378-1119(00)00596-5. [DOI] [PubMed] [Google Scholar]
- 60.Beer M., Montani M., Gerhardt J., Wild P.J., Hany T.F., Hermanns T., Müntener M., Kristiansen G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate. 2012;72:318–325. doi: 10.1002/pros.21434. [DOI] [PubMed] [Google Scholar]
- 61.Bartholdi M.F., Wu J.M., Pu H., Troncoso P., Eden P.A., Feldman R.I. In situ hybridization for gastrin-releasing peptide receptor (GRP receptor) expression in prostatic carcinoma. Int. J. Cancer. 1998;79:82–90. doi: 10.1002/(sici)1097-0215(19980220)79:1<82::aid-ijc16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 62.Markwalder R., Reubi J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 63.Bologna M., Festuccia C., Muzi P., Biordi L., Ciomei M. Bombesin stimulates growth of human prostatic cancer cellsin vitro. Cancer. 1989;63:1714–1720. doi: 10.1002/1097-0142(19900501)63:9<1714::aid-cncr2820630912>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 64.Shimoda J. Effects of bombesin and its antibody on growth of human prostatic carcinoma cell lines. Nihon Hinyokika Gakkai Zasshi. 1992;83:1459–1468. doi: 10.5980/jpnjurol1989.83.1459. [DOI] [PubMed] [Google Scholar]
- 65.Zellweger T., Ninck C., Bloch M., Mirlacher M., Koivisto P.A., Helin H.J., Mihatsch M.J., Gasser T.C., Bubendorf L. Expression patterns of potential therapeutic targets in prostate cancer. Int. J. Cancer. 2005;113:619–628. doi: 10.1002/ijc.20615. [DOI] [PubMed] [Google Scholar]
- 66.Li R., Younes M., Wheeler T.M., Scardino P., Ohori M., Frolov A., Ayala G. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate. 2004;58:193–199. doi: 10.1002/pros.10321. [DOI] [PubMed] [Google Scholar]
- 67.Inoue Y., Ojima E., Watanabe H., Hiro J., Toiyama Y., Kobayashi M., Miki C., Kusunoki M. Does preoperative chemo-radiotherapy enhance the expression of vascular endothelial growth factor in patients with rectal cancer? Oncol. Rep. 2007;18:369–375. [PubMed] [Google Scholar]
- 68.Bellone S., Siegel E.R., Cocco E., Cargnelutti M., Silasi D.A., Azodi M., Schwartz P.E., Rutherford T.J., Pecorelli S., Santin A.D. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: Implications for epithelial cell adhesion molecule-specific immunotherapy. Int. J. Gynecol. Cancer. 2009;19:860–866. doi: 10.1111/IGC.0b013e3181a8331f. [DOI] [PubMed] [Google Scholar]
- 69.Koomagi R., Zintl F., Sauerbrey A., Volm M. Vascular endothelial growth factor in newly diagnosed and recurrent childhood acute lymphoblastic leukemia as measured by real-time quantitative polymerase chain reaction. Clin. Cancer Res. 2001;7:3381–3384. [PubMed] [Google Scholar]
- 70.Ananias H.J., de Jong I.J., Dierckx R.A., van de Wiele C., Helfrich W., Elsinga P.H. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Curr. Pharm. Des. 2008;14:3033–3047. doi: 10.2174/138161208786404335. [DOI] [PubMed] [Google Scholar]
- 71.Afshar-Oromieh A., Malcher A., Eder M., Eisenhut M., Linhart H.G., Hadaschik B.A., Holland-Letz T., Giesel F.L., Kratochwil C., Haufe S., et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 72.Cho S.Y., Gage K.L., Mease R.C., Senthamizhchelvan S., Holt D.P., Jeffrey-Kwanisai A., Endres C.J., Dannals R.F., Sgouros G., Lodge M., et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai W., Chen X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008;49:113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 74.Hsieh W.J., Liang C.J., Chieh J.J., Wang S.H., Lai I.R., Chen J.H., Chang F.H., Tseng W.K., Yang S.Y., Wu C.C., et al. In vivo tumor targeting and imaging with anti-vascular endothelial growth factor antibody-conjugated dextran-coated iron oxide nanoparticles. Int. J. Nanomed. 2012;7:2833–2842. doi: 10.2147/IJN.S32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song Y., Zhu Z., An Y., Zhang W., Zhang H., Liu D., Yu C., Duan W., Yang C.J. Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal. Chem. 2013;85:4141–4149. doi: 10.1021/ac400366b. [DOI] [PubMed] [Google Scholar]