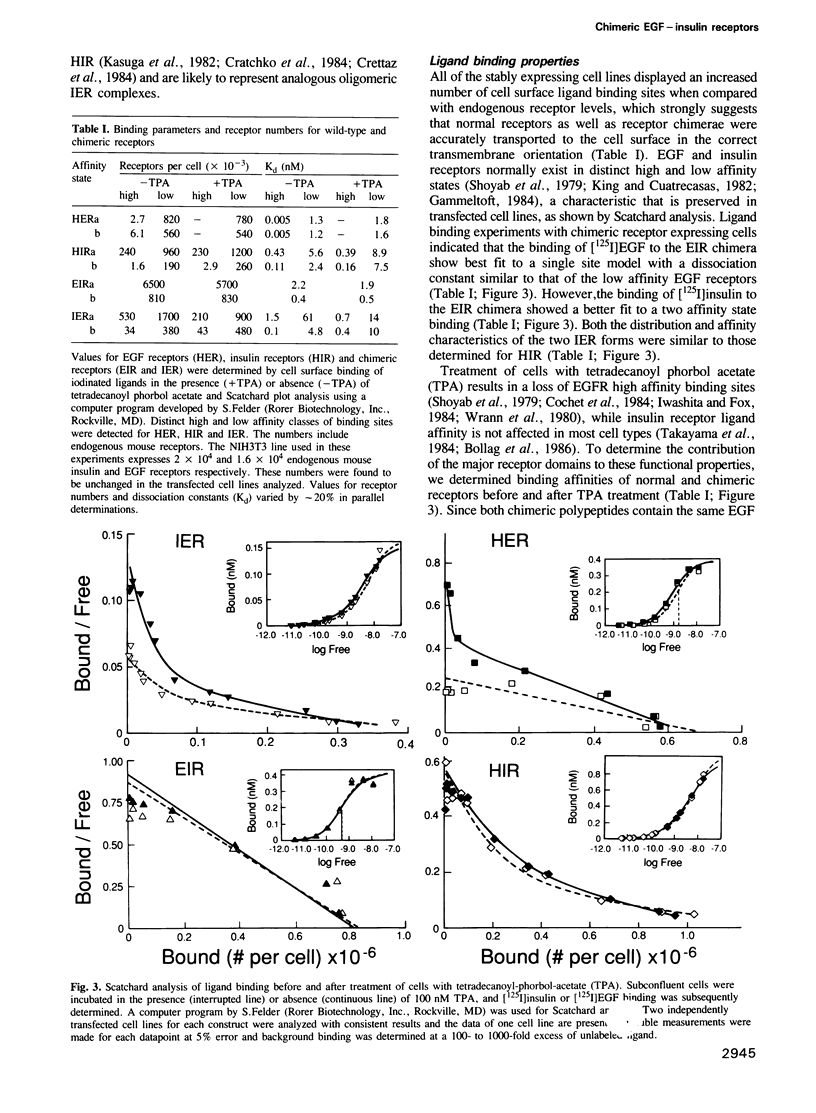
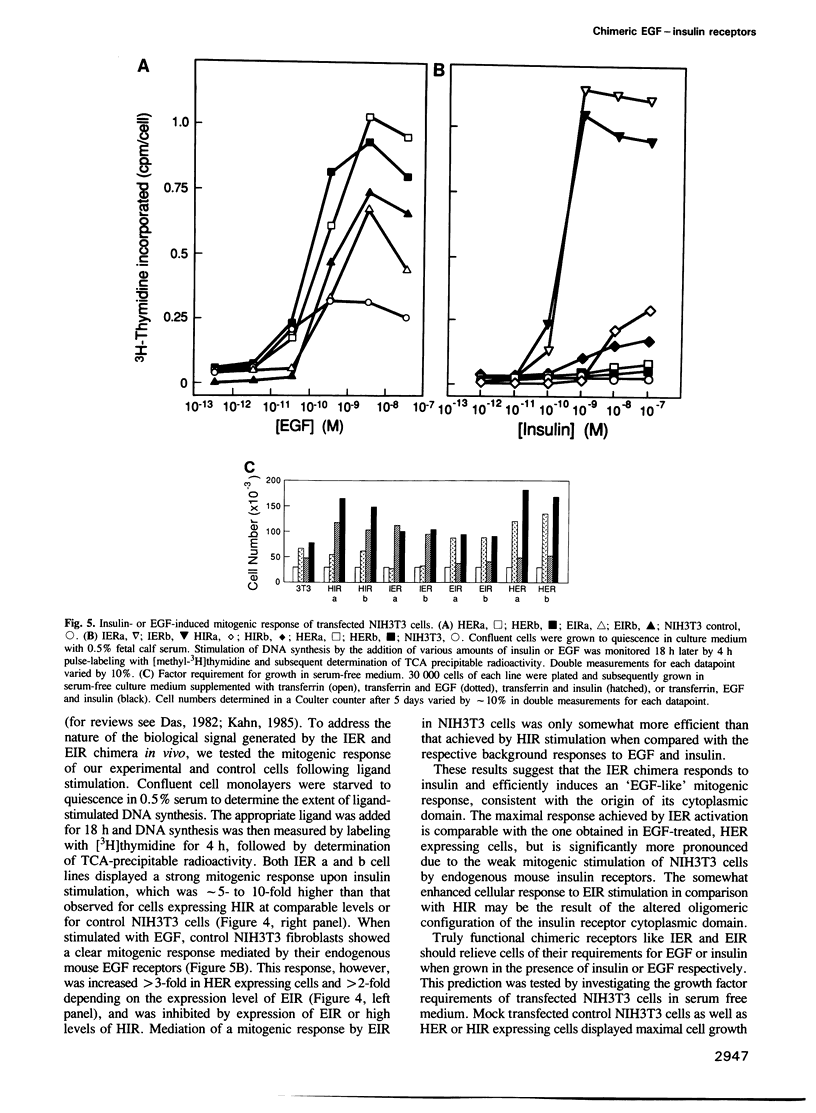
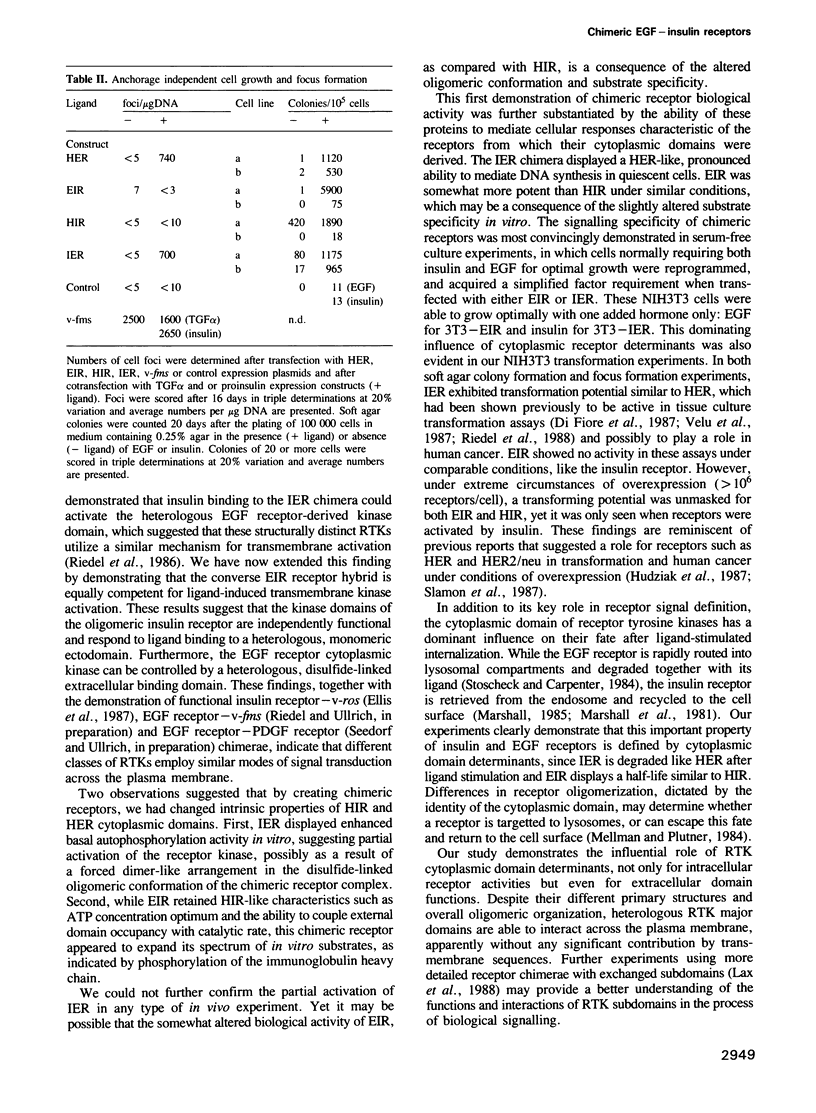
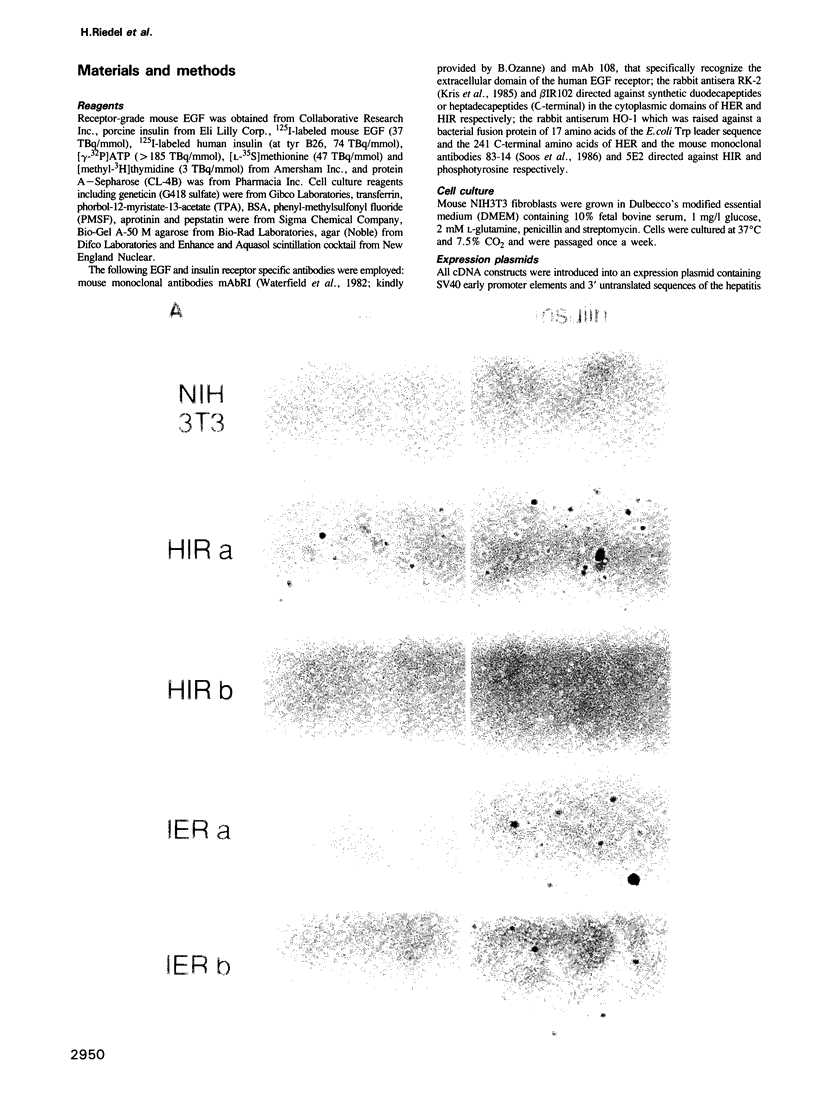
Abstract
The cell surface receptors for insulin and epidermal growth factor (EGF) both employ a tyrosine-specific protein kinase activity to fulfil their distinct biological roles. To identify the structural domains responsible for various receptor activities, we have generated chimeric receptor polypeptides consisting of major EGF and insulin receptor structural domains and examined their biochemical properties and cellular signalling activities. The EGF-insulin receptor hybrids are properly synthesized and transported to the cell surface, where they form binding competent structures that are defined by the origin of their extracellular domains. While their ligand binding affinities are altered, we find that these chimeric receptors are fully functional in transmitting signals across the plasma membrane and into the cell. Thus, EGF receptor and insulin receptor cytoplasmic domain signalling capabilities are independent of their new heterotetrameric or monomeric environments respectively. Furthermore, the cytoplasmic domains carry the structural determinants that define kinase specificity, mitogenic and transforming potential, and receptor routing.
Full text
PDF


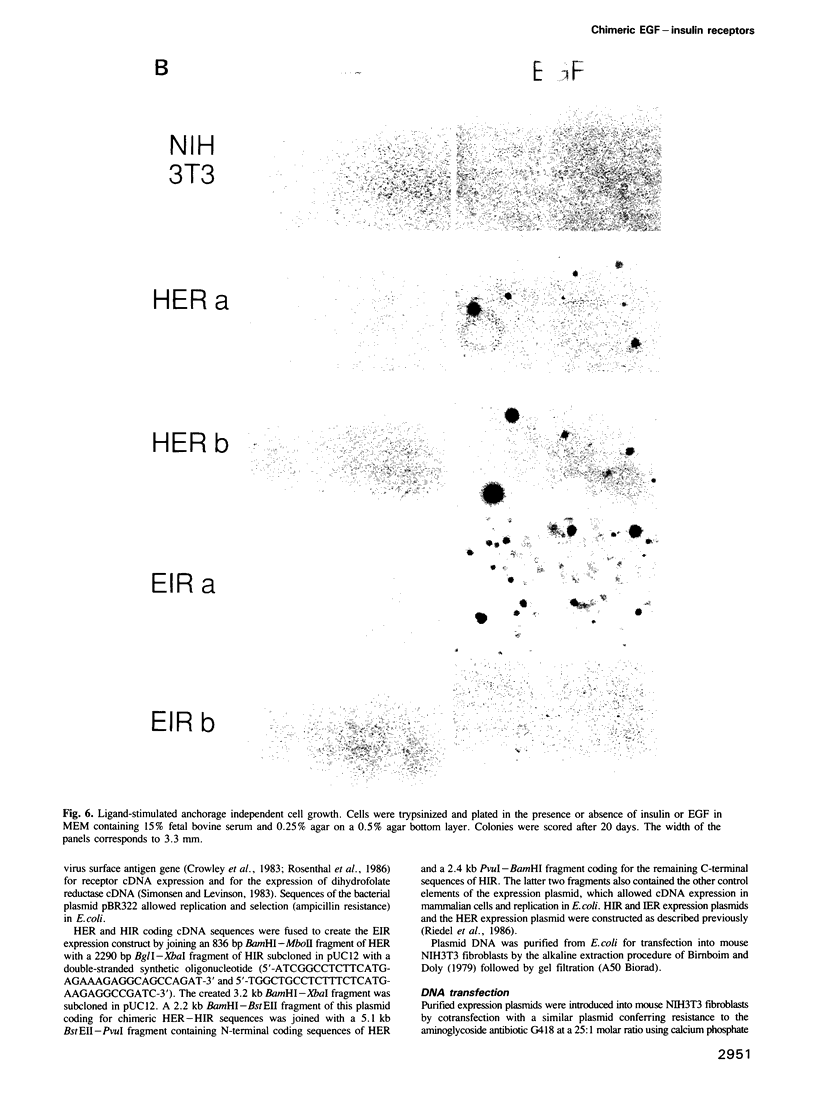
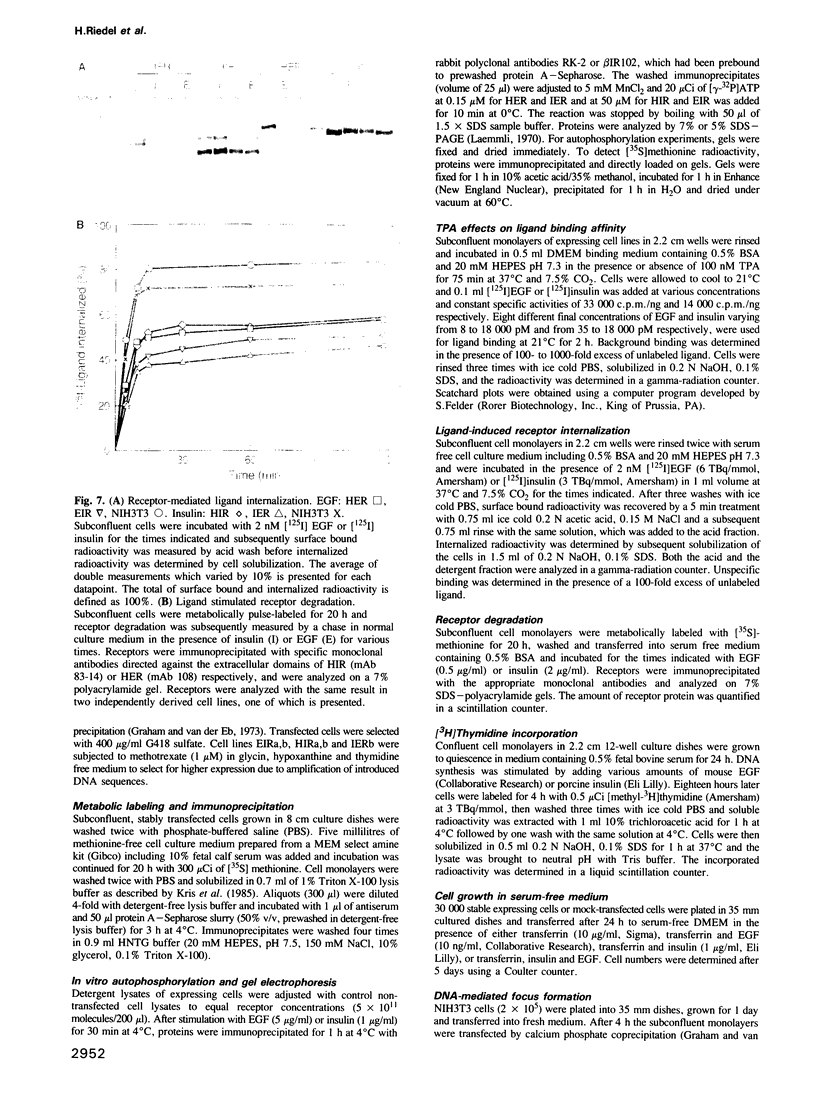


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguinot L., Lyall R. M., Willingham M. C., Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Cruz J., Khan M. N., Posner B. I. Uptake of insulin and other ligands into receptor-rich endocytic components of target cells: the endosomal apparatus. Annu Rev Physiol. 1985;47:383–403. doi: 10.1146/annurev.ph.47.030185.002123. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Insulin binds to and promotes the phosphorylation of a Mr 210 000 component of its receptor in detergent extracts of rat liver microsomes. FEBS Lett. 1983 Jul 25;158(2):243–246. doi: 10.1016/0014-5793(83)80587-0. [DOI] [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F., Bouscarel B., Slaton J., Blackmore P. F., Exton J. H. Epidermal growth factor mimics insulin effects in rat hepatocytes. Biochem J. 1986 Nov 1;239(3):523–530. doi: 10.1042/bj2390523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Chvatchko Y., Gazzano H., Van Obberghen E., Fehlmann M. Subunit arrangement of insulin receptors in hepatoma cells. Mol Cell Endocrinol. 1984 Jun;36(1-2):59–65. doi: 10.1016/0303-7207(84)90085-6. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Jialal I., Kasuga M., Kahn C. R. Insulin receptor regulation and desensitization in rat hepatoma cells. The loss of the oligomeric forms of the receptor correlates with the change in receptor affinity. J Biol Chem. 1984 Sep 25;259(18):11543–11549. [PubMed] [Google Scholar]
- Crowley C. W., Liu C. C., Levinson A. D. Plasmid-directed synthesis of hepatitis B surface antigen in monkey cells. Mol Cell Biol. 1983 Jan;3(1):44–55. doi: 10.1128/mcb.3.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M. Epidermal growth factor: mechanisms of action. Int Rev Cytol. 1982;78:233–256. doi: 10.1016/s0074-7696(08)60107-2. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Dunn W. A., Connolly T. P., Hubbard A. L. Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes: receptor pathway. J Cell Biol. 1986 Jan;102(1):24–36. doi: 10.1083/jcb.102.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Jong S. M., Wang L. H., Roth R. A., Rutter W. J. Heterologous transmembrane signaling by a human insulin receptor-v-ros hybrid in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5101–5105. doi: 10.1073/pnas.84.15.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Cohen S., Garbers D. L. The kinetics of tyrosine phosphorylation by the purified epidermal growth factor receptor kinase of A-431 cells. J Biol Chem. 1983 Apr 10;258(7):4137–4142. [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Freemark M. Epidermal growth factor stimulates glycogen synthesis in fetal rat hepatocytes: comparison with the glycogenic effects of insulin-like growth factor I and insulin. Endocrinology. 1986 Aug;119(2):522–526. doi: 10.1210/endo-119-2-522. [DOI] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Gammeltoft S. Insulin receptors: binding kinetics and structure-function relationship of insulin. Physiol Rev. 1984 Oct;64(4):1321–1378. doi: 10.1152/physrev.1984.64.4.1321. [DOI] [PubMed] [Google Scholar]
- Giugni T. D., James L. C., Haigler H. T. Epidermal growth factor stimulates tyrosine phosphorylation of specific proteins in permeabilized human fibroblasts. J Biol Chem. 1985 Dec 5;260(28):15081–15090. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Szapary D., Schmidt A., Lyall R., Van Obberghen E., Dull T. J., Ullrich A., Schlessinger J. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol. 1987 Dec;7(12):4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak R. M., Schlessinger J., Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Hedo J. A., Yamada K. M., Kahn C. R. The structure of insulin receptor and its subunits. Evidence for multiple nonreduced forms and a 210,000 possible proreceptor. J Biol Chem. 1982 Sep 10;257(17):10392–10399. [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lax I., Bellot F., Howk R., Ullrich A., Givol D., Schlessinger J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989 Feb;8(2):421–427. doi: 10.1002/j.1460-2075.1989.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Pike L. J., Freidenberg G. R., Cordera R., Stith B. J., Olefsky J. M., Krebs E. G. Increased phosphorylation of ribosomal protein S6 following microinjection of insulin receptor-kinase into Xenopus oocytes. Nature. 1986 Apr 3;320(6061):459–461. doi: 10.1038/320459a0. [DOI] [PubMed] [Google Scholar]
- Marshall S., Green A., Olefsky J. M. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981 Nov 25;256(22):11464–11470. [PubMed] [Google Scholar]
- Marshall S. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J Biol Chem. 1985 Apr 10;260(7):4136–4144. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenoff R. A., Gunsalus J. R., Avruch J. An insulin-stimulated (ribosomal S6) protein kinase from soluble extracts of H4 hepatoma cells. Arch Biochem Biophys. 1986 Feb 15;245(1):196–203. doi: 10.1016/0003-9861(86)90205-5. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Perrotti N., Accili D., Marcus-Samuels B., Rees-Jones R. W., Taylor S. I. Insulin stimulates phosphorylation of a 120-kDa glycoprotein substrate (pp120) for the receptor-associated protein kinase in intact H-35 hepatoma cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3137–3140. doi: 10.1073/pnas.84.10.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Khan M. N., Bergeron J. J. Endocytosis of peptide hormones and other ligands. Endocr Rev. 1982 Summer;3(3):280–298. doi: 10.1210/edrv-3-3-280. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Hedo J. A., Zick Y., Roth J. Insulin-stimulated phosphorylation of the insulin receptor precursor. Biochem Biophys Res Commun. 1983 Oct 31;116(2):417–422. doi: 10.1016/0006-291x(83)90539-9. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Taylor S. I. An endogenous substrate for the insulin receptor-associated tyrosine kinase. J Biol Chem. 1985 Apr 10;260(7):4461–4467. [PubMed] [Google Scholar]
- Reiss N., Kanety H., Schlessinger J. Five enzymes of the glycolytic pathway serve as substrates for purified epidermal-growth-factor-receptor kinase. Biochem J. 1986 Nov 1;239(3):691–697. doi: 10.1042/bj2390691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H., Dull T. J., Schlessinger J., Ullrich A. A chimaeric receptor allows insulin to stimulate tyrosine kinase activity of epidermal growth factor receptor. Nature. 1986 Nov 6;324(6092):68–70. doi: 10.1038/324068a0. [DOI] [PubMed] [Google Scholar]
- Riedel H., Massoglia S., Schlessinger J., Ullrich A. Ligand activation of overexpressed epidermal growth factor receptors transforms NIH 3T3 mouse fibroblasts. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1477–1481. doi: 10.1073/pnas.85.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Lindquist P. B., Bringman T. S., Goeddel D. V., Derynck R. Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell. 1986 Jul 18;46(2):301–309. doi: 10.1016/0092-8674(86)90747-6. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Quantitative ultrastructural analysis of receptor-mediated insulin uptake into adipocytes. J Cell Physiol. 1983 May;115(2):199–207. doi: 10.1002/jcp.1041150215. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- White M. F., Haring H. U., Kasuga M., Kahn C. R. Kinetic properties and sites of autophosphorylation of the partially purified insulin receptor from hepatoma cells. J Biol Chem. 1984 Jan 10;259(1):255–264. [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Wrann M., Fox C. F., Ross R. Modulation of epidermal growth factor receptors on 3T3 cells by platelet-derived growth factor. Science. 1980 Dec 19;210(4476):1363–1365. doi: 10.1126/science.6254158. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987 Mar 10;26(5):1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987 Mar 10;26(5):1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]