Abstract
Sexual differentiation in Bombyx mori is controlled by sex-specific splicing of Bmdsx, which results in the omission of exons 3 and 4 in a male-specific manner. In B. mori, insulin-like growth factor II mRNA-binding protein (Imp) is a male-specific factor involved in male-specific splicing of Bmdsx. Male-specific Imp mRNA results from the male-specific inclusion of exon 8. To verify the link between histone methylation and alternative RNA processing in Imp, we examined the effects of RNAi-mediated knockdown of several histone methyltransferases on the sex-specific mRNA expression of Imp. As a result, male-specific expression of Imp mRNA was completely abolished when expression of the H3K79 methyltransferase DOT1L was repressed to <10% of that in control males. Chromatin immunoprecipitation-quantitative PCR analysis revealed a higher distribution of H3K79me2 in normal males than in normal females across Imp. RNA polymerase II (RNAP II) processivity assays indicated that RNAi knockdown of DOT1L in males caused a twofold decrease in RNAP II processivity compared to that in control males, with almost equivalent levels to those observed in normal females. Inhibition of RNAP II-mediated elongation in male cells repressed the male-specific splicing of Imp. Our data suggest the possibility that H3K79me2 accumulation along Imp is associated with the male-specific alternative processing of Imp mRNA that results from increased RNAP II processivity.
Keywords: alternative splicing, DOT1L, histone H3 methylation, sex determination
1. Introduction
Alternative splicing of pre-mRNA is an essential mechanism in the regulation of differential gene expression that can produce functionally distinct proteins from a single gene based on the developmental or physiological state of the cells in a multicellular organism [1,2]. Recent studies estimate that 90% of human genes are alternatively spliced [3], and several thousand different mRNA isoforms can be produced from a single gene. Although many examples describe how alternative splicing regulates gene expression, the mechanisms involved are less well understood [4–8].
Alternative splicing is thought to be regulated by the interaction of splicing factors and splicing enhancers (or silencers). Alternative splicing regulatory mechanisms have been investigated, structural models of spliceosomes have been proposed, and many RNA regulatory elements have been characterized; however, the emerging complexity of alternative splicing regulation suggests that these approaches have not sufficiently described how alternative splicing is regulated. Recent provocative studies point to a key function of chromatin structure and histone modification in alternative splicing regulation [9–11]. For example, the nucleosome occupancy level was lower in cassette exons than in constitutively spliced exons [12–14]. H3K36me3 and H3K9ac were related to the exon-skipping event of NCAM [15] and the levels of H3K36me3 differed in mutually exclusive exons of several genes among different cell types. Moreover, a genome-wide study across different species revealed that H3K36me3 was depleted in skipped exons [13,16]. Other histone modifications such as H3K4me1, H3K4me3, H3K27me3, and H3K9me1 have been associated with the alternative splicing events of FGFR2 [17]. H3K4me3 was suggested to affect the alternative splicing events of CHD1 [18], while H3K9me3 was associated with multiple exon skipping of CD44 [19]. A recent genome-wide chromatin immunoprecipitation (ChIP)-seq analysis of histone H3 methylation in mammals revealed that alternative exons are preferentially marked with H3K4me1, H3K27me3, and H3K79me2, while being marked with H3K4me2, H3K4me3, and H3K36me3 at significantly lower levels [20].
In the silkworm Bombyx mori, the chromosomal sex determination mechanism is distinct from that of Drosophila melanogaster, with female (ZW) being the heterogametic sex and male (ZZ) the homogametic sex. The female sex in B. mori is determined by the presence of a dominant feminizing factor, Feminizer (Fem), on the W chromosome [21]. Note that no sex-specific regulatory Sxl homolog has been isolated from B. mori [22], and no tra homolog has been found in the Bombyx genome [23]. Despite these differences, a B. mori dsx homolog (Bmdsx) has been implicated in sex determination [24]. The primary transcript of the Bmdsx gene is alternatively spliced in males and females to yield sex-specific mRNAs that encode male-specific (BmDSXM) and female-specific (BmDSXF) polypeptides [25]. We found that unlike Drosophila dsx, the Bmdsx female exon is devoid of putative TRA/TRA-2 binding sites [25]. Instead, the splicing inhibitor BmPSI and a B. mori insulin-like growth factor II mRNA-binding protein (Imp) regulate male-specific splicing of Bmdsx [26,27]. Imp is localized on the Z chromosome and is expressed in a male-specific manner in various tissues. In male cells, the male-specific Imp mRNA is formed as a result of the inclusion of exon 8 and the promoter-distal poly(A) site choice, whereas non-sex-specific polyadenylation occurs at the promoter-proximal poly(A) site downstream of exon 7 [28]. The molecular mechanisms underlying the sex-specific splicing regulation of this gene remain unclear.
To verify the link between histone methylation and alternative RNA processing in Imp mRNA production, we investigated the effects of RNAi-mediated knockdown of several histone methyltransferases (HMTases) on sex-specific mRNA expression of Imp. Notably, the male-specific expression of Imp mRNA was completely abolished when expression of the H3K79 methyltransferase DOT1L was repressed to <10% of that in control males. Here, we provide several lines of evidence suggesting that H3K79me2 accumulation along Imp is associated with male-specific alternative RNA processing in Imp mRNA production, resulting from increased RNAP II processivity. To our knowledge, this is the first report to associate histone modification with the regulation of sex-specific alternative splicing.
2. Results and Discussion
2.1. Results
2.1.1. Knockdown of DOT1L Abolished Male-Specific Expression of the Imp mRNA
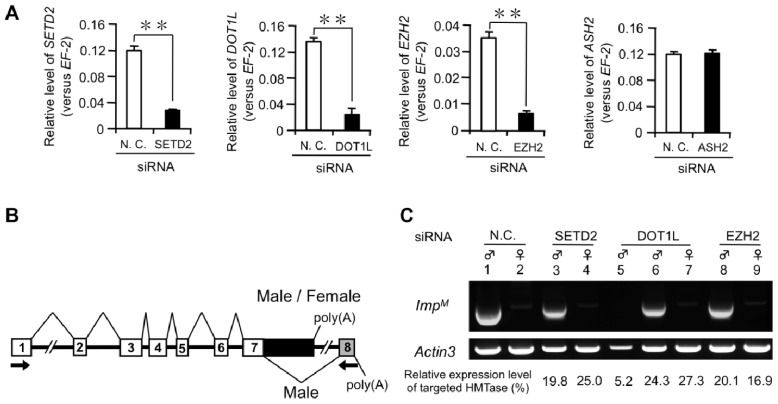
Recent genome-wide ChIP-seq analyses revealed that alternatively spliced exons are preferentially marked with H3K4me1, H3K27me3, and H3K79me2 [20]. Furthermore, a genome-wide study across different species revealed that H3K36me3 was depleted in skipped exons [13,16]. To investigate whether these epigenetic marks are associated with male-specific splicing of Imp pre-mRNA, we performed RNAi knockdown of several histone methyl transferases (HMTases) such as ASH2, EZH2, SETD2, and DOT1L known to modify H3K4, H3K27, H3K36, and H3K79, respectively, in embryos. Microinjection of dsRNA into B. mori embryos has been used successfully in many studies, although silencing levels vary [29]. siRNAs were injected into eggs during the early embryonic stage 6–8 h after oviposition, a developmental period known to be sensitive to RNAi-mediated gene knockdown [30]. Total RNA was extracted from each egg 4 days after injection. As shown in Figure 1A, qRT-PCR confirmed a significant reduction in EZH2, SETD2, and DOT1L transcript levels in embryos injected with siRNAs targeting these HMTase-coding genes. Injection of ASH2 siRNA failed to reduce the level of the target gene mRNA even though we used several siRNA sequences. Therefore, we focused on the knockdown effects of SETD2, DOT1L, and EZH2 on the expression of the male-specific Imp mRNA (ImpM). RNAi knockdown of SETD2 and EZH2 had no influence on the expression of ImpM (Figure 1C, lanes 3, 4, 8, and 9). Notably, the expression of ImpM was completely abolished when the DOT1L expression level was repressed to <10% of that in control males (Figure 1C, lane 5). Five of six examined individuals whose DOT1L level was less than 10% also showed the disappearance of male-specific Imp expression. Further study is required to determine whether a similar effect on the expression of ImpM occurs when the expression levels of SETD2 and EZH2 are repressed <10% of that in control males.
Figure 1.
The effect of histone methyltransferase (HMTase) knockdown on sex-specific splicing of Imp. (A) Quantification of HMTase mRNA expression 4 days after siRNA injections using quantitative reverse transcription (qRT)-PCR. Elongation factor 2 (EF-2) served as an internal standard. Error bar: SD; n = 8–24 individuals. ** p < 0.01, Student’s t-test; (B) A schematic diagram of alternative splicing in Imp pre-mRNA. Exons are numbered and displayed as boxes. The gray box indicates the male-specific exon. The V-shaped lines above and below the diagram denote the splice variants observed in males and females. Imp contains two poly(A) sites. The proximal promoter site located within intron 7 is utilized in a non-sex-specific manner. The distal promoter site is selected in a male-specific manner and exists near the end of exon 8. The arrows indicate the approximate location of primers used for RT-PCR in C; (C) The male-specific Imp mRNA (ImpM) was detected by RT-PCR and analyzed in a 1% agarose gel. The upper panel depicts expression of ImpM, and the lower panel shows the amplification of the Actin3 transcript, which served as a positive control for RNA extraction and RT-PCR. Sex identification of each egg was performed by PCR amplification of the W-specific random amplified polymorphic DNA (RAPD) marker Rikishi. The expression levels of targeted HMTases relative to the negative control embryos in each individual examined are indicated below each lane.
2.1.2. DOT1L Knockdown Affects Male-Specific Splicing of Imp Pre-mRNA
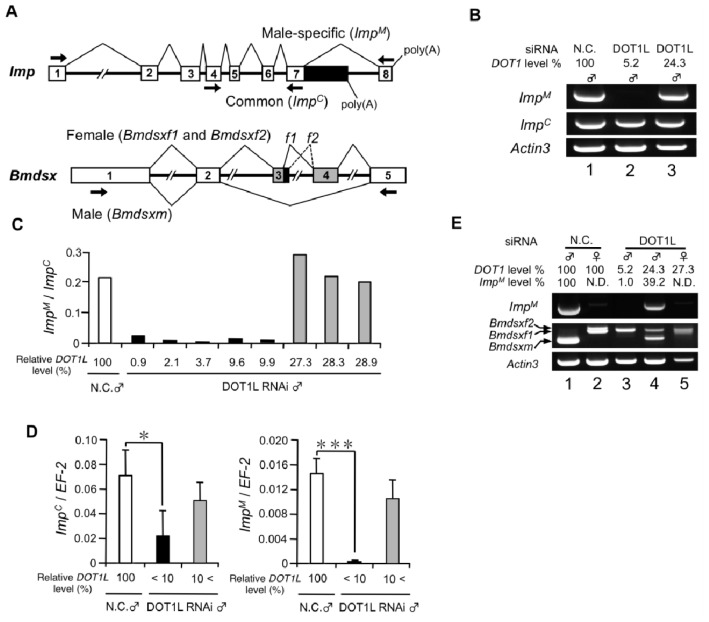
The above results indicate that DOT1L knockdown led to the loss of male-specific Imp expression in males. Two possible explanations may account for this phenomenon: DOT1L knockdown repressed Imp transcription or downregulation of DOT1L inhibited the splicing between exons 7 and 8 in Imp pre-mRNA. To examine these possibilities, we performed comparative analyses of ImpM and the Imp transcript common to both sexes (ImpC). RT-PCR analyses using primers described in Figure 2A demonstrated that DOT1L knockdown had little or no effect on the ImpC mRNA expression (Figure 2B). In contrast, ImpM transcript was not detected in a male with the DOT1L expressed to <10% of that in a control male (Figure 2B, lane 2). Next, using qRT-PCR, ImpM mRNA expression was compared with that of the ImpC mRNA. As shown in Figure 2C, extremely low expression of ImpM mRNAs were observed in all males with DOT1L levels <10% of those in control males relative to ImpC mRNA. These results indicate that DOT1L knockdown exclusively decreased the expression of the ImpM transcript. To rule out the possibility that down-regulation of DOT1L by RNAi affects Imp RNA transcription, qRT-PCR was performed to measure the ImpC mRNA level that represents total Imp mRNA expression. As shown in Figure 2D, the ImpC mRNA level in DOT1L knockdown males with DOT1L expression <10% of that in control males was decreased to a level less than one-third of that in control males (Figure 2D, left panel). In contrast, a more prominent reduction in ImpM mRNA was observed in the same DOT1L knockdown males (Figure 2D, right panel). These results indicate that DOT1L knockdown affects Imp RNA transcription but the male-specific splicing of Imp is repressed more severely by DOT1 depletion, leading to the loss of the ImpM expression. These results highlight the link between DOT1L and male-specific alternative splicing of Imp pre-mRNA. To investigate whether the decreased level of the ImpM mRNA caused by DOT1L depletion affects sex-specific splicing of Bmdsx, RT-PCR analysis was performed using primers designed to allow discrimination between female- and male-specific Bmdsx transcripts (Figure 2A, lower panel). As shown in Figure 2E, decreased expression of ImpM induced the expression of the female-specific Bmdsx (Bmdsxf1 and Bmdsxf2) mRNAs in addition to the male-specific Bmdsx (BmdsxM) mRNA (Figure 2E, lane 4). Only Bmdsxf1 expression was observed males whose ImpM expression was severely diminished by DOT1L depletion (Figure 2E, lane 3). These results were consistent with our previous data showing that downregulation of ImpM by RNAi in male cells increases female-specific splicing of Bmdsx [27]. Sometimes a doublet band was seen in the DOT1L-knockdown females (Figure 2E, lane 5). The upper band corresponded to Bmdsxf1 and the lower band was expected to be a splice variant that lacks the third exon. At present we do not know exactly the reason for the appearance of the splice variant that lacks exon 3 in the DOT1L-knockdown female.
Figure 2.
Effect of DOT1L knockdown on male-specific splicing of Imp. (A) The upper panel shows the location of the reverse transcription (RT)-PCR primers used for detection of Imp mRNA. The arrows above the diagram indicate the primers that were used for amplification of ImpM, and the arrows below the diagram show the primers used to amplify Imp mRNA transcribed from a region common to both sexes (ImpC). The lower panel indicates a schematic diagram of alternative splicing in the Bmdsx pre-mRNA. The gray boxes indicate the female-specific exons. The arrows point to the approximate locations of the primers used for RT-PCR in E; (B) Expression of ImpM and ImpC was detected by RT-PCR with primers illustrated in A and analyzed in a 1% agarose gel. The cDNA samples examined in lanes 1, 2, and 3 were identical to those used in lanes 1, 5, and 6, respectively, in Figure 1C. The bottom panel shows amplification of the Actin3 transcript, which served as a positive control for RNA extraction and RT-PCR. The expression of DOT1L relative to the negative control embryos in each individual is indicated above each lane; (C) The ratio of ImpM to ImpC was analyzed by qRT-PCR; (D) Quantification of ImpC (left panel) or ImpM mRNA expression (right panel) by qRT-PCR. The elongation factor 2 (EF-2) served as an internal standard. SD; n = 5 individuals. * p < 0.05, *** p < 0.001, Student’s t-test; (E) Female- or male-specific splicing of Bmdsx pre-mRNA was detected by RT-PCR and analyzed on a 1% agarose gel. The upper panel shows expression of ImpM, and the middle panel indicates the female- and male-specific splicing products of Bmdsx (Bmdsxf1, Bmdsxf2 and Bmdsxm, respectively). The lower panel shows amplification of the Actin3 transcript, which served as a positive control for RNA extraction and RT-PCR. The expression levels of DOT1L and ImpM relative to those of the negative control embryos in each individual are indicated above each lane.
2.1.3. High Levels of H3K79me2 Favor Inclusion of Male-Specific Exon of Imp
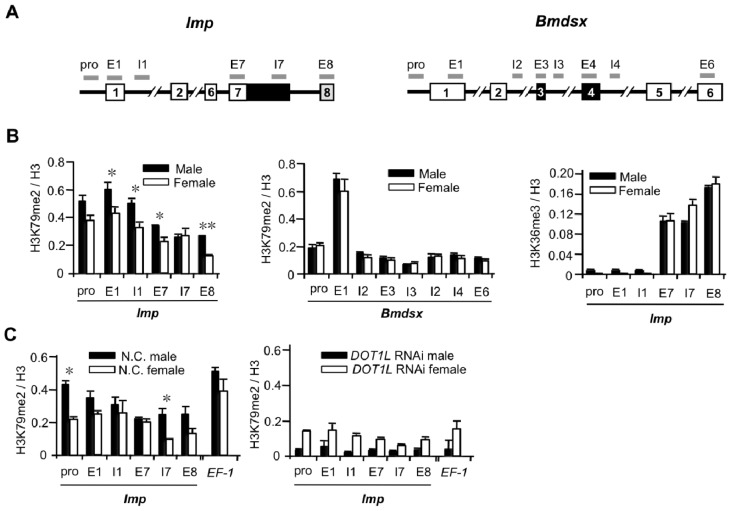
The above results support the possibility that H3K79 methylation marks are directly or indirectly associated with the regulation of the male-specific splicing of Imp pre-mRNA. We next performed comparative mapping of H3K79me2 across the alternatively spliced regions of Imp in females and males by ChIP-qPCR. As shown in Figure 3B, higher distributions of H3K79me2 were observed in males than in females across Imp (Figure 3B, left panel). Although the H3K79me2 enrichments were not limited to the alternatively spliced exon 8, most of the significant differences between males and females were observed in exon 8. In contrast, no significant differences in the levels of H3K79me2 over Bmdsx were observed (Figure 3B, middle panel). To investigate whether the distribution of the other histone marks across Imp differed between males and females, we performed comparative mapping of H3K36me3 across Imp in females and males by ChIP-qPCR. As a result, no significant difference between males and females was observed in the levels of H3K36me3 over Imp (Figure 3B, right panel). To examine whether siRNA-mediated knockdown of DOT1L reduces the level of H3K79me2 accumulation, DOT1L siRNA-injected embryos were subjected to ChIP-qPCR analyses. Although higher distributions of H3K79me2 were observed in males than in females across Imp in negative control individuals (Figure 3C, left panel), DOT1L knockdown exerted a marked influence on the accumulation of H3K79me2, with an at least 10-fold reduction throughout the regions examined in males (Figure 3C, right panel). Western blotting analysis demonstrated that DOT1L siRNA-injection efficiently reduced the total H3K79me2 level (Figure 4C). Since Dot1 in yeast and its homologs appear to be solely responsible for H3K79 methylation [31], reduction in the accumulation of H3K79me2 across Imp by DOT1L depletion could be attributed to a reduction in the amount of DOT1L interacting with Imp. These results provide overwhelming support for the specificity of the ChIP data. However, reduced accumulation of H3K79me2 in females was less than that in males. This difference in the extent of reduction in H3K79me2 between males and females could be attributed to differences in DOT1L-knockdown efficiencies between males and females, as shown in Figure 1C and Figure 2E. Mean DOT1L expression relative to control individuals was 27.6% in DOT1L knockdown females and 15.1% in DOT1L-knockdown males. At present we cannot explain the sexual difference in knockdown efficiencies. Although loss of H3K79me2 may affect indirectly the distribution of H3K4me1, H3K27me3, or H3K36me3, such indirect effect seems unlikely to affect the male-specific splicing of Imp since RNAi knockdown of ASH2, EZH2, and SETD2 had no influence on the expression pattern of Imp mRNA (Figure 1C). Overall we conclude that methylation of histone H3 across Imp involves the HMTase DOT1L and high levels of H3K79me2 favor inclusion of exon 8.
Figure 3.
Increased H3K79 methylation across Imp in males. (A) Schematic representation of Imp and Bmdsx genes showing the distribution of quantitative (q) PCR amplicons used in the analysis; (B) Mapping of H3K79me2 across Imp (left panel) and Bmdsx (middle panel) and of H3K36me3 across Imp (right panel) in female (black) and male (white) larval tissues by chromatin immunoprecipitation (ChIP)-qPCR. Values represent the means ± SE of six qPCR values from one representative of five independent experiments. * p < 0.05, ** p < 0.01, Student’s t-test; (C) ChIP assays with antibodies to H3K79me2 and H3 and chromatin prepared from 60-pooled negative control embryos of each sex (left panel) or 60-pooled DOT1L siRNA-injected embryos of each sex (right panel). The relative enrichment of H3K79me2 on EF-2 exon2 or along Imp was quantified by qPCR using primer sets indicated in A and expressed as a fraction of histone H3. Values represent the means ± SE of two independent qPCR assays from one representative of two independent experiments. * p < 0.05, Student’s t-test. The percentage of input was normalized to unmodified H3.
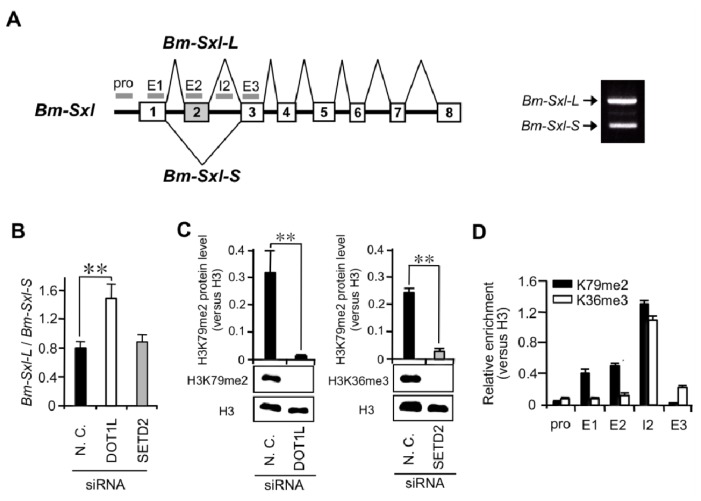
Figure 4.
Effect of DOT1L knockdown on alternative splicing of Bm-Sxl and distribution of H3K79me2 around the alternatively spliced exon in Bm-Sxl. (A) Schematic representation of Bm-Sxl showing the distribution of qPCR amplicons used in the analysis; (B) The ratio of Bm-Sxl-L to Bm-Sxl-S was analyzed by qRT-PCR. SD; n = 5 individuals. ** p < 0.01, Student’s t-test; (C) Western blotting analysis of H3K79me2 protein extracted from negative control or DOT1L siRNA-injected embryos using anti-H3K79me2 and anti-H3 antibodies (lower left panel). Quantification of H3K79me2 protein levels, as detected by Western blotting analysis (upper left panel). The intensity of each band was measured using Bioimage Analyser LAS1000. H3K79me2 protein level was normalized to the H3 protein level. Values represent the means ± SE of six bands from one representative of two independent experiments. ** p < 0.01, Student’s t-test. The same analysis was performed on H3K36me3 protein extracted from negative control or SETD2 siRNA-injected embryos using anti-H3K79me2 and anti-H3 antibodies (upper and lower right panels); (D) Mapping of H3K79me2 (black) and H3K36me3 (white) around Bm-Sxl exon 2 in larval tissues by ChIP-qPCR. Values represent the means ± SE of six qPCR values from one representative of five independent experiments.
To verify the link between the high distributions of H3K79me2 and inclusion of alternative exons in genes other than Imp, we examined the effects of DOT1L-knockdown on a Bombyx homolog of Sex-lethal gene (Bm-Sxl). The primary transcript of the Bm-Sxl gene is alternatively spliced to yield two isoforms, Bm-Sxl-L and Bm-Sxl-S [22]. Bm-Sxl-L consists of eight exons, while Bm-Sxl-S is a splice variant that lacks the second exon (Figure 4A). qRT-PCR analysis demonstrated that Bm-Sxl-L/Bm-Sxl-S ratio was more than twofold higher in DOT1L-knockdown embryos than in negative control embryos (Figure 4B). This result indicated that the DOT1L-knockdown relatively increased the inclusion of alternatively spliced exon (exon 2). In contrast, SETD2-knockdown caused no influence on the Bm-Sxl-L/Bm-Sxl-S ratio. Western blotting analysis demonstrated that DOT1L siRNA-injection and SETD2 siRNA-injection efficiently reduced the total H3K79me2 level and total H3K36me3 level, respectively (Figure 4C). To investigate whether the high distributions of H3K79me2 is correlated with DOT1L-dependent inclusion of Bm-Sxl exon 2, we performed mapping of H3K79me2 around the exon 2 by ChIP-qPCR. The H3K79me2 mark was specifically enriched on the regions around exon 2 as compared with the H3K36me3 mark (Figure 4D). These results suggest that enrichment of H3K79me2 could be correlated with DOT1L-dependent inclusion of alternative exons.
2.1.4. Male and Female Differences in RNAP II Processivity in the Imp Gene
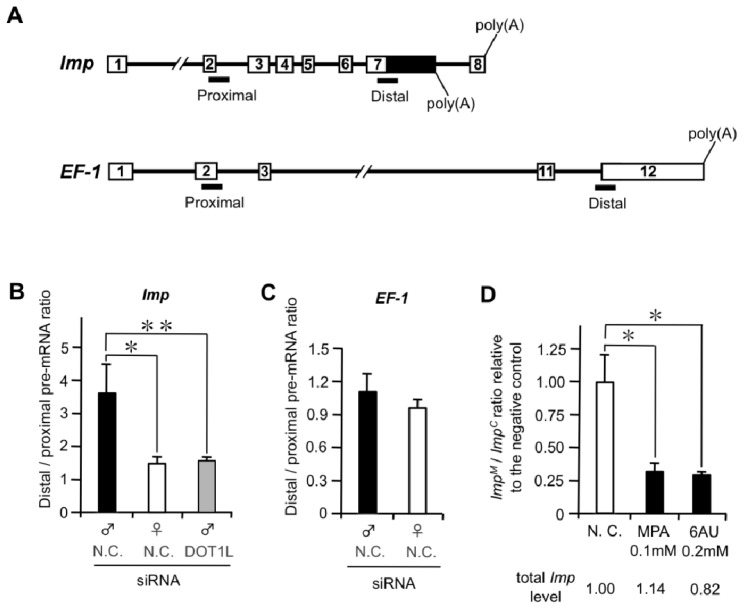
Our data suggest a link between male-specific alternative splicing of Imp pre-mRNA and higher accumulation of H3K79me2. H3K79me2 modification is tightly associated with active transcription [32–34]. Moreover, several observations have suggested a close relationship between RNAP II dynamics and alternative splicing [35,36]. Based on these observations, we analyzed whether RNAP II processivity in Imp differs between males and females. To this end, we utilized an observation previously identified by others that transcription by a slower mutant RNAP II results in an increase in the ratio between promoter-proximal and promoter-distal pre-mRNA [15,36]. qRT-PCR of Imp pre-mRNA with primer sets located at each end of Imp (Figure 5A) showed that the distal/proximal (D/P) pre-mRNA ratio was more than twofold higher in males than in females (Figure 5B). In contrast, no significant difference in the D/P ratio was observed in the control gene, B. mori elongation factor-1 (EF-1) (Figure 5C). These data suggest that a link between male-specific alternative splicing of Imp pre-mRNA and higher RNAP II processivity at this locus. As shown in Figure 5B, DOT1L knockdown reduced the D/P ratio in the male to a level similar to that in the normal female. Taken together with the ChIP-qPCR data indicated in Figure 3C, this result supports the possibility that higher distribution of H3K79me2 across Imp results in increased RNAP II processivity at this locus.
Figure 5.
Male and female differences in RNAP II processivity in Imp. (A) Schematic diagram of Imp and EF-1 showing the distribution of the proximal and distal amplicons (black bars) used for qPCR analysis; (B,C) RNAP II processivity was determined as a ratio of the proximal and distal pre-mRNA sequences (distal/proximal) of Imp (B) and EF-1 (C). The abundance of each pre-mRNA was determined by quantitative (q)PCR. Values represent the means ± SE of six qPCR values from three individuals; (D) Inhibitors of nucleotide biosynthesis suppress male-specific splicing of Imp pre-mRNA. qRT-PCR analysis was performed to calculate the ratio of ImpM to ImpC. The ImpM/ImpC ratio in male cells treated with 0.1 mM mycophenolic acid (MPA) or with 0.2 mM 6-azauracil (6AU) (C) is relative to that in the negative control cells in each experiment. Values represent the means ± SE from three individual experiments. * p < 0.05, ** p < 0.01, Student’s t-test. Values below each graph indicate total Imp mRNA expression in the cells examined relative to those in the negative control cells.
2.1.5. Suppression of Male-Specific Imp Pre-mRNA Splicing by Inhibitors of Nucleotide Biosynthesis
Sensitivity to MPA and the base analog 6AU are hallmarks of transcription elongation defects in yeast [37–39]. Both drugs cause the depletion of cellular nucleotide substrate pools required by RNA polymerases [40,41]. In vitro, RNAP II complexes pause and arrest more frequently under conditions of limiting amounts of nucleotides [42], suggesting that the in vivo hypersensitivity to these compounds is due to increased dependence on factors that promote elongation by RNAP II. Treatment with these drugs causes inhibition of exon skipping [36,43] because the extent of skipping of alternative exons correlates with the elongation rate of RNAP II [36]. Based on these reports, we investigated the effects of these inhibitors on male-specific splicing of Imp pre-mRNA. Male cultured cells (NIAS-Bm-M1) that were not growth-arrested were treated with these inhibitors at concentrations established previously [43]. The efficiency of male-specific splicing was estimated by the ratio of ImpM/ImpC mRNA. The ImpM/ImpC ratio in male cells treated with each drug relative to that in the negative control cells is indicated in Figure 5D. In the MPA experiment, an approximately threefold decrease in the ImpM/ImpC ratio was observed. A similarly high reduction in the ImpM/ImpC ratio was observed when the male cells were treated with 6AU. Importantly, no significant difference was detected in the level of total Imp mRNA between control cells and inhibitor-treated cells, indicating that the reduction in the ImpM/ImpC ratio represented simply the reduction in male-specific Imp mRNA expression. Taken together with measurements of RNAP II processivity in Figure 5B, the simplest explanation for these results is that drug-induced reduction in transcript elongation led to the inhibition of the male-specific splicing of Imp pre-mRNA.
2.1.6. Embryonic Lethality Caused by DOT1L Knockdown
Above results indicated that DOT1L knockdown caused decreased expression of ImpM, leading to the expression of the female-specific Bmdsx mRNA in male embryos (Figure 2E). To evaluate whether DOT1L does indeed play an important role in sexual differentiation, we investigated the effect of DOT1L knockdown on the development of sexual phenotypes. The highest hatch rate of the control siRNA-injected embryos in six trials was 21.7% (Table 1), which was still lower than that reported by another group [30]. Presumably, this difference was caused by technical issues related to microinjection. Similarly low hatchability in negative control dsRNA-injected eggs is reported by the other group [44]. Compared with the control embryos, nearly all the embryos injected with DOT1L siRNA did not hatch, suggesting embryonic lethality (Table 1). One hatched larva was obtained from a male egg injected with DOT1L siRNA that survived to the adult stage. This male had normal fertility and its genital organs showed no abnormalities when viewed under a dissecting microscope (data not shown).
Table 1.
Effects of DOTIL siRNA injection on egg development.
| siRNA | Injected eggs | Early or mid-stage embryonic lethal | Late-stage embryonic lethal | Viable |
|---|---|---|---|---|
| N. C. siRNA | 115 | 58 (50.4%) | 32 (27.8%) | 25 (21.7%) |
| DOT1L siRNA | 263 | 188 (71.5%) | 74 (28.1%) | 1 (0.4%) (male) |
2.2. Discussion
Imp in B. mori has been identified as a male-specific RNA-binding protein involved in the regulation of male-specific splicing of Bmdsx [27]. The pre-mRNA of Imp undergoes sex-specific RNA processing. In male cells, male-specific Imp mRNA contains exon 8 and the distal promoter poly(A) site choice, whereas non-sex-specific polyadenylation occurs at the proximal promoter poly(A) site downstream of exon 7. Here, we found that DOT1L knockdown affects male-specific splicing of Imp pre-mRNA, leading to loss of male-specific Imp expression (Figure 2B,C). In support of this result, higher distributions of H3K79me2 were observed in males than in females or in DOT1L knockdown males across Imp (Figure 3B,C). Strong link between enrichment of H3K79me2 and DOT1L-dependent inclusion of alternative exons was also observed in Bm-Sxl (Figure 4). Comparative analysis of RNAP II processivity indicated that higher distribution of H3K79me2 across Imp was correlated to increased RNAP II processivity at this locus (Figure 5B). Inhibition experiments using inhibitors of RNAP II elongation suggested that the higher elongation rate was tightly associated with male-specific RNA processing of Imp pre-mRNA (Figure 5D). Together, our data suggest that H3K79me2 accumulation along Imp is associated with male-specific alternative RNA processing in Imp mRNA production, resulting from increased RNAP II processivity.
The alternative RNA processing pattern of Imp pre-mRNA closely resembles that found in Drosophila polo pre-mRNA. Polo, which is a cell cycle gene, also contains a proximal and a distal poly(A) site in the 3′untranslated region (UTR) to produce alternative mRNA that differ in their 3′UTR length [45]. In a mutant Drosophila strain that displays a reduced RNAP II elongation rate, RNAP II occupancy along polo is altered and the proximal poly(A) site is used 3.5-fold more efficiently than in wild-type flies [46]. An increase in proximal poly(A) site usage was also observed in five other alternatively polyadenylated transcripts in Drosophila. These results in Drosophila show that the kinetics of RNAP II can determine alternative poly(A) site selection. As shown in Figure 5D, significant reduction in the ImpM/ImpC ratio was observed when the male cells were treated with inhibitors of RNAP II elongation. This result indicates that drug-induced repression of transcript elongation leads to the reduction in utilization of distal poly(A) site, resulting in the relative increase in proximal poly(A) site usage. Slow RNAP II presumably exposes the proximal poly(A) site on the nascent transcript to the polyadenylation machinery for a longer time before RNAP II transcribes the distal poly(A) site [46]. Therefore, the proximal poly(A) site is processed before the distal poly(A) site is transcribed, suggests a “first come, first served” mechanism. This resembles the extra domain I (EDI) alternative splicing mechanism described previously, whereby a slow RNAP II preferentially included the normally excluded alternative EDI exon because it allowed ample assembly time for the spliceosome machinery [36,47].
Eleven-nineteen lysine-rich leukemia gene (ELL) family proteins are essential components of the super elongation complex (SEC) and increase the catalytic rate of transcription elongation by RNA polymerase II [48–50]. ELL2 knockdown by siRNA affects the alternative pre-mRNA processing of the immunoglobulin heavy chain (IgH) gene, which is accompanied by reduced H3K4 and H3K79 methylation [50]. In addition to the ELLs, the SEC contains the MLL translocation partners AF4/FMR2 family member 1 (AFF1; also known as AF4), AFF4, eleven-nineteen leukemia (ENL) and ALL1-fused gene from chromosome 9 (AF9) [47]. In this complex, ENL is linked, not only with all members of the AF4 protein family that occur as MLL fusion partners, but also with pTEFb and DOT1L [51,52]. Notably, several frequent MLL fusion partners seem to coordinate DOT1L activity with a protein complex that stimulates the elongation phase of transcription by phosphorylating the carboxy-terminal repeat domain of RNA polymerase II [49,50].
H3K79me2 modification is tightly associated with active transcription [32,33]. Milcarek et al. speculated that conversion of monomethylated H3K79 into di- and trimethylated forms is correlated with the transition from low to high level gene transcription, due most likely to a decrease in the histone-DNA interaction [53]. Because H3K79 resides within the histone core, its methylation may facilitate DNA unwinding from the histone, allowing the downstream chromatin to open more readily and be transcribed more efficiently.
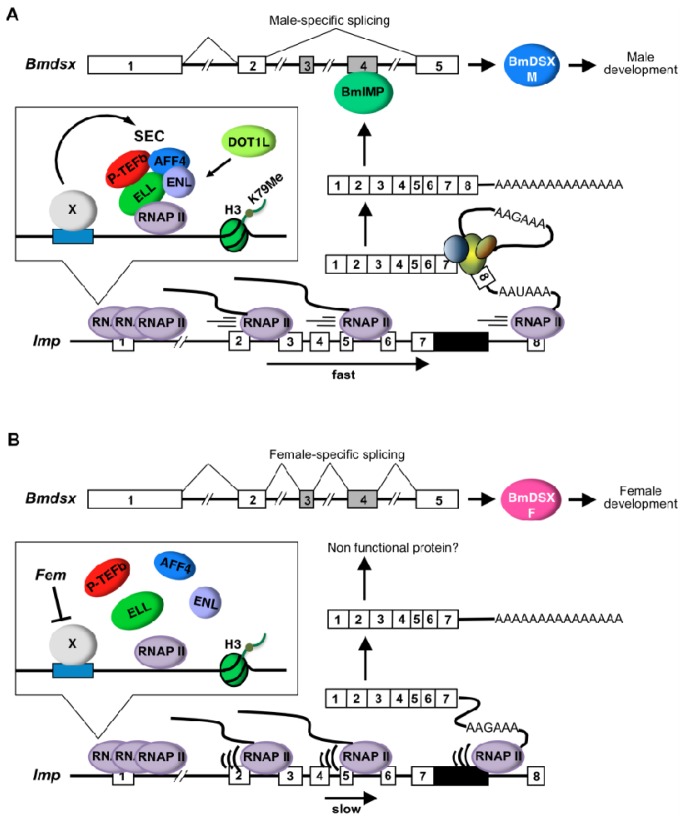
Based on these previous findings, we propose a possible model for the regulatory mechanism underlying sex-specific alternative splicing of Imp pre-mRNA (Figure 6). In male cells, exclusive expression of a transcription elongation factor (X), such as ELL family proteins, promotes the formation of SEC on Imp, causing H3K79 methylation by DOT1L. Higher RNAP II processivity due to H3K79me2 accumulation does not allow enough time to complete 3′-end processing at the proximal, non-sex-specific poly(A) site, leading to exclusive use of the 3′ splice site of exon 8. The male-specific Imp protein induces the male-specific splicing of Bmdsx pre-mRNA (Figure 6A). We have found recently that male-specific Imp bound immediately downstream of the proximal poly(A) site and promoted male-specific splicing of its pre-mRNA [28]. Therefore, after the male-specific Imp protein has been produced, the protein product may inhibit use of the proximal poly(A) site and promote the splicing of intron 7, leading to exclusive use of the 3′ splice site of exon 8. In female cells, Fem directly or indirectly represses the expression of X, leading to failure of SEC formation. Decreased accumulation of H3K79me2 caused by the loss of SEC slows RNAP II processivity, providing sufficient time to recruit cleavage factors such as CF I, CF II, and/or poly(A) polymerase (PAP) for 3′-end processing at the proximal poly(A) site. The absence of the male-specific Imp induces the female-specific splicing of Bmdsx pre-mRNA (Figure 6B).
Figure 6.
Model for the regulation of alternative splicing of Imp pre-mRNA by H3K79me2 and DOT1L. (A) In males, a transcription elongation factor (X) promotes the formation of SEC on Imp, causing H3K79 methylation by DOT1L. Higher RNAP II processivity due to H3K79me2 accumulation does not allow enough time to complete 3′-end processing at the proximal poly(A) site (AAGAAA), leading to exclusive use of the distal 3′ splice site of exon 8 and the distal poly(A) site (AAUAAA). While the distal poly(A) signal sequence is perfectly matched to the most canonical poly(A) signal hexamer, the proximal poly(A) signal sequence is consistent with a single-nucleotide variant of the canonical hexamer. Therefore, the polyadenylation machinery prefers the distal poly(A) site rather than the proximal poly(A) site when both poly(A) sites are present on the nascent transcript simultaneously. The protein product from the male-specific Imp transcript induces the male-specific splicing of Bmdsx pre-mRNA; (B) In females, the presence of a dominant feminizing factor, Fem, on the W chromosome directly or indirectly represses the expression of X, leading to failure of SEC formation. Decreased accumulation of H3K79me2 caused by the loss of SEC slows RNAP II processivity, providing sufficient time to recruit cleavage factors for 3′-end processing at the proximal poly(A) site. The absence of the male-specific Imp induces the female-specific splicing of Bmdsx pre-mRNA.
In this model, both the proximal and distal poly(A) sites are exposed to the polyadenylation factors at the same time in male cells. How then is the distal poly(A) site exclusively selected? While the distal poly(A) signal sequence is perfectly matched to the most canonical poly(A) signal hexamer AAUAAA, the proximal poly(A) signal sequence is AAGAAA, which is consistent with a single-nucleotide variant of the canonical hexamer. The motifs that are functional in vertebrates are AAUAAA and its highly conserved variants (e.g., AUUAAA, UAUAAA, AGUAAA, AAGAAA) [54]. Among those hexamers, the canonical AAUAAA was reported to be present in 53% of the mRNAs; in contrast, a single-nucleotide variant AAGAAA was found in only 3% of the mRNA [55]. Therefore, one can reasonably suppose that the polyadenylation machinery prefers the distal poly(A) site rather than the proximal poly(A) site when both poly(A) sites are present on the nascent transcript simultaneously. This scenario is consistent with the kinetic coupling model for the regulation of alternative splicing by RNAP II elongation [56]. In this model, when a proximal suboptimal (weak) 3′ splice site and a downstream canonical (strong) 3′ splice site are presented simultaneously to the splicing machinery, the strong 3′ splice site could easily outcompete the weak site, resulting in alternative exon skipping. Our results indicate that both alternative polyadenylation and alternative splicing depend on RNAP II kinetics. In the present study, we were unable to assess precisely whether DOT1L plays a crucial role in regulating sex determination or sexual differentiation of B. mori because nearly all the embryos injected with DOT1L siRNA died before hatching. Consistent with our results, Dot1L-deficient embryos died between 9.5–10.5 days post coitum due to developmental abnormalities, including growth impairment, angiogenesis defects in the yolk sac, and cardiac dilation [57]. In D. melanogaster, grappa (gpp) is an ortholog of Dot1L [58]. gpp is an essential gene identified in a genetic screen for dominant suppressors of pairing-dependent silencing where a Polycomb-group (Pc-G)-mediated silencing mechanism necessary for the maintenance of parasegment identity during embryo development [59]. As is the case in D. melanogaster, DOT1L in B. mori may be required to maintain developmental gene expression through Pc-G-mediated mechanism.
Assuming that our model presented above is valid, an ELL family protein—such as ELL2—might be a key factor in facilitating male differentiation as a result of inducing the male-specific splicing of Imp pre-mRNA. Recently, we found that an ELL2 homolog is expressed in embryos in early developmental stages. Ongoing investigations are aimed at determining whether the ELL2 homolog is involved in regulating the sex-specific splicing of Imp pre-mRNAs.
3. Experimental Section
3.1. Silkworm Strains
The Bombyx mori non-diapausal and white egg strain (pnd-w1) was kindly provided by Kenichi Moto of RIKEN (Wako, Osaka, Japan). The S-2 strain, in which the females have the T (W; 2, 5) pB + re (black egg, black larvae) genotype and the males have the + pB, re (red egg, white larvae) genotype, was established in our laboratory. The former strain was used primarily for RNAi experiments and the latter for ChIP-quantitative PCR (qPCR) and gene expression analysis. The developing eggs were enclosed in a plastic case and incubated at 25 ± 2 °C with sufficient humidity. Larvae were reared on an artificial diet (Nihon Nosan, Yokohama, Japan) at 25 ± 2 °C.
3.2. Preparation of siRNAs
cDNA sequences predicted to encode ASH2, EZH2, SETD2, and DOT1L were retrieved by tBLASTn searches of the KAIKObase (http://sgp.dna.affrc.go.jp/KAIKObase/) using human ASH2, EZH2, SETD2, and DOT1L as query sequences (Figure 7). siRNA targeted to four HMTases (ASH2, EZH2, SETD2, DOT1L) were designed as described previously [60]. All sequences used in RNAi experiments are listed in Table 2. Each siRNA was synthesized using the custom select siRNA synthesis service provided by Ambion (Austin, TX, USA). Silencer negative control #1 siRNA (Ambion, Austin, TX, USA) was used as a negative control in the siRNA experiments.
Figure 7.
Predicted amino acid sequences of Bombyx mori ASH2, EZH2, SETD2, and DOT1L. (A) Alignment of Bombyx mori ASH2 (KAIKObase China Gene Model Gene No. BGIBMGA008025), Drosophila melanogaster ASH2, and human ASH2. The putative SPRY domain is denoted by horizontal line above the amino acid alignment; (B) Alignment of Bombyx mori EZH (KAIKObase EST clone ID: FS904534); (C) Drosophila melanogaster EZH, and human EZH; (C) Alignment of Bombyx mori SETD2 (KAIKObase China Gene Model Gene No. BGIBMGA003106), Drosophila melanogaster SETD2, and human SETD2. The putative AWS and SET domains are denoted by horizontal lines above the amino acid alignment; (D) Alignment of Bombyx mori DOT1L (KAIKObase FLcDNA clone name: ffbm34A09), Drosophila melanogaster Gpp (DOT1L ortholog), and human DOT1L. The putative DOT1 domain is denoted by horizontal line above the amino acid alignment. Amino acid identity is denoted by black boxes.
Table 2.
siRNA sequences used in RNAi experiments.
| Target gene | siRNA | Sense | Antisense |
|---|---|---|---|
| SETD2 | 393 | UGCCAGCUCUGAGUCUGAUUCAAU | AUUGAAUCAGACUCAGAGCUGGCA |
| 490 | CAGUGUAGCUCAAGAGAUATT | UAUCUCUUGAGCUACACUGTT | |
| DOT1L | 243 | UUCCAAAGCAACUACAGAAUCGAUG | CAUCGAUUCUGUAGUUGCUUUGGAA |
| 353 | AUUUACUCGCUUUACUUUGTT | CAAAGUAAAGCGAGUAAAUTT | |
| EZH2 | 38 | GACAACCCAACAGGUACCAAUAAGA | UCUUAUUGGUACCUGUUGGGUUGUC |
| 224 | CGACGGGAAAGUGCAUGGUGAUAAA | UUUAUCACCAUGCACUUUCCCGUCG | |
| ASH2 | 157 | GACCGGCCUCUAGUCAAAUUCAAGA | UCUUGAAUUUGACUAGAGGCCGUC |
| 167 | UAGUCAAAUUCAAGAGCCACCUGUA | UACAGGUGGCUCUUGAAUUUGACUA |
3.3. Injection of siRNAs into Eggs
siRNAs were injected into eggs as described previously [60]. Negative control siRNA, EZH2 siRNA, and ASH2 siRNA were injected at 50 μM, and SETD2 siRNA and DOT1L siRNA were injected at 50 or 100 μM.
3.4. Extraction of Total RNA and Genomic DNA
Total RNA was extracted from each egg and from NIAS-Bm-M1 cells using Isogen (Nippon Gene, Tokyo, Japan) according to the protocol described previously [60]. Genomic DNA was recovered by ethanol precipitation from the intermediate and organic phases obtained in the RNA extraction process. The precipitated DNA was purified using the SimplePrep® reagent for DNA (Takara, Kyoto, Japan) according to the manufacturer’s instructions. To perform molecular sexing of each egg, PCR was performed using primers specific to the W chromosome genomic sequence according to the protocol described previously [60].
3.5. Reverse Transcription (RT)-PCR Analyses
RT-PCR reactions performed according to the protocol described previously [60]. The primer sequences and PCR conditions utilized in this study are indicated in Supplementary Table 3.
Table 3.
Primer sequences and PCR conditions utilized in this study.
| Target gene | Primers | Sequence (5′ to 3′) | Denature | Annealing | Elongation | N° cycles |
|---|---|---|---|---|---|---|
| Bmdsx | FDSX-F2 | CGCCTTACCGCAGACAGGCAG | 98 °C | 57 °C | 57 °C | 35 |
| FDSX-R4 | GCGCAGTGTCGTCGCTACAAGG | 10 s | 30 s | 60 s | ||
| ImpM | BmIMPF1 | ATGGACGGTGACATGTCTCAAG | 98 °C | 55 °C | 57 °C | 35 |
| BmIMPR1 | TCATCCCGCCTCAGACGATTG | 10 s | 30 s | 90 s | ||
| ImpC | IMPE4F1 | TCCCATAATAATCTCATTGGAC | 98 °C | 55 °C | 57 °C | 35 |
| IMPE7R1 | AATGTGAACGGTGGTCTCGTG | 10 s | 30 s | 90 s | ||
| Actin3 | BA3F1 | AGATGACCCAGATCATGTTCG | 98 °C | 57 °C | 57 °C | 26 |
| BASR1 | GAGATCCACATCTGTTGGAAG | 10 s | 30 s | 30 s | ||
| m | BmSxlF1 | ATTAATCATCATAAAGCTACG | 98 °C | 57 °C | 57 °C | 35 |
| BmSxlR1 | AATCCGTAACTGTAGCCAGTC | 10 s | 30 s | 30 s |
3.6. Quantitative Real-Time RT-PCR (qRT-PCR)
qRT-PCR assays were performed according to the protocol described previously [60]. All primer sequences used in this study are listed in Table 4. The BmEF-2F1 and BmEF-2R1 primers were used to amplify elongation factor-2 (EF-2) as an internal standard for quantification [61].
Table 4.
Sequences of primers used for qRT-PCR.
| Target gene | Primers | Sequence (5′ to 3′) |
|---|---|---|
| SETD2 | SETD2qPCRF1 | CCTACAGGACATCTGGAGTTAC |
| SETD2qPCRR1 | GAATCAGTACCAGCATTTAGATG | |
| DOT1L | dot1qPCRF1 | AGAATCCGAACGACTCGACAG |
| dot1qPCRR1 | CTGTTCTTGGTCTTCGTTCAAC | |
| EZH2 | EZH2F1 | GGTGTAGTGACAACCCAACAG |
| EZH2R1 | TCTTAACTCCTGAGCTGTTCC | |
| ASH2 | ASHF2 | GGGGACCAGGTTCCACGAGTC |
| ASHR2 | TACAGGTGGCTCTTGAATTTG | |
| Bmdsx promoter | BmdsxproF1 | TGCATGTTTCTTATTAATCAGCTAG |
| BmdsxproR1 | GTAAATTTCGTAAAAGCTGACCAG | |
| Bmdsx E1 | ChIPEx1F | CCTGTACCACCAGTGGTGAAG |
| ChIPEx1R | CTGACGGCGGTGGAGCGTATG | |
| Bmdsx I2 | ChIPI2F | CTACATGAACAGTACCAGTCAG |
| ChIPI2R | GTAAGTACAAACTAAATAGCGTTC | |
| Bmdsx E3 | ChIPEx3F | GTCGACGAGTACGCGAGGAAG |
| ChIPEx3R | TGTGATGCATGTATCTGTCGC | |
| Bmdsx I3 | ChIPI3F | GTAACTGACCTTCTTGCTAATC |
| ChIPI3R | CTGTGCCATTTTATTAATATCGTC | |
| Bmdsx E4 | ChIPEx4F | ATATAAGTGGTGTACTGTCTTC |
| ChIPEx4R | CCATAGATCCAATGTTACGAC | |
| Bmdsx I4 | ChIPI4F | GTTCAAACACATCGAAGCTAC |
| ChIPI4R | GTCCGAGATAGACTGGCCTTG | |
| Bmdsx E6 | ChIPEx6F | GGCACAGCGCCGACAAGTAAG |
| ChIPEx6R | ATTGTCTGTAGATATTCGTGATC | |
| BmIMP promoter1S | IMPproF2 | TTAAGCATTTAATTATAAGAAGATC |
| IMPproR2 | CTAGAATCTGCGATTACATAC | |
| BmIMP E1S | IMPE1F1 | TCCGTTCAGTACTCGCTATAC |
| IMPE1R1 | TCTTACCTATCGTCATAGATTC | |
| BmIMP I1S | IMPI1F2 | ATTTGGTAAAATAGTCTCGTATC |
| IMPI1R2 | ACCTTGTGATACGGGGTTAAC | |
| BmIMP pro1L | IMPE1LproF1 | GCTGCCCCACCCTTTAAACCG |
| IMPE1LproR1 | CTCGATCGTGCTGACTCTAGC | |
| BmIMP E1L | IMPE1F1 | TTTCAAGTATACTCCTTCTATAG |
| IMPE1R1 | TTCGCCATTTTGAGCAGATTG | |
| BmIMP I1L | IMPI1F3 | CAAATGGGCACATATTGTTGG |
| IMPI1R3 | GTTTAAGCGCTTTCGTGATGG | |
| BmIMP E7 | IMPE7F1 | ATGCGGGAAGAAGGTTTTATG |
| IMPE7R1 | AATGTGAACGGTGGTCTCGTG | |
| BmIMP I7 | IMPI7F1 | GTGCATAAATCCACAGAACAG |
| IMPI7R1 | TTACTCAGAAACTCAGAAGTAC | |
| BmIMP E8 | IMPE8F1 | CGTCTGAGGCGGGATGAGAAC |
| IMPE8R1 | TAAATTCGCCGCAATCAGCAG | |
| EF1 promoter | EF-1proF1 | TATATCAATTTTGGTGCAAGAATGG |
| EF-1proR1 | GTAATAATATTCTATTCTATCCACCG | |
| EF-1 E2 | EF-1E2F1 | TGGCGATGGAGGCGGAGAAG |
| EF-1E2R1 | CTCAACTTCCCAGCTGTCTGC | |
| EF-1 I3 | EF-1I3F1 | ACTTACTTATTTATGATCATGCGTC |
| EF-1I3R1 | GCTAACCACAATTATATTTGTGGAG | |
| EF-1 E6 | EF-1E6F1 | TACAGGTCATTTCTGCACGTAAG |
| EF-1E6R1 | TCATCCCAGTTAACTGTTGGATC | |
| EF-1 I11 | EF-1I11F1 | TTCATGGACTACATTTTACCTTGG |
| EF-1I11R1 | CTAAGCTCTTCTAAAAGAGATGAGC | |
| EF-1 I12 | EF-1I12F1 | GCATTAATATTAATTCCACCACAAG |
| EF-1I12R1 | CACACCTCACTGCTCTTCCGC | |
| Bm-Sxl promoter | Sxli1F1 | GGCTAAACTATCTTCAACAAG |
| Sxli1R1 | CGGTCACCGTTCTCGTGAAAG | |
| Bm-Sxl E1 | Sxle1F1 | GCCAGTCCAAATGGACGAATC |
| Sxle1R1 | GTTCACTGACTTTCGAGTGAG | |
| Bm-Sxl E2 | Sxle2F1 | GCCTACTCGAACAATAAAAAAG |
| Sxle2R1 | TTCCAAAGAATTGAAACTCCTG | |
| Bm-Sxl I2 | Sxli2F1 | TGAATCAGAACATCTCATTTGG |
| Sxli2R1 | CCAAGCCGCTGCCTACCTAAC | |
| Bm-Sxl E3 | Sxle3F1 | CGAGGCAGAGCGGGTTCGAAC |
| Sxle3R1 | CCTTCATCACTCGACAGCTCTC | |
| BmIMP Proximal | IMPE2F1 | ATCCTCAAAGGTACTCATCAG |
| IMPE2I2R | GCATGCATCACTCAACAATAC | |
| BmIMP Distal | IMPE7F2 | GGATCATCGGCAAAGGCGGAC |
| IMPI7R2 | AGCACTTGGATCATTCATACC | |
| EF-1 Proximal | EF-1E2F1 | TGGCGATGGAGGCGGAGAAGG |
| EF-1E2I2R | GAAAAAGAAGAAAGCATTCATGC | |
| EF-1 Distal | EF-1I11E12F | ATGATACTGTATTAACTGCATTC |
| EF-1E12R2 | TTCAGGATTTTGAGACCCTGG | |
| BmIMP E7-E8 | IMPE7F1 | ATGCGGGAAGAAGGTTTTATG |
| BmIMPR1 | TCATCCCGCCTCAGACGATTG | |
| BmIMP E1-E3 | BmIMPF1 | ATGGACGGTGACATGTCTCAAG |
| IMPE3R1 | CATCCATTCAACCCGTTTATG | |
| BmIMP E1S | BmIMPF1 | ATGGACGGTGACATGTCTCAAG |
| IMPE2R2 | GCCTGCTCTGGACTCTCGAAG | |
| BmIMP E1L | IMPE1LF1 | AATCTGCTCAAAATGGCGAAG |
| IMPE2R2 | GCCTGCTCTGGACTCTCGAAG | |
| Bm-Sxl S | BmSxlF2 | ACTCGCGTTACCTATTTAAC |
| BmSxlSR1 | GTACTGCTGTTGGATTTGGTC | |
| Bm-Sxl L | BmSxlF2 | ACTCGCGTTACCTATTTAAC |
| BmSxlLR1 | CTGCTGTTGGATTTGATTTTC |
3.7. ChIP Experiments
The fifth instar day-3 larvae fat bodies were treated with 1× cold phosphate-buffered saline (PBS) containing 1% protease inhibitor cocktail (Roche, Basel, Switzerland). To cross-link samples, a formaldehyde solution (50 mM HEPES-KOH [pH 7.5], 100 mM NaCl, 1 mM EDTA [pH 8.0], 0.5 mM EGTA [pH 8.0], 11% formaldehyde) was added to a final concentration of 1% for 15 min at room temperature. Eggs were mashed with a pestle in the formaldehyde solution to prepare samples for ChIP experiments. The reaction was stopped by adding a glycine solution to a final concentration of 125 mM for 5 min at room temperature and washed twice with 1× cold PBS. The fixed fat bodies were disrupted with a Polytron (KINEMATICA, Lucern, Switzerland) (10,000 rpm, 20 s) and centrifuged at 2800 rpm for 5 min at 4 °C. The samples were resuspended in lysis buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA [pH 8.0], 0.5 mM EGTA [pH 8.0], 0.1% Na-deoxycholate, 0.5% N-lauroylsarcosine, 1% protease inhibitor) and sonicated (pulsed 30 s, paused 1 min × 7 sets) on ice to avoid overheating. Triton-X was added to the resulting lysate at a final concentration of 1% and centrifuged at 15,000 rpm for 10 min at 4 °C. An aliquot (200 μL) of each sonicated sample served as an input DNA control. For ChIP, 300 μL of the supernatant was incubated with Dynabeads Protein G (Invitrogen, Carlsbad, CA, USA) with the appropriate antibody overnight at 4 °C. The beads were washed twice with 1× cold PBS. Next, 5 μg of antibodies were added in 300-μL blocking solution (0.5% bovine serum albumin [BSA]/PBS) and incubated overnight at 4 °C. The antibodies used in this study were anti-H3 antibody (ab1791, Abcam, Cambridge, UK), anti-H3K36me3 antibody (ab9050, Abcam, Cambridge, UK), anti-H3K79me2 antibody (ab3594, Abcam, Cambridge, UK), and anti-rabbit IgG antibody (12–370, Millipore, Billerica, MA, USA). The beads were washed once with low salt buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA [pH 8.0], 0.1% SDS, 1% Triton X-100), twice with high salt buffer (20 mM Tris-HCl [pH 8.0], 400 mM NaCl, 2 mM EDTA [pH 8.0], 0.1% SDS, 1% Triton X-100), five times with RIPA buffer (50 mM HEPES-KOH [pH 7.5], 500 mM LiCl, 1 mM EDTA [pH 8.0], 1% NP-40, 0.7% Na-deoxycholate), and once with TE (Tris + EDTA) + 50 mM NaCl. To elute the histone-DNA complex, elution buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0], 1% SDS) was added to the beads and incubated at 65 °C for 15 min followed by centrifugation to obtain the supernatant. The supernatants were further incubated at 65 °C for 6 h, and then incubated with RNase A (Roche, Basel, Switzerland) at 37 °C for 2 h followed by incubation with proteinase K (20 mg/mL, Takara, Kyoto, Japan) at 55 °C for 2 h. Finally, the DNA was extracted with phenol chloroform and diluted in ddH2O. To calculate the amount of target sequence in the immunoprecipitated chromatin, we performed real-time qPCR as described above.
3.8. Mycophenolic Acid (MPA) and 6-Azauracil (6AU) Treatments
NIAS-Bm-M1 cells were maintained in IPL-41 (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (HyClone, Thermo Scientific, Waltham, MA, USA) under a humidifying atmosphere at 26 ± 1 °C and 2 mL of cultured cells were seeded onto a 35-mm dish. For MPA treatment, 2.5 μL of MPA (16 mg/mL in dimethyl sulfoxide (DMSO); Wako, Osaka, Japan) were added to each dish. For the 6AU treatment, 2.5 μL of 6AU (2 mg/mL in DMSO; Wako, Osaka, Japan) were added to each dish. The concentration of each inhibitor was determined as reported previously [43]. Total RNA was isolated at 1 or 3 days after treatment, respectively, according to the protocol described above.
4. Conclusions
Male-specific alternative splicing of Imp pre-mRNA was repressed by DOT1L depletion in male embryos. Consistent with this finding, higher distributions of H3K79me2 were observed in males than in females across Imp. Comparative analysis of RNAP II processivity indicated that RNAP II processivity was higher in males than in females at this locus. Inhibition experiments using inhibitors of RNAP II elongation suggested that the higher elongation rate was closely associated with male-specific splicing of Imp pre-mRNA. Taken together, our data suggest that greater accumulation of H3K79me2 along Imp in males causes increased RNAP II processivity, leading to male-specific alternative RNA processing in Imp mRNA production. Furthermore, knockdown of DOT1L caused embryonic lethality.
Acknowledgments
We are grateful to J. Joe Hull for his helpful comments on the manuscript. We also thank Ken-ich Moto and Takeshi Yokoyama for providing the silkworm strains. We are grateful to Hiroki Sakai for technical support with microinjection. This work was supported by a Grant-in-Aid for Scientific Research (B), 23380032, 2011.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Author Contributions
Masataka Suzuki wrote and prepared all parts of this manuscript. He designed and arranged all the experiments presented in this manuscript. He acquired government funds to perform this study.
Haruka Ito performed all the experiments described above. Some experiments were originally designed based on her idea.
Fugaku Aoki made a great contribution to perform ChIP-qPCR analysis and gave us helpful comments on preparing this manuscript based on his deep knowledge about chromatin modifications.
References
- 1.Bandziulis R.J., Swanson M.S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989;3:431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- 2.Green M.R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu. Rev. Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- 3.Wang E.T., Sandberg R., Luo S., Khrebtukova I., Zhang L., Mayr C., Kingsmore S.F., Schroth G.P., Burge C.B. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black D.L. Finding splice sites within a wilderness of RNA. RNA. 1995;1:763–771. [PMC free article] [PubMed] [Google Scholar]
- 5.Chou M.Y., Rooke N., Turck C.W., Black D.L. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol. Cell. Biol. 1999;19:69–77. doi: 10.1128/mcb.19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Expert-Bezancon A., Le Caer J.P., Marie J. Heterogeneous nuclear ribonucleoprotein (hnRNP) K is a component of an intronic splicing enhancer complex that activates the splicing of the alternative exon 6A from chicken beta-tropomyosin pre-mRNA. J. Biol. Chem. 2002;277:16614–16623. doi: 10.1074/jbc.M201083200. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Shambaugh M.E., Rottman F.M., Bokar J.A. SR proteins Asf/SF2 and 9G8 interact to activate enhancer-dependent intron D splicing of bovine growth hormone pre-mRNAin vitro. RNA. 2000;6:1847–1858. doi: 10.1017/s1355838200000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modafferi E.F., Black D.L. Combinatorial control of a neuron-specific exon. RNA. 1999;5:687–706. doi: 10.1017/s1355838299990155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alló M., Schor I.E., Muñoz M.J., de la Mata M., Agirre E., Valcárcel J., Eyras E., Kornblihtt A.R. Chromatin and alternative splicing. Cold Spring Harb. Symp. Quant. Biol. 2010;75:103–111. doi: 10.1101/sqb.2010.75.023. [DOI] [PubMed] [Google Scholar]
- 10.Luco R.F., Allo M., Schor I.E., Kornblihtt A.R., Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144:16–26. doi: 10.1016/j.cell.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luco R.F., Misteli T. More than a splicing code: Integrating the role of RNA, chromatin and non-coding RNA in alternative splicing regulation. Curr. Opin. Genet. Dev. 2011;21:366–372. doi: 10.1016/j.gde.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spies N., Nielsen C.B., Padgett R.A., Burge C.B. Biased chromatin signatures around polyadenylation sites and exons. Mol. Cell. 2009;36:245–254. doi: 10.1016/j.molcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz S., Meshorer E., Ast G. Chromatin organization marks exon-intron structure. Nat. Struct. Mol. Biol. 2009;16:990–995. doi: 10.1038/nsmb.1659. [DOI] [PubMed] [Google Scholar]
- 14.Tilgner H., Nikolaou C., Althammer S., Sammeth M., Beato M., Valcárcel J., Guigó R. Nucleosome positioning as a determinant of exon recognition. Nat. Struct. Mol. Biol. 2009;16:996–1001. doi: 10.1038/nsmb.1658. [DOI] [PubMed] [Google Scholar]
- 15.Schor I.E., Rascovan N., Pelisch F., Alló M., Kornblihtt A.R. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc. Natl. Acad. Sci. USA. 2009;106:4325–4330. doi: 10.1073/pnas.0810666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolasinska-Zwierz P., Down T., Latorre I., Liu T., Liu X.S., Ahringer J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nat. Genet. 2009;41:376–381. doi: 10.1038/ng.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luco R.F., Pan Q., Tominaga K., Blencowe B.J., Pereira-Smith O.M., Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims R.J., 3rd, Millhouse S., Chen C.F., Lewis B.A., Erdjument-Bromage H., Tempst P., Manley J.L., Reinberg D. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol. Cell. 2007;28:665–676. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saint-André V., Batsché E., Rachez C., Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat. Struct. Mol. Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y., Lu Y., Tian W. Epigenetic features are significantly associated with alternative splicing. BMC Genomics. 2012;13:123. doi: 10.1186/1471-2164-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto H. The role of the W chromosome for sex determination in the silkwormBombyx mori. Jpn. J. Genet. 1933;8:245–258. [Google Scholar]
- 22.Niimi T., Sahara K., Oshima H., Yasukouchi Y., Ikeo K., Traut W. Molecular cloning and chromosomal localization of the Bombyx Sex-lethal gene. Genome. 2006;49:263–268. doi: 10.1139/g05-108. [DOI] [PubMed] [Google Scholar]
- 23.Mita K., Kasahara M., Sasaki S., Nagayasu Y., Yamada T., Kanamori H., Namiki N., Kitagawa M., Yamashita H., Yasukochi Y., et al. The genome sequence of silkwormBombyx mori. DNA Res. 2009;11:27–35. doi: 10.1093/dnares/11.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M.G., Funagume S., Kanda T., Tamura T., Shimada T. Role of the male BmDSX protein in the sexual differentiation ofBombyx mori. Evol. Dev. 2005;7:58–68. doi: 10.1111/j.1525-142X.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M.G., Ohbayashi F., Mita K., Shimada T. The mechanism of sex-specific splicing at the doublesex gene is different between Drosophila melanogaster andBombyx mori. Insect Biochem. Mol. Biol. 2001;31:1201–1211. doi: 10.1016/s0965-1748(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M.G., Imanishi S., Dohmae N., Nishimura T., Shimada T., Matsumoto S. Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol. Cell. Biol. 2008;28:333–343. doi: 10.1128/MCB.01528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki M.G., Imanishi S., Dohmae N., Asanuma M., Matsumoto S. Identification of a male-specific RNA binding protein that regulates sex-specific splicing of Bmdsx by increasing RNA binding activity of BmPSI. Mol. Cell. Biol. 2010;30:5776–5786. doi: 10.1128/MCB.00444-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki M.G., Kobayashi S., Aoki F. Male-specific splicing of the silkworm Imp gene is maintained by an autoregulatory mechanism. Mech. Dev. 2014;131:47–56. doi: 10.1016/j.mod.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Terenius O., Papanicolaou A., Garbutt J.S., Eleftherianos I., Huvenne H., Kanginakudru S., Albrechtsen M., An C., Aymeric J.L., Barthel A., et al. RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011;57:231–245. doi: 10.1016/j.jinsphys.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi J., Mizoguchi T., Fujiwara H. siRNAs induce efficient RNAi response in Bombyx mori. PLoS One. 2011;6:e25469. doi: 10.1371/journal.pone.0025469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen A.T., Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 2011;25:1345–1358. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schübeler D., MacAlpine D.M., Scalzo D., Wirbelauer C., Kooperberg C., van Leeuwen F., Gottschling D.E., O’Neill L.P., Turner B.M., Delrow J., et al. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. 2004;18:1263–1271. doi: 10.1101/gad.1198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morillon A., Karabetsou N., Nair A., Mellor J. Dynamic lysine methylation on histone H3 defines the regulatory phase of gene transcription. Mol. Cell. 2005;18:723–734. doi: 10.1016/j.molcel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Pokholok D.K., Harbison C.T., Levine S., Cole M., Hannett N.M., Lee T.I., Bell G.W., Walker K., Rolfe P.A., Herbolsheimer E., et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Kadener S., Fededa J.P., Rosbash M., Kornblihtt A.R. Regulation of alternative splicing by a transcriptional enhancer through RNA pol II elongation. Proc. Natl. Acad. Sci. USA. 2002;99:8185–8190. doi: 10.1073/pnas.122246099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De la Mata M., Alonso C.R., Kadener S., Fededa J.P., Blaustein M., Pelisch F., Cramer P., Bentley D., Kornblihtt A.R. A slow RNA polymerase II affects alternative splicingin vivo. Mol. Cell. 2003;12:525–532. doi: 10.1016/j.molcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi T., Nakano A., Nomura K., Sekimizu K., Natori S. Purification, gene cloning, and gene disruption of the transcription elongation factor S-II inSaccharomyces cerevisiae. J. Biol. Chem. 1992;267:13200–13204. [PubMed] [Google Scholar]
- 38.Nakanishi T., Shimoaraiso M., Kubo T., Natori S. Structure-function relationship of yeast S-II in terms of stimulation of RNA polymerase II, arrest relief, and suppression of 6-azauracil sensitivity. J. Biol. Chem. 1995;270:8991–8995. doi: 10.1074/jbc.270.15.8991. [DOI] [PubMed] [Google Scholar]
- 39.Powell W., Reines D. Mutations in the second largest subunit of RNA polymerase II cause 6-azauracil sensitivity in yeast and increased transcriptional arrest in vitro. J. Biol. Chem. 1996;271:6866–6873. doi: 10.1074/jbc.271.12.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin T.J., Cook J.M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem. J. 1969;113:515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Exinger F., Lacroute F. 6-Azauracil inhibition of GTP biosynthesis inSaccharomyces cerevisiae. Curr. Genet. 1992;22:9–11. doi: 10.1007/BF00351735. [DOI] [PubMed] [Google Scholar]
- 42.Uptain S.M., Kane C.M., Chamberlin M.J. Basic mechanisms of transcript elongation and its regulation. Annu. Rev. Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 43.Howe K.J., Kane C.M., Ares M., Jr. Perturbation of transcription elongation influences the fidelity of internal exon inclusion inSaccharomyces cerevisiae. RNA. 2003;9:993–1006. doi: 10.1261/rna.5390803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masumoto M., Yaginuma T., Niimi T. Functional analysis of Ultrabithorax in the silkworm, Bombyx mori, using RNAi. Dev. Genes Evol. 2009;219:437–444. doi: 10.1007/s00427-009-0305-9. [DOI] [PubMed] [Google Scholar]
- 45.Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B.A., Gonzalez C., Karess R.E., Glover D.M., Sunkel C.E. polo encodes a protein kinase homolog required for mitosis inDrosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 46.Pinto P.A., Henriques T., Freitas M.O., Martins T., Domingues R.G., Wyrzykowska P.S., Coelho P.A., Carmo A.M., Sunkel C.E., Proudfoot N.J., et al. RNA polymerase II kinetics in poilo polyadenylation signal selection. EMBO J. 2011;30:2431–2444. doi: 10.1038/emboj.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De la Mata M., Lafaille C., Kornblihtt A.R. First come, first served revisited: Factors affecting the same alternative splicing event have different effects on the relative rates of intron removal. RNA. 2010;16:904–912. doi: 10.1261/rna.1993510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Z., Lin C., Shilatifard A. The super elongation complex (SEC) family in transcriptional control. Nat. Rev. Mol. Cell Biol. 2012;13:543–547. doi: 10.1038/nrm3417. [DOI] [PubMed] [Google Scholar]
- 49.Shilatifard A., Lane W.S., Jackson K.W., Conaway R.C., Conaway J.W. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 50.Shilatifard A., Duan D.R., Haque D., Florence C., Schubach W.H., Conaway J.W., Conaway R.C. ELL2, a new member of an ELL family of RNA polymerase II elongation factors. Proc. Natl. Acad. Sci. USA. 1997;94:3639–3643. doi: 10.1073/pnas.94.8.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bitoun E., Oliver P.L., Davies K.E. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum. Mol. Gen. 2007;16:92–106. doi: 10.1093/hmg/ddl444. [DOI] [PubMed] [Google Scholar]
- 52.Mueller D., Bach C., Zeisig D., Garcia-Cuellar M.P., Monroe S., Sreekumar A., Zhou R., Nesvizhskii A., Chinnaiyan A., Hess J.L., et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. 2007;110:4445–4454. doi: 10.1182/blood-2007-05-090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milcarek C., Albring M., Langer C., Park K.S. The eleven-nineteen lysine-rich leukemia gene (ELL2) influences the histone H3 protein modifications accompanying the shift to secretory immunoglobulin heavy chain mRNA production. J. Biol. Chem. 2011;286:33795–33803. doi: 10.1074/jbc.M111.272096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colgan D.F., Manley J.L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. doi: 10.1101/gad.11.21.2755. [DOI] [PubMed] [Google Scholar]
- 55.Tian B., Hu J., Zhang H., Lutz C.S. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kornblihtt A.R. Chromatin, transcript elongation and alternative splicing. Nat. Struct. Mol. Biol. 2006;13:5–7. doi: 10.1038/nsmb0106-5. [DOI] [PubMed] [Google Scholar]
- 57.Jones B., Su H., Bhat A., Lei H., Bajko J., Hevi S., Baltus G.A., Kadam S., Zhai H., Valdez R., et al. The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet. 2008;4:e1000190. doi: 10.1371/journal.pgen.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanower G.A., Muller M., Blanton J.L., Honti V., Gyurkovics H., Schedl P. Characterization of the grappa gene, the Drosophila histone H3 lysine 79 methyltransferase. Genetics. 2005;169:173–184. doi: 10.1534/genetics.104.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brock H.W., van Lohuizen M. The Polycomb group—No longer an exclusive club. Curr. Opin. Genet. Dev. 2001;11:175–181. doi: 10.1016/s0959-437x(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki M.G., Suzuki K., Aoki F., Ajimura M. Effect of RNAi-mediated knockdown of the Bombyx mori transformer-2 gene on the sex-specific splicing of Bmdsx pre-mRNA. Int. J. Dev. Biol. 2012;56:693–699. doi: 10.1387/ijdb.120049ms. [DOI] [PubMed] [Google Scholar]
- 61.Koike Y., Mita K., Suzuki M.G., Maeda S., Abe H., Osoegawa K., deJong P.J., Shimada T. Genomic sequence of a 320-kb segment of the Z chromosome of Bombyx mori containing a kettin ortholog. Mol. Genet. Genomics. 2003;269:137–149. doi: 10.1007/s00438-003-0822-6. [DOI] [PubMed] [Google Scholar]