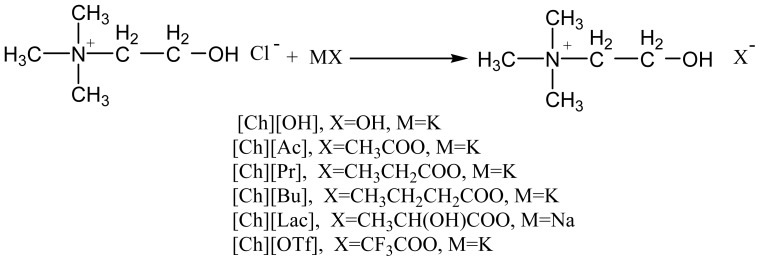Abstract
A mild, efficient, and environmentally benign protocol for the synthesis of tetrahydrobenzo[b]pyran derivatives in the presence of readily accessible, biodegradable, and choline hydroxide based ionic liquid as catalyst has been established. The key features of the reported methodology include good to excellent yields of desired products, simple work-up procedure and good recyclability of catalysts, which may be a practical alternative to the existing conventional processes for the preparation of 4-H pyrans to cater to the requirements of academia as well as industry.
Keywords: pyran, choline hydroxide, ionic liquid, cost-effectiveness, biodegradability, recyclability
1. Introduction
As an important class of oxygen-containing heterocycles, tetrahydrobenzo[b]pyrans are widely employed as potential biodegradable agrochemicals [1], photoactive materials [2], cosmetics and pigments [3]. Because of the inherent reactivity of the pyran ring, 4-H pyrans are versatile synthons, which can be easily converted into pyridine compounds as pharmacologically important calcium antagonists [4,5]. Tetrahedronhydro[b]pyran compounds themselves have a broad spectrum of biological properties [6,7], such as spasmolytic, anticancer, diuretic, anticoagulant, and antiancaphylactia activities [8,9]. They can also be used as cognitive enhancers not only for the treatment of neurodegenerative disease, for example, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, AIDS associated dementia and Down syndrome but also for the treatment of schizophrenia and myoclonus [10]. Due to the versatile utilization of the pyran derivatives in the field of organic synthesis as well as in medicinal chemistry, many researchers have been encouraged to develop highly efficient procedures for the preparation of these kinds of compounds.
Multi-components reactions (MCRs), an important subclass of tandem reactions, are one-pot processes in which complicated molecules, especially various heterocycles with biological activities, can be produced in a very fast, efficient, and economic manner without the isolation of any intermediate [11–13]. Hence, considering the fascinating advantages of MCRs, some methodologies for the synthesis of tetrahedrondydro[b]pyrans via three components one-pot reactions catalyzed by 1,4-diazabicyclo[2.2.2]octane [14], N-methylimidazole [15], tetra-methyl ammonium hydroxide [16], (NH4)2HPO4 [17] K3PO4 [18], ZnO-beta zeolite [19], nanosized Ce1MgXZr1−xO2 [20], Ru(II) complex [21], Na2SeO4 [22], S-proline [23], l-proline [24], 1,8-diazabicyclo[5.4.0]undec-7-ene [25], sulfonic acid functionalized silica [26], phenylboronic acid [27], caro’s acid-silica gel [28], and cerium(III) chloride [29] have been reported. In addition, procedures of catalyst-free and 2,2,2-trifluoroethanol as reaction medium [30], microwave and ultrasonic irradiation [9,31,32] could also give satisfactory yields of pyrans. Each of the above methods has its own merits, while some are plagued by one or more limitations of tedious work-up, poor product yields, long reaction times, effluent pollution, unavailability of the catalyst and vast employment of organic solvents, which is significantly harmful to environment. Consequently, gaps remain in terms of the search for alternative methods for synthesis of 4-H pyrans which are both high efficiency and environmentally friendly.
Ionic quids (ILs) with the unique properties of low volatility, good and tunable solubility, non-flammability, and excellent recyclability are receiving considerable global attention and used extensively in organic synthesis as green reaction media or designable catalysts [33–40]. Thus, some task-specific ionic liquids were successfully introduced as catalysts for the preparation of the desired tetrahydrobenzo[b]pyran derivates [41–44]. However, disadvantages associated with high price, a little toxicity and difficulty of biodegradability, to some extent, limit these methodologies at the industrial scale. Choline chloride (ChCl), a member of the vitamin B family, is a very cheap, commercially available, biodegradable and non-toxic quarternary ammonium salt, which can be simply produced by gas phase reaction or directly extracted from biomass [45,46]. Compared to the intensive studies of ChCl-derived deep eutectic solvents as catalysts for organic transformations [45,46] the research with regard to the application of ChCl-based ionic liquids to organic synthesis is very rare [47]. As part of our ongoing pursuit for the establishment of “green organic synthesis” [48–56], we herein prepared a type of non-toxic, cheap and biodegradable ChCl-derived ionic liquids and present their usage as catalysts for the synthesis of 4H-benzo[b]pyrans via MCRs of aldehydes, methylene active compounds and 5,5-dimethyl-1,3-cyclohexanedione in aqueous medium. These type of ionic liquids can be readily prepared through anion exchange reactions (Scheme 1) and they are all liquids at room temperature (25 °C).
Scheme 1.
Preparation of choline hydroxide based ionic liquids.
2. Results and Discussion
Initially, the reaction of p-anisaldehyde, malononitrile and dimedone was selected as model for the optimization of reaction conditions. As shown in Table 1, [bmim]BF4 and [bmim]PF6, two traditional ionic liquids frequently used as reaction media for organic transformations, gave significantly lower yields of products (Table 1, entries 1–2). Moreover, new DBU derived ILs were found to be less suitable as catalysts for the preparation of the pyran product while they exhibited excellent catalytic activities for aza-Michael addition [48–50] and Knoevenagel condensation [48–56] (Table 1, entries 3 and 4). We then turn our attention to the cost-effective and biodegradable ILs, [Ch][X]. Among the ILs [Ch][X] examined as catalysts for the model reaction, [Ch][OH] could efficiently catalyze to form tetrahydro[b]pyrans with excellent yield in short time, and the remained ILs, including [Ch][Ac], [Ch][Lac], [Ch][Pr], and [Ch][OTf] exhibited relatively lower catalytic activities (Table 1, entries 5–11). For comparison, [bmim]OH, a basic ionic liquid widely used in organic catalysis, gave slightly lower yield (86%). Combination of ILs’s accessibility, availability and their catalytic efficiency, [Ch][OH] was selected as the best catalyst for the preparation of the functionalized pyrans. Upon investigating the influence of the amount of catalyst on the reaction, it was found that 10 mol % of [Ch][OH] was sufficient to promote the one-pot reaction (Table 1, entries 12–15). Moreover, a brief screening of solvents showed that the much better results in terms of both reaction rate and yield were observed with water as reaction medium than those performed in organic solvents (Table 1, entries 13 and 16–19).
Table 1.
Optimization of reaction conditions for the synthesis of tetrahydrobenzo[b]pyran a.

| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Ionic Liquids | Mol % | Solvents (10 mL) | Time (h) | Yield (%) b |
| 1 | [bmim]BF4 | 20 | Water | 5 | 17 |
| 2 | [bmim]PF6 | 20 | Water | 5 | 8 |
| 3 | [DBU][Ac] | 20 | Water | 2 | 31 |
| 4 | [DBU][Lac] | 20 | Water | 2 | 28 |
| 5 | [Ch][Ac] | 20 | Water | 3 | 52 |
| 6 | [Ch][Pr] | 20 | Water | 3 | 47 |
| 7 | [Ch][Bu] | 20 | Water | 3 | 55 |
| 8 | [Ch][Lac] | 20 | Water | 3 | 55 |
| 9 | [Ch][OTf] | 20 | Water | 3 | 38 |
| 10 | [Ch][OH] | 20 | Water | 2 | 95 |
| 11 | [bmim]OH | 20 | Water | 3 | 86 |
| 12 | [Ch][OH] | 10 | Water | 2 | 96 |
| 13 | [Ch][OH] | 5 | Water | 5 | 83 |
| 14 | [Ch][OH] | 30 | Water | 2 | 95 |
| 15 | [Ch][OH] | 50 | Water | 2 | 96 |
| 16 c | [Ch][OH] | 10 | CH2Cl2 | 5 | Trace |
| 17 c | [Ch][OH] | 10 | THF | 3 | Trace |
| 18 c | [Ch][OH] | 10 | Ethanol | 2 | 82 |
| 19 c | [Ch][OH] | 10 | Methanol | 2 | 68 |
All reactions were carried out as follows: the mole ratio of p-anisaldehyde: malononitrile: dimedone was 1:1:1 and the reaction temperature was 80 °C;
Isolated yields;
The reactions were conducted in the presence of reflux conditions.
To extend the scope and demonstrate the generality of the present method, we explored the reaction of various aromatic aldehydes with malononitrile and dimedone under the optimized reaction conditions to furnish respective substituted pyrans and the results are demonstrated at Table 2. To our pleasure, the reactions proceeded smoothly and good to excellent yields of desired products were obtained within several hours. The electronic nature of substituents on the aromatic ring has some effects on the transformation. The aromatic aldehydes bearing electron-donating groups such as MeO, OH, 3,4-2(Me) reacted much slower with malononitrile and dimedone than other aromatic aldehydes substituted with NO2, CN, F, Cl, Br (Table 2, entries 1–10). The heterocyclic aldehydes such as 2-thienyl and furyl aldedydes were also demonstrated to be efficient reagents for this reaction (Table 2, entries 12 and 14). However, when pyridine and 2-naphthyl aldehydes were subjected to the procedures, only intermediates-Knoevenagel condensation products were detected (Table 2, entries 11 and 13). In addition, the reaction of trans-cinnamaldehyde with malononitrile and dimedone was performed under the same conditions and some lower yield (69%) was observed (Table 2, entry 14). It is noteworthy to mention that aliphatic aldehydes were suitable for the condensation achieving good product yields (Table 2, entries 16 and 17).
Table 2.
Synthesis of pyran derivatives via condensation of aromatic aldehydes, malononitrile and dimedone using [Ch][OH] as catalyst in water.

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Entry | R1 | Time (h) | Products | Yields (%) a | Mp (°C) Measured | Mp (°C) References |
| 1 | C6H5 | 1 | 2a | 92 | 232–234 | 234–235 [57] |
| 4-NO2-C6H4 | 30 min | 2b | 88 | 176–177 | 177–178 [27] | |
| 2 | 3-NO2-C6H4 | 20 min | 2c | 95 | 213–214 | 213–215 [27] |
| 3 | 4-Cl-C6H4 | 1 | 2d | 86 | 212–214 | 209–210 [19] |
| 4 | 4-F-C6H4 | 10 min | 2e | 90 | 198–200 | 200 [28] |
| 5 | 4-Br-C6H4 | 1 | 2f | 91 | 165–168 | 169–170[30] |
| 6 | 4-CN-C6H4 | 30 min | 2g | 90 | 227–229 | 224–226 [18] |
| 7 | 4-OH-C6H4 | 2 | 2h | 86 | 206–298 | 207–209 [28] |
| 8 | 4-MeO-C6H4 | 3 | 2i | 95 | 198–200 | 199–200 [28] |
| 9 | 4-N(Me)2-C6H4 | 5 | 2j | 98 | 216–218 | 217–218 [57] |
| 10 | 3,4-2(MeO)-C6H3 | 3 | 2k | 92 | 184–185 | 182–184 [14] |
| 11 c | 3-Pyridine | 10 | 2l | 0 | - | - |
| 12 | 2-Furyl | 3 | 2m | 82 | 223–226 | 222–224 [57] |
| 13 c | 2-Naphthyl | 10 | 2n | 0 | - | - |
| 14 | 2-Thienyl | 1 | 2o | 85 | 210–212 | 210–212 [18] |
| 15 b | C6H5-CH=CH- | 5 | 2p | 68 | 205–207 | 208–210 [27] |
| 16 b | CH3CH2 | 3 | 2q | 85 | 190–192 | 190–194 [22] |
| 17 b | CH3CH2CH2 | 3 | 2r | 79 | 192–194 | 192–193 [58] |
Isolated yields;
20 Mol % amount of [Ch][OH] and reaction temperature of 100 °C were required;
Only Knoevenagel condensation products were detected.
Encouraged by the exciting results from the reactions of malononitrile, we decided to further extend the substrate scope and two CN substituted active methylene compounds, cyanoacetate and 2-(benzo[d]thiazol-2-yl)acetonitrile were tested (Scheme 2). As outlined in Scheme 2, the novel ionic liquid [Ch][OH] as catalyst is suitable for cyanoacetate as well as 2-(benzo[d]thiazol-2-yl)acetonitrile and can afford the corresponding heterocyclic compounds in good yields (Scheme 2, 4a–i).
Scheme 2.
[Ch][OH] promoted one-pot synthesis of 4H-benzo[b]pyrans via the reaction of aromatic aldehydes, cyanoacetate or 2-(benzo[d]thiazol-2-yl)acetonitrile, and dimedone.
In order to demonstrate the industrial applicability of this methodology, the aqueous one-pot synthesis of 4H-benzo[b]pyrans via the reaction of p-anisaldehyde, malononitrile, dimedone catalyzed by [Ch]][OH] was carried out on a larger scale (100 mmol). The reaction was completed in 2 h. The excellent yield of 98% for the conjugate product was obtained. On the same scale, the recyclability of the catalytic system was investigated using the same reaction as model reaction. Upon the completion of the reaction, the product was isolated by filtration while the filtrate was dried to remove water at 80 °C under vacuum. The recovered ionic liquid was reused in subsequent reactions. As shown in Figure 1, the ionic liquid [Ch][OH] can be recycled 5 times without considerable decrease of activity and the used ionic liquid remained intact (1H NMR).
Figure 1.
Reuse of ionic liquid for three components condensation of p-anisaldehyde (100 mmol), malononitrile and dimedone in the presence of ionic liquid [Ch]][OH] in aqueous solution.
A plausible mechanism for the reactions is illustrated in Scheme 3. Due to the strong basicity of hydroxyl anion of the IL (see Table S1) and hydrogen bond formed between hydroxyl group of side chain of Ch and carboxyl moiety of aldehydes, the dual activation of methylene ingredients and aldehydes facilitate the formation of a Knoevenagel condensation product 5. Dimedone can be easily converted to its enolic form in the presence of [Ch][OH] that could readily react with acrylonitrile 5, affording the Michael addition product 6, followed by tautomerism, intramolecular O-cyclization and proton transfer reactions under dual activities of the IL to give the desired product 4. 13C NMR data of benzaldehyde and the mixture of IL [Ch][OH] and benzaldehyde also provided the catalytic role of the hydrogen bond (see Figures S1 and S2).
Scheme 3.
The proposed mechanism for [Ch][OH] promoted synthesis of tetrahydrobenzo[b]pyrans.
3. Experimental Section
3.1. Materials and General Methods
NMR spectra were recorded Bruker Advance DPX 400 MHz spectrometer (Bruker BioSpin Corporation, Fällanden, Switzerland) with chemical shift values (δ) in parts per million, relative to the internal standard of tetramethylsilane (TMS). Melting points were determined using YRT-3 apparatus (Reliant Instument, Tianjin, China) and were not corrected. All chemical were purchased from Aladdin, Aldrich or Fluka (Buches SG, Switzerland). All reactions were monitored by thin layer chromatography (TLC).
3.2. General Procedure for the Synthesis of the ChCl-Based Ionic Liquids
A mixture of ChCl (20 mmol), MX (20 mmol) and methanol (25 mL) was stirred at reflux for 12 h. Then methanol was evaporated at 60 °C in high vacuum until the weight of the residue remained constant. The resulting liquids were the [Ch]X. The ionic liquids were characterized by 1H NMR, 13C NMR (Bruker BioSpin Corporation, Fällanden, Switzerland).
[Ch][OH] 1H NMR (400 M, D2O): δ4.05 (m, 2H), 3.49 (t, 2H, J = 5.2 Hz), 3.20 (s, 9H); 13C NMR (100 M, D2O): δ 67.86, 55.73, 53.91, 53.84, 48.92.
[Ch][Ac] 1H NMR (400 M, D2O): δ3.98 (m, 2H), 3.44 (t, 2H, J = 4.8 Hz), 3.13 (s, 9H), 1.83 (s, 3H); 13C NMR (100 M, D2O): δ 180.90, 67.42, 55.57, 53.84, 23.46.
[Ch][Pr] 1H NMR (400 M, D2O): δ4.03 (m, 2H), 3.48 (t, 2H, J = 5.2 Hz), 3.16 (s, 9H), 2.12 (t, 2H, J = 7.2 Hz), 0.86 (t, 3 H, J = 7.2 Hz); 13C NMR (100 M, D2O): δ 183.73, 67.49, 55.68, 54.02, 53.97, 53.94, 39.73, 13.46.
[Ch][Bu] 1H NMR (400 M, D2O): δ 3.98 (m, 2H), 3.44 (t, 2H, J = 4.8 Hz), 3.13 (s, 9H), 2.08 (t, 2H, J = 7.2 Hz), 1.48 (m, 2H), 0.82 (t, 3H, J = 7.2 Hz); 13C NMR (100 M, D2O): δ 183.78, 67.21, 55.58, 53.90, 53.97, .53.86, 39.67, 19.44, 13.43.
[Ch][Lac] 1H NMR (400 M, D2O): δ3.82 (m, 3H), 3.26 (t, 2H, J = 4.8 Hz), 2.98 (s, 9H), 1.07 (d, 3H, J = 6.8 Hz), 0.86 (t, 3H, J = 7.2 Hz); 13C NMR (100 M, D2O): δ 182.40, 68.53, 67.47, 55.67, 53.96, 53.93, 53.89, 20.22.
[Ch][OTf] 1H NMR (400 M, D2O): δ 4.02 (m, 2H), 3.48 (t, 2H, J = 4.8 Hz), 3.16 (s, 9H); 13C NMR (100 M, D2O): δ 70.42, 58.61, 56.83.
3.3. Typical Procedure for One-Pot Synthesis of Tetrahydrobenzo[b]Pyrans
To a mixture of aromatic aldehyde (5 mmol), methyl active compound (5 mmol), and dimedone (5 mmol) in water (10 mL), [Ch][OH] was added. Upon addition, the reaction was stirred at reflux until the disappearance of starting material by TLC. After the completion of the reaction, the reaction mixture was filtrated to obtain a solid, which was recrystallized in 95% ethanol to give pure product. The ionic liquid was recovered from the remaining filtrate, subsequently remove water in vacuum at 80 °C and reused several times without further purification. The product was characterized by melting point measurement and NMR.
3.4. Spectroscopic Data of Typical Products
2-Amino-3-cyano-4-phenyl-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (2a): mp 233–235 °C. IR (KBr): 3310, 2966, 2188, 1658, 1372 cm−1; 1H NMR (200 MHz, CDCl3) (ppm): 1.04 (s, 3H, CH3), 1.11 (s, 3H, CH3), 2.23 (s, 2H, CH2), 2.46 (s, 2H, CH2), 4.40 (s, 1H, CH), 4.58 (s, 2H, NH2), 7.21–7.30 (m, 5H, Ar-H); ESI-MS: m/z 294 [M]+.
2-Amino-3-cyano-4-(4-hydroxyphenyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (2h): mp 205–206 °C. IR (KBr): 3353, 2958, 2170, 1660, 1395, 1218 cm−1; 1H NMR (200 MHz, CDCl3) (ppm): 1.04 (s, 3H, CH3), 1.25 (s, 3H, CH3), 2.23 (s, 2H, CH2), 2.44 (s, 2H, CH2), 4.35 (s, 1H, CH), 4.49 (s, 2H, NH2), 6.71–7.27 (m, 4H, Ar-H); ESI-MS: m/z 333 [M + Na]+.
2-Amino-3-cyano-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (2i): mp 198–201 °C. IR (KBr): 3356, 2970, 2158, 1656, 1385, 1219 cm−1; 1H NMR (200 MHz, CDCl3) (ppm): 1.04 (s, 3H, CH3), 1.22 (s, 3H, CH3), 2.34–2.39 (t, J = 10 Hz, 2H, CH2), 3.77 (s, 1H, CH), 6.78–7.26 (m, 4H, Ar-H); ESI-MS: m/z 324 [M + Na]+.
2-Amino-3-(benzo[d]thiazol-2-yl)-4-(4-fluorophenyl)-7,7-dimethyl-5-carbonyl-5,6,7,8-4H-benzo[b] pyran (4f): mp 190–192 °C. IR (KBr): 3325, 1665, 1617, 1482, 1450 cm−1; 1H NMR (200 MHz, DMSO-d6) (ppm): 1.01 (s, 3H, CH3), 1.12 (s, 3H, CH3), 2.23 (d, 1H, J = 6.8 Hz), 2.28 (d, 1H, J = 6.6 Hz), 2.45 (d, 1H, J = 7.2 Hz), 2.42 (d, 1H, J = 7.2 Hz), 4.85 (s, 1H), 6.89–7.68 (m, 8H, Ar-H), 8.49 (s, 2H, NH2); ESI-MS: m/z 421 [M + H]+.
2-Amino-3-(benzo[d]thiazol-2-yl)-4-(4-methyoxyphenyl)-7,7-dimethyl-5-carbonyl-5,6,7,8-4H-benzo[ b]pyran (4i): mp 176–178 °C. IR (KBr): 3398, 1668, 1654, 1623, 1474, 756, 664 cm−1; 1H NMR (200 MHz, DMSO-d6) (ppm): 0.89 (s, 3H, CH3), 1.05 (s, 3H, CH3), 2.12 (d, 1H, J = 6.8 Hz), 2.25 (d, 1H, J = 6.8 Hz), 2.46 (d, 1H, J = 7.2 Hz), 2.58 (d, 1H, J = 7.2 Hz), 3.69 (s, 3H, OCH3), 4.55 (s, 1H), 6.76–7.93 (m, 8H, Ar-H), 8.38 (s, 2H, NH2); ESI-MS: m/z 433 [M + H]+.
4. Conclusions
In summary, a simple, efficient and environmentally benign protocol for preparation of tetrahydrobenzo[b]pyrans was developed using novel basic, biodegradable, and cost-effective ionic liquid [Ch][OH] as catalyst in aqueous solution. Compared with the traditional imidazole derived ionic liquids, [Ch][OH] not only produced comparative or better results in terms of reaction rate and product yield but also is biodegradable, cheap and can be reused six times without significant loss of its catalytic efficiency. The applications of the novel ChCl-derived ionic liquids for other organic transformations are currently being investigated in our lab.
Supplementary Information
Acknowledgments
We are grateful for the financial support for this research by the National Natural Science Foundation of China (Grant No. 21106090 and 21272169), the Zhejiang Provincial Natural Science Foundation of China (No. LY12B02004), and the Foundation of Low Carbon Fatty Amine Engineering Research Center of Zhejiang Province (2012E10033).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hafez E.A.A., Elnagdi M.H., Elagemey A.G.A., EI-Taweel F.M.A.A. Nitriles in heterocyclic synthesis-novel synthesis of benzo[c]coumarin and of benzo[c]pyrano[3,2-c]quinoline derivatives. Heterocycles. 1987;26:903–907. [Google Scholar]
- 2.Armesto D., Horspool W.M., Martin N., Ramos A., Seaone C. Synthesis of cyclobutenes by the novel photochemical ring contraction 4-sbustituted 2-amino-3,5-dicyano-6-phenyl-4H-pyrans. J. Org. Chem. 1989;54:3069–3072. [Google Scholar]
- 3.Ellis G.P. In: The Chemistry of Heterocyclic Compounds Chromenes, Chromanes and Chromones. Weissberger A., Taylor E.C., editors. John Wiley; New York, NY, USA: 1977. pp. 11–139. [Google Scholar]
- 4.Martin N., Martin G., Seoane C., Maco J.L., Albert A., Cano F.H. Michael addition of malononitrile to α-acetylcinnamamides. Ann. Chem. 1993;7:801. [Google Scholar]
- 5.Marco J.L., Martin N., Grau A.M., Seoane C., Albert A., Cano F.H. Development of methods for the synthesis of chiral, highly functionalized 2-aminno-4-aryl-4H-pyrans. Tetrahedron. 1994;50:3509. [Google Scholar]
- 6.Green G.R., Evans J.M., Vong A.K. In: Comprehensive Heterocyclic Chemistry II. Ktritsky A.R., Rees C.W., Scriven E.F.V., editors. Vol. 5. Permagon Press; Oxford UK: 1995. p. 469. [Google Scholar]
- 7.Jin T.S., Wang A.Q., Wang X., Zhang J.S., Li T.S. A clean one-pot synthesis of tetrahydrobenzo[b]pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synlett. 2004;5:871–873. [Google Scholar]
- 8.Bonsignore L., Loy G., Secci D., Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur. J. Med. Chem. 1993;28:517–520. [Google Scholar]
- 9.Devi I., Bhuyan P.J. Sodium bromide catalysed one-pot synthesis of tetrahydrobenzo[b]pyrans via a three-component cyclocondensation under microwave irradiation and solvent free conditions. Tetrahedron Lett. 2004;45:8625–8627. [Google Scholar]
- 10.Konkoy C.S., Fick D.B., Cai S.X., Lan N.C., Keana J.F.W. Substituted 5-oxo-5,6,7,8-tetrahydro-4h-1-benzopyrans and benzothiopyrans and the use thereof as potentiators of ampa. WO0075123 PCT Int. Appl. 2000; Chem. Abstr. 2001;134:29313a. [Google Scholar]
- 11.Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 12.Ganem B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009;42:463–472. doi: 10.1021/ar800214s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toure B.B., Hall D.G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]
- 14.Tahmassebi D., Bryson J.A., Binz S.I. 1,4-Diazabicyclo[2.2.2]octane as an efficient catalyst for a clean, one-pot synthesis of tetrahydrobenzo[b]pyran derivatives via multicomponent reaction in aqueous media. Synth. Commun. 2011;41:2701–2711. [Google Scholar]
- 15.Lian X.Z., Huang Y., Li Y.Q., Zheng W.J. A green synthesis of tetrahydrobenzo[b]pyran derivatives through three-component condensation using N-methylimidazole as organocatalyst. Monatsh. Chem. 2008;139:129–131. [Google Scholar]
- 16.Balalaie S., Sheikh-Ahmadi M., Bararjanian M. Tetra-methyl ammonium hydroxide: An efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media. Catal. Commun. 2007;8:1724–1728. [Google Scholar]
- 17.Balalaie S., Bararjanian M., Sheikh-Ahmadi M. Diammonium hydrogen phosphate: An efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media. Synth. Commun. 2007;37:1097–1108. [Google Scholar]
- 18.Pore D.M., Undale K.A., Dongare B.B., Desai U.V. Potassium phosphate catalyzed a rapid three-component synthesis of tetrahydrobenzo[b]pyran at ambient temperature. Catal. Lett. 2009;132:104–108. [Google Scholar]
- 19.Katkar S.S., Lande M.K., Arbad B.R., Gaikwad S.T. A recyclable and highly effective ZnO-beta zeolite as a catalyst for one-pot three-component synthesis of tetrahydrobenzo[b]pyrans. Chin. J. Chem. 2011;29:199–202. [Google Scholar]
- 20.Rathod S., Arbad B., Land M. Preparation, characterization, and catalytic application of a nanosized Ce1Mgx Zr1−x O2 solid heterogeneous catalyst for the synthesis of Tetrahydrobenzo[b]pyran derivatives. Chin. J. Catal. 2010;31:631–636. [Google Scholar]
- 21.Tabatabaeian K., Heidari H., Mamaghani M., Mahmoodi N.O. Ru(II) complexes bearing tertiary phosphine ligands: A novel and efficient homogeneous catalyst for one-pot synthesis of dihydropyrano[3,2-c]chromene and tetrahydrobenzo[b]pyran derivatives. Appl. Organometal. Chem. 2012;26:56–61. [Google Scholar]
- 22.Hekmatshoar R., Majedi S., Bakhtiari K. Sodium selenate catalyzed simple and efficient synthesis of tetrahydro benzo[b]pyran derivatives. Catal. Commun. 2008;9:307–310. [Google Scholar]
- 23.Balalaie S., Bararjanian M., Amani A.M., Movassagh B. (S)-Proline as a neutral and efficient catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media. Synlett. 2006:263–266. [Google Scholar]
- 24.Li Y., Chen H., Shi C., Ji S. Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. J. Comb. Chem. 2010;12:231–237. doi: 10.1021/cc9001185. [DOI] [PubMed] [Google Scholar]
- 25.Khurana J.M., Nand B., Saluja P. DBU: A highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H benzo[g]chromenes in aqueous medium. Tetrahedron. 2010;66:5637–5641. [Google Scholar]
- 26.Ziarani G.M., Abbasi A., Badiei A., Aslani Z. An efficient synthesis of tetrahydrobenzo[b]pyran derivatives using sulfonic acid functionalized silica as an efficient catalyst. E-J. Chem. 2011;8:293–295. [Google Scholar]
- 27.Nemouchi S., Boulcina R., Carboni B., Debache A. Phenylboronic acid as an efficient and convenient catalyst for a three-component synthesis of tetrahydrobenzo[b]pyrans. C. R. Chim. 2012;15:394–397. [Google Scholar]
- 28.Oskooie H.A., Heravi M.M., Karimi N., Zadeh M.E. Versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives. Synth. Commun. 2011;41:436–440. [Google Scholar]
- 29.Sabitha G., Arundhathi K., Sudhakar K., Sastry B.S., Yadav J.S. Cerium(III) chloride-catalyzed one-pot synthesis of tetrahydrobenzo[b]pyrans. Synth. Commun. 2009;39:433–442. [Google Scholar]
- 30.Khaksar S., Rouhollahpour A., Talesh S.M. A facile and efficient synthesis of 2-amino-3-cyano-4H-chromenes and tetrahydrobenzo[b]pyrans using 2,2,2-trifluoroethanol as a metal-free and reusable medium. J. Fluor. Chem. 2012;141:11–15. [Google Scholar]
- 31.Feng C., Wang Q., Lu C., Yang G., Chen Z. Green Synthesis of Tetrahydrobenzo[b]Pyrans by Microwave Assisted Multi-Component One-Pot Reactions in PEG-400. Comb. Chem. High Throughput Screen. 2012;15:100–103. doi: 10.2174/138620712798280790. [DOI] [PubMed] [Google Scholar]
- 32.Li J.T., Xu W.Z., Yang L.C., Li T.S. One-pot synthesis of 2-amino-4-aryl-3- carbalkoxy-7,7-dimethyl-5,6,7,8-tetrahydrobenzo[b]pyran derivatives catalyzed by KF/basic Al2O3 under ultrasound irradiation. Synth. Commun. 2004;34:4565–4571. [Google Scholar]
- 33.Zhang Q., Zhang S., Deng Y. Recent advances in ionic liquid catalysis. Green Chem. 2011;13:2619–2637. [Google Scholar]
- 34.Niedermeyer H., Hallett J.P., Villar-Garcia I.J., Hunt P.A., Welton T. Mixtures of ionic liquids. Chem. Soc. Rev. 2012;41:7780–7802. doi: 10.1039/c2cs35177c. [DOI] [PubMed] [Google Scholar]
- 35.Pereira M.M.A. Immobilized Ionic Liquids in Organic Chemistry. Curr. Org. Chem. 2012;16:1680–1710. [Google Scholar]
- 36.Tang S., Baker G.A., Zhao H. Ether- and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012;41:4030–4066. doi: 10.1039/c2cs15362a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payagala T., Armstrong D.W. Chiral ionic liquids: A compendium of syntheses and applications 2005–2012. Chirality. 2012;24:17–53. doi: 10.1002/chir.21975. [DOI] [PubMed] [Google Scholar]
- 38.Isambert N., Duque M.M.S., Plaquevent J.-C., Génisson Y., Rodriguez J., Constantieux T. Multicomponent reactions and ionic liquids: A perfect synergy for eco-compatible heterocyclic synthesis. Chem. Soc. Rev. 2011;40:1347–1357. doi: 10.1039/c0cs00013b. [DOI] [PubMed] [Google Scholar]
- 39.Ying A.-G., Ye W.-D., Liu L., Wu G.-F., Chen X.-Z., Qian S., Zhang Q.-P. Progress in the application of ionic liquids to organic synthesis. Chin. J. Org. Chem. 2008;28:2081–2094. [Google Scholar]
- 40.Ying A.-G., Chen X.-Z., Ye W.-D., Zhang D.-F., Liu L., Chen J.-H. Application of ionic liquids in organic synthesis promoted by microwave irradiation. Prog. Chem. 2008;20:1642–1650. [Google Scholar]
- 41.Salvi P.P., Mandhare A.M., Sartape A.S., Pawar D.K., Han H.S., Kolekar S.S. An efficient protocol for synthesis of tetrahydrobenzo[b]pyrans using amino functionalized ionic liquid. C. R. Chim. 2011;14:878–882. [Google Scholar]
- 42.Wen L.-R., Xie H.-Y., Li M. A basic ionic liquid catalyzed reaction of benzothiazole, aldehydes, and 5,5-dimethyl-1,3-cyclohexanedione: Efficient synthesis of tetrahydrobenzo[b]pyrans. J. Heterocycl. Chem. 2009;46:954–959. [Google Scholar]
- 43.Zheng J., Li Y. Basic ionic liquid-catalyzed multicomponent synthesis of tetrahydrobenzo[b]pyrans and pyrano[c]chromenes. Mendeleev Commun. 2011;21:280–281. [Google Scholar]
- 44.Shaterian H.R., Arman M., Rigi F. Domino Knoevenagel condensation, Michael addition, and cyclization using ionic liquid, 2-hydroxyethylammonium formate, as a recoverable catalyst. J. Mol. Liq. 2011;158:145–150. [Google Scholar]
- 45.Ruβ C., König B. Low melting mixtures in organic synthesis—An alternative to ionic liquids? Green Chem. 2012;14:2969–2982. [Google Scholar]
- 46.Zhang Q., Vigier K.D.O., Royer S., Jérôme F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012;41:7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- 47.Abelló S., Medina F., Rodríguez X., Cesteros Y., Salagre P., Sueiras J.E.S., Tichit D., Coq B. Supported choline hydroxide (ionic liquid) as heterogeneous catalyst for aldol condensation reactions. Chem. Commun. 2004:1096–1097. doi: 10.1039/b401448k. [DOI] [PubMed] [Google Scholar]
- 48.Ying A.-G., Liu L., Wu G.-F., Chen G., Chen X.-Z., Ye W.-D. Aza-Michael addition of aliphatic or aromatic amines to alpha,beta-unsaturated compounds catalyzed by a DBU-derived ionic liquid under solvent-free conditions. Tetrahedron Lett. 2009;50:1653–1657. [Google Scholar]
- 49.Ying A.-G., Wang L.-M., Deng H.-X., Chen J.-H., Chen X.-Z., Ye W.-D. Green and efficient aza-Michael additions of aromatic amines to alpha,beta-unsaturated ketones catalyzed by DBU based task-specific ionic liquids without solvent. Arkivoc. 2009:288–298. [Google Scholar]
- 50.Ying A.-G., Liu L., Wu G.-F., Chen X.-Z., Ye W.-D., Chen J.-H., Zhang K.-Y. Knoevenagel condensation catalyzed by DBU brønsted ionic liquid without solvent. Chem. Res. Chin. Univ. 2009;25:876–881. [Google Scholar]
- 51.Ying A.-G., Wang L.-M., Wang L.-L., Chen X.-Z., Ye W.-D. Green and efficient Knoevenagel condensation catalysed by a DBU based ionic liquid in water. J. Chem. Res. 2010:30–33. [Google Scholar]
- 52.Ying A.-G., Chen X.-Z., Wu C.-L., Zheng R.-H., Liang H.-D., Ge C.-H. Novel task-specific ionic liquids as solvent for Michael addition of methylene active compounds to chalcones without any catalyst. Synth. Commun. 2012;42:3455–3462. [Google Scholar]
- 53.Ying A.-G., Zhang Q.-H., Li H.-M., Shen G.-F., Gong W.-Z., He M.-J. An environmentally benign protocol: Catalyst-free Michael addition of aromatic amines to alpha,beta-unsaturated ketones in glycerol. Res. Chem. Intermed. 2013;39:517–525. [Google Scholar]
- 54.Ying A., Xu S., Liu S., Ni Y., Yang J., Wu C. Novel multiple-acidic ionic liquids: Catalysts for environmentally friendly benign synthesis of trans-beta-nitrostyrenes under solvent-free conditions. Ind. Eng. Chem. Res. 2014;53:547–552. [Google Scholar]
- 55.Ying A., Ni Y., Xu S., Liu S., Yang J., Li R. Novel DABCO based ionic liquids: Green and efficient catalysts with dual catalytic roles for aqueous Knoevenagel condensation. Ind. Eng. Chem. Res. 2014;53:5678–5682. [Google Scholar]
- 56.Ying A., Liu S., Ni Y., Qiu F., Xu S., Tang W. Ionic tagged DABCO grafted on magnetic nanoparticles: A water-compatible catalyst for aqueous aza-Michael addition of amines to α,β-unsatuated amides. Catal. Sci. Technol. 2014 doi: 10.1039/C4CY00232F. [DOI] [Google Scholar]
- 57.Gao S., Tsai C.H., Tseng C., Yao C.F. Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tetrahedron. 2008;64:9143–9149. [Google Scholar]
- 58.Banerjee S., Horn A., Khatri H., Sereda G. A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst. Tetrahedron Lett. 2011;52:1878–1881. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.