Abstract
While childhood malnutrition is associated with increased morbidity and mortality, less well understood is how early childhood growth influences height and body composition later in life. We revisited 152 Peruvian children who participated in a birth cohort study between 1995 and 1998, and obtained anthropometric and bioimpedance measurements 11 to 14 years later. We used multivariable regression models to study the effects of childhood anthropometric indices on height and body composition in early adolescence. Each standard deviation decrease in length-for-age at birth was associated with a decrease in adolescent height-for-age of 0.7 SD in both boys and girls (all p<0.001) and 9.7 greater odds of stunting (95% CI 3.3 to 28.6). Each SD decrease in length-for-age in the first 30 months of life was associated with a decrease in adolescent height-for-age of 0.4 in boys and 0.6 standard deviation in girls (all p<0.001) and with 5.8 greater odds of stunting (95% CI 2.6 to 13.5). The effect of weight gain during early childhood on weight in early adolescence was more complex to understand. Weight-for-length at birth and rate of change in weight-for-length in early childhood were positively associated with age- and sex-adjusted body mass index and a greater risk of being overweight in early adolescence. Linear growth retardation in early childhood is a strong determinant of adolescent stature, indicating that, in developing countries, growth failure in height during early childhood persists through early adolescence. Interventions addressing linear growth retardation in childhood are likely to improve adolescent stature and related-health outcomes in adulthood.
Keywords: Stunting, obesity, development origins
Childhood malnutrition is one of the leading causes of mortality and morbidity in low and middle income countries (United Nations, 2000). Stunting, severe wasting, and intrauterine growth restriction combined are responsible for 2.2 million deaths and 21 percent of disability-adjusted life-years lost for children under five years of age (Black et al., 2008). Moreover, it is estimated that 178 million children under five in developing countries are stunted (Black et al., 2008). Malnutrition is a major contributor to the intergenerational poverty plaguing low and middle income countries and it has been identified as an international priority as part of the United Nations Millennium Development Goals (United Nations, 2000).
The long-term consequences of linear growth retardation during childhood on adolescent, adult and intergenerational health outcomes are less well understood and have emerged as a topic of importance to the field of evolutionary biology. Linear growth retardation in utero and during early childhood contributes to shorter adolescent and adult stature (Coly et al., 2006; Billewicz and McGregor, 1982; Sachdev et al., 2005; Victora et al., 2008), increased risk of overweight (Popkin et al., 1996; Law et al., 1992), cognitive impairments (Niehaus et al., 2002; Berkman et al., 2002) and reduced human capital (Victora et al., 2008; Chen and Zhou, 2007). Furthermore, recent evidence suggests that stunting followed by rapid growth in childhood may increase the risk of developing cardiovascular disease (Forsen et al., 2004; Singhal et al., 2004; Mamun et al., 2009; Eriksson et al., 1999), obesity (Mamun et al., 2009, Monteiro et al., 2003), type 2 diabetes mellitus (Barker et al., 2002), and other symptoms of metabolic syndrome in childhood (Taveras et al., 2009) and adulthood (Victora et al., 2008). Investigations into the physiologic basis of these findings have found that while there is no difference in the resting and total energy expenditure between stunted and non-stunted children (Hoffman et al. 2000a), stunted children have lower rates of fat oxidation (Hoffman et al. 2000b), contributing to an increased deposition of fats and increased levels of adiposity later in life (Frisancho 2003; Sawaya et al. 2003). Phenotypic variations in response to prenatal environmental stimuli illustrate an adaptive signaling mechanism from mother to child, indicating a malleable metabolic strategy early in life (Kuzawa and Pike 2005).
Epidemiologic studies that have assessed the contribution of early growth to later stature and body composition have typically based their conclusions using a few anthropometric measurements during childhood (Lucas et al., 1999; Keijzer-Veen et al., 2005). We sought to characterize the effect of longitudinal growth patterns in the first three years of life on height and body composition attained in early adolescence. To achieve this aim, we revisited a birth cohort of Peruvian children who were followed daily for diarrheal surveillance and measured monthly for anthropometric growth in the first 30 months of life.
MATERIALS AND METHODS
Study site
We conducted a follow-up study of a birth cohort of children living in Pampas de San Juan de Miraflores, a peri-urban community (pueblo joven) located 25 kilometers south from the city-center of Lima, Peru. Established in 1965, it remained a sparsely populated shanty town until the 1980's when it began to receive a large and continuous influx of indigenous immigrants from the highlands during the period of political violence and instability in Peru. As is common throughout the developing world, these rural transplants initially relied to a large extent on a grey market of economic production without government support or access to basic utilities. Infection and undernutrition were exceedingly common with high rates of diarrhea and stunting in children (Checkley et al., 2004).
In the last two decades Pampas has undergone many economic and social developments. In 1989, most homes were temporary structures constructed of wooden poles and woven thatch, without water or sewage lines. By 2008, over 75 percent of homes were constructed from brick or cement with in-home water and sewage lines. Recent community development has also decreased the homogeneity in socioeconomic status within the community since the initial follow-up. More recently established residents live higher in the Andean foothills on more steeply sloped land which lacks the infrastructure found in the flatter regions closer to Lima. The community is described in more detail elsewhere (Berkman et al., 2002; Gilman et al., 1993; Checkley et al., 2003).
Original study
The original study took place between February 1995 and December 1998. Women in their third trimester of pregnancy were asked to participate in a study aimed at describing the epidemiology of diarrheal disease in early childhood (Checkley et al., 2002; Checkley et al., 2003; Checkley et al. 2004). In our earlier study, we found that children who were ill with diarrhea for 10% of the time during the first 24 months were 1.5 cm shorter than children who never had diarrhea (Checkley et al., 2003). Follow-up began in the first six months of life and consisted of daily surveillance for diarrheal diseases and monthly anthropometric measurements. We measured length or height to the nearest 0.1 cm with a locally made wooden platform and sliding footboard/headboard and weight to the nearest 0.1 kg with Salter scales (Salter Housewares LTD, Tonbridge, England). We measured length in children who were less than two years of age, and height in those who were two years of age and older. For the purpose of our analysis, however, and to differentiate early childhood length/height measurements from adolescent height, we will refer to any childhood measurements of height as length. We calculated length-for-age (LAZ) and weight-for-length (WLZ) using the 2006 WHO anthropometric growth standards for children (Bloem, 2007).
Follow-up study
Between August 2008 and April 2009, we tracked and revisited participants who were enrolled no later than three months of age and completed at least 12 months of longitudinal follow-up. Of the original cohort, we tracked 152 participants who met these criteria and requested parental written informed consent and child assent to perform a follow-up visit. Follow-up consisted of a socioeconomic survey and measurement in triplicate of weight, height and bioelectrical impedance. We measured height to the nearest 0.1 cm with a locally constructed wooden stadiometer and sliding headboard, and weight to the nearest 0.01 kg with a digital scale (Tanita, Tokyo, Japan). We measured resistance and reactance using a Quantum II Handheld Bioelectrical Impedance Analyzer (RJL Systems, Clinton Township, Michigan, USA). We used the mean of the three values for analysis.
We used the anthropometric data collected during the follow-up study to calculate body mass index (kg/height2), and calculated height-for-age (HAZ) and body mass index-for-age (BAZ) using the 2007 WHO anthropometric reference for children and adolescents (de Onis et al., 2007). We calculated percent body fat, fat free mass and fat mass indices using an aggregate of four separate algorithms validated for children from 9-13 years of age (Houtkooper et al., 1992; Deurenberg et al., 1992; Mellits et al., 1970; Pietrobelli et al., 2003), in a methodology shown to be robust (Wells et al., 2009). We calculated fat and fat-free mass indices by dividing each respective raw mass value by height2 (Wells et al., 2002).
Definitions
We defined the beginning of a diarrheal episode as a day in which the mother indicated the child had diarrhea and the child had passed three or more liquid or semi-liquid stools. We defined the end of a diarrheal episode after recording two consecutive days without diarrhea. We calculated longitudinal prevalence of diarrhea as the number of days of diarrhea divided by the number of days of diarrhea surveillance in the first year of life multiplied by 100. We defined stunting as a LAZ < −2 standard deviations (SD), wasting as a WLZ < −2 SD, and overweight and obesity as a BAZ > 1 SD and > 2 SD, respectively.
Biostatistical methods
We analyzed our data in two stages. In the first stage, we estimated the change for LAZ and WLZ per month of age using a random-effects model (Laird and Ware, 1982), adjusted for sex and the longitudinal prevalence of diarrhea. In exploratory analyses, we found the change in LAZ per month of age to be relatively linear; therefore, we were able to summarize the age-related changes in LAZ using a random intercept (per SD) and a random slope (per SD/30 months). In contrast, the change of WLZ per month of age was not linear. Instead there were two slopes with an inflexion at approximately six months of age. As a result, we estimated the change in WLZ using a random intercept (per SD), and two random slopes, one from 0 to 5 months of age (per SD/6 months), and another from 6 to 30 months of age (per SD/24 months). Subject-specific intercepts were used as proxies for LAZ and WLZ at birth.
In the second stage, we used multiple linear and logistic regression models to assess the contributions of the estimated subject-specific intercept and subject-specific slopes on our outcomes of interest, adjusted for age in early adolescence, sex, maternal education (no education beyond primary school as reference), and number of people per room in 2008 (lowest two tertiles as reference). Maternal education serves as a proxy for socioeconomic status in infancy as more educated women tend to have healthier babies (Raum et al. 2001). Number of people per room, a measure of residential crowding, has been found to be a proxy of household income (Evans and Kantrowitz, 2002; Raum et al., 2001). We did not include the longitudinal prevalence of diarrhea in this stage because it was included in the first stage. Outcomes of interest in 2008 were HAZ, BAZ, BMI, fat mass index, and fat free mass index, and stunted and overweight status.
We conducted our analyses in STATA (Stata Corp., College Station, TX) and R (www.r-project.org).
Ethics
The original study was approved by the Internal Review Boards of A.B. PRISMA, Lima, Peru, and the Johns Hopkins School of Public Health, Baltimore, MD. The follow-up study was approved by the Internal Review Boards of A.B. PRISMA, Lima, Peru. Planning and implementation of this study was conducted without a conflict of interest.
RESULTS
Cohort characteristics
Of the 333 individuals invited to participate in the original cohort, 196 met the inclusion criteria for follow-up. We were able to contact 156 (80%) of these individuals of whom 152 (78%) completed follow-up (Figure 1). We did not find differences in early growth in height (p=0.82), weight (p=0.53), maternal height (p=0.12), the percentage of households without sanitation facilities (p=0.34) water connection (p=0.27) or in the house flooring material (p = 0.60) between the 152 participants included in the follow-up and the 44 (22%) participants of the original cohort that met inclusion criteria but who did not participate in this follow-up study.
Figure 1.
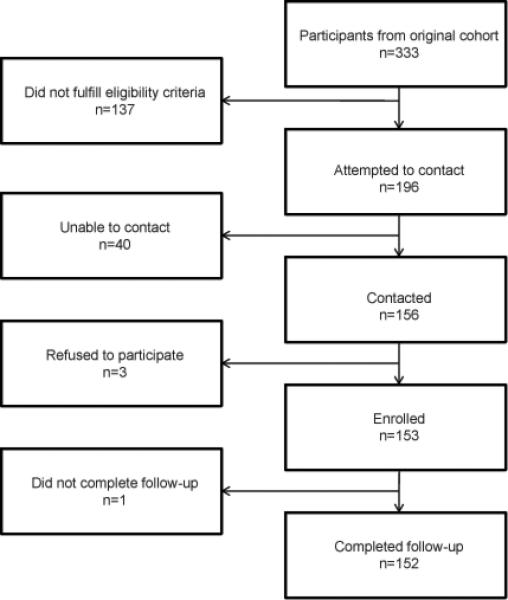
Follow-up of participants from original cohort.
We display baseline characteristics of the participants stratified by sex in Table 1. The mean age of entry into the original study was 13.9 days (range 0 to 54 days). There were no cases of early childhood wasting. In 2008, we found no differences in age, height, body mass index for age Z score (BAZ) or socioeconomic indicators between the sexes (Table 1); however boys had higher mean fat free mass index than did girls. Girls had higher relative mean weight, fat mass index and percent body fat than did boys. At the time of follow-up, mean height-for-age Z score (HAZ) was −1.0 SD in boys and −1.1 SD in girls, and the prevalence of stunting was 8.1% and 19.4%, respectively.
Table 1.
Baseline characteristics of adolescent participants at follow-up; 2008-2009, Lima, Peru.
| Total | Male | Female | p-value | |
|---|---|---|---|---|
| Sample size | 152 | 85 | 67 | |
| Age in years (SD) | 12.5 (0.8) | 12.5 (0.8) | 12.6 (0.7) | 0.41 |
| Mean height-for-age score (SD) | −1.0 (0.9) | −1.0 (0.9) | −1.1 (0.9) | 0.72 |
| Height-for-age < −1, % (SD) | 50.7 (77) | 51.8 (44) | 49.3 (33) | 0.76 |
| Stunted (Height-for-age < −2), % (n) | 13.1% (20) | 8.1 (7) | 19.4 (13) | 0.04 |
| Mean body mass for index score (SD) | 0.6 (1.1) | 0.5 (1.2) | 0.7 (1.0) | 0.37 |
| Overweight (Body mass index-for age≥1), % (n) | 34.8 (53) | 28.2 (24) | 43.3 (29) | 0.07 |
| Obese (Body mass index-for age≥2), % (n) | 11.1 (17) | 13.95 (12) | 7.5 (5) | 0.21 |
| Fat Mass Index (SD) | 5.4 (2.4) | 4.7 (2.1) | 6.3 (2.5) | <0.01 |
| Fat Free Mass Index (SD) | 14.8 (1.3) | 15.0 (1.4) | 14.5 (0.9) | 0.02 |
| Percent Body Fat % (SD) | 25.6 (7.7) | 22.7 (6.6) | 29.2 (7.5) | <0.01 |
| Mean age of entry into study, days | 13.9 (14.6) | 15.0 (15.4) | 12.2 (13.6) | 0.26 |
| Educationally-deprived mother, % (n) | 69.3 (106) | 72.1 (62) | 66.0 (44) | 0.40 |
| Repeated a grade, % (n) | 26.8 (41) | 22.1 (19) | 32.8 (22) | 0.14 |
| Upper tertile of people per room % (n) | 30.1 (46) | 30.2 (26) | 29.9 (20) | 0.96 |
| In home water % (n) | 86.9 (133) | 87.2 (75) | 86.6 (58) | 0.92 |
| In home sewage % (n) | 88.2 (135) | 89.5 (77) | 88.6 (58) | 0.61 |
p values derived from linear regression comparing difference in prevalence of each measurement by gender.
Longitudinal growth patterns during early childhood
24% of participants entered the study at less than one month of age, and 71% entered at less than two months of age. All participants were still in the study at 12 months of age, and 72% were still available for follow-up at 2 years. There were no significant differences by sex in the rate of change of length-for-age from birth through 30 months of age. At birth, 71% of boys and 70% of girls were between −1 SD and 1 SD in LAZ. LAZ decreased linearly with age, with an average rate of change in LAZ from 0 to 30 months of −1.8 SD and −1.6 SD in boys and girls, respectively. In contrast, average weight-for-length Z score (WLZ) values showed greater sex-based variation. Boys had greater birth WLZ, higher rates of change in WLZ in the first six months of life, and decreased more in WLZ from 6 to 30 months of age. At birth, 67.1% of boys and 47.8% of girls had WLZ values between −1 SD and 1 SD. Mean WLZ in the first six months of life increased by 0.5 SD for boys and 0.3 SD for girls; and mean WLZ between 6 and 30 months of age decreased by 0.2 SD for both boys and girls.
Effects of growth during early childhood on adolescent height
Individuals who were shorter at birth and who grew less in length during early childhood were more likely to be stunted in early adolescence. Both LAZ at birth and the rate of change in LAZ per month of age during early childhood were approximately linear with adolescent HAZ (Figure 2). Specifically, each 1 SD decrease in LAZ at birth was associated with a decrease in adolescent HAZ of 0.7 SD in both boys and girls (Table 2; all p<0.001); and, each 1 SD decrease in LAZ in the first 30 months of life was associated with an decrease in adolescent HAZ of 0.4 SD in boys and 0.6 SD in girls (Table 2; all p<0.001). Coefficients of determination for the association between childhood linear growth and adolescent height ranged from 60% in boys to 66% in girls. Children with a lower LAZ at birth and those with a lower or more negative rate of change in LAZ during early childhood had significantly increased odds of being stunted in early adolescence, even after adjusting for socioeconomic status (Table 3). Since we did not find an interaction effect between sex and either LAZ at birth or the rate of change in LAZ (p=0.17), combined regression analyses revealed that each SD decrease in LAZ at birth was associated with a 9.7 greater odds of stunting (95% CI 3.3 to 28.6) and each SD decrease in LAZ in the first 30 months of life was associated with 5.8 greater odds of stunting (95% CI 2.6 to 13.5). We did not find associations between either WLZ at birth or the rate of change in WLZ from 6-30 months on adolescent HAZ (Table 2) or on the risk of stunting in early adolescence (Table 3). Coefficients of determination for the association between childhood ponderal growth and adolescent height ranged from 25% in girls to 26% in boys.
Figure 2.
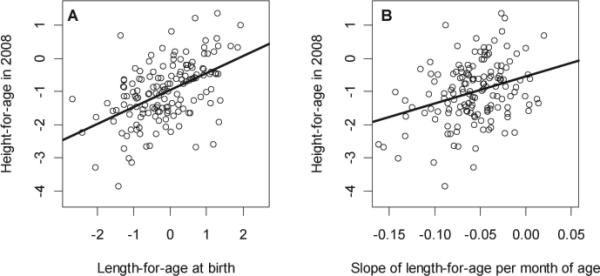
(a) Scatter plot of (a) length-for-age intercept and (b) rate of change in length-for-age during early childhood, by adolescent height-for-age in 2008; 2008-2009, Lima, Peru.
Table 2.
Effects of growth parameters in early childhood on adolescent height-for-age and body mass for index for age; 2008-2009, Lima, Peru.
| Height-for-age (95% CI) | p | Body mass index for age (95% CI) | p | |
|---|---|---|---|---|
| Boys | ||||
| Length-for-age at birth (per SD) | 0.7 (0.6 to 0.9) | <0.001 | 0.3 (0.1 to 0.6) | 0.02 |
| Change in length-for-age 0-29 mo (per SD / 30 mo) | 0.4 (0.3 to 0.5) | <0.001 | 0.3 (0.1 to 0.5) | 0.01 |
| Weight-for-length at birth (per SD) | 0.3 (−0.0 to 0.6) | 0.07 | 0.9 (0.6 to 1.3) | <0.001 |
| Change in weight-for-length 0-5 months (per SD / 6 mo) | 0.5 (0.2 to 0.8) | <0.01 | 0.3 (0.1 to 0.4) | <0.001 |
| Change in weight-for-length 6-30 months (per SD / 24 mo) | 0.1 (−0.2 to 0.4) | 0.58 | 0.5 (0.1 to 0.8) | 0.02 |
| Girls | ||||
| Length-for-age at birth (per SD) | 0.7 (0.6 to 0.9) | <0.001 | 0.2 (−0.1 to 0.5) | 0.14 |
| Change in length-for-age 0-29 mo (per SD / 30 mo) | 0.6 (0.5 to 0.8) | <0.001 | 0.2 (−0.1 to 0.5) | 0.24 |
| Weight-for-length at birth (per SD) | 0.1 (−0.3 to 0.4) | 0.61 | 0.6 (0.2 to 1.0) | <0.01 |
| Change in weight-for-length 0-5 months (per SD / 6 mo) | 0.4 (0.1 to 0.7) | 0.01 | 0.3 (0.1 to 0.5) | 0.001 |
| Change in weight-for-length 6-30 months (per SD / 24 mo) | 0.1 (−0.3 to 0.4) | 0.77 | 0.5 (0.1 to 1.0) | 0.02 |
Table 3.
Log odds ratio of adolescent stunting and overweight status by estimated LAZ and WLZ values at birth, and rate of change in LAZ and WLZ in early childhood; 2008-2009, Lima, Peru.
| Log odds ratio (95% CI) | ||||
|---|---|---|---|---|
| Stunted | p | Overweight | p | |
| Boys | ||||
| Length-for-age at birth (per SD) | −1.9 (−3.5 to −0.3) | 0.02 | 0.7 (−0.02 to 1.4) | 0.06 |
| Change in length-for-age 0-29 mo (per SD/30 mo) | −1.2 (−2.1 to −0.2) | 0.02 | 0.5 (0.02 to 1.1) | 0.04 |
| Weight-for-length at birth (per SD) | −0.02 (−1.9 to 1.8) | 0.43 | 1.8 (0.5 to 3.1) | <0.01 |
| Change in weight-for-length 0-5 mo (per SD/6 mo) | −1.2 (−3.1 to 0.6) | 0.19 | 1.7 (0.6 to 2.9) | <0.01 |
| Change in weight-for-length 6-29 mo (per SD/24 mo) | 0.4 (−1.6 to 2.3) | 0.37 | 0.5 (−0.6 to 1.6) | 0.38 |
| Girls | ||||
| Length-for-age at birth (per SD) | −3.6 (−5.8 to −1.3) | <0.01 | 0.6 (−0.04 to 1.2) | 0.07 |
| Change in length-for-age 0-29 mo (per SD/30 mo) | −3.4 (−5.5 to −1.2) | <0.01 | 0.4 (−0.2 to 1.0) | 0.20 |
| Weight-for-length at birth (per SD) | −0.5 (−1.7 to 0.7) | 0.40 | 1.0 (−0.04 to 2.0) | 0.06 |
| Change in weight-for-length 0-5 mo (per SD/6 mo) | −0.9 (−2.1 to 0.3) | 0.15 | 1.3 (0.3 to 2.3) | <0.01 |
| Change in weight-for-length 6-29 mo (per SD/24 mo) | 0.02 (−1.2 to 1.3) | 0.97 | 0.8 (−0.3 to 1.9) | 0.15 |
We display patterns of longitudinal growth during early childhood stratified by adolescent HAZ tertiles (Figure 3A) and adolescent stunted status (Figure 3B). Specifically, stunted adolescents and those in the lowest tertile of adolescent HAZ had lower LAZ values throughout early childhood when compared with non-stunted individuals and those in the upper tertile of adolescent HAZ. This effect was even greater in girls. Our findings strongly suggest that height deficits accumulate over childhood and persist into early adolescence. Among 27 participants who were stunted at 12 months of age, 11 (40.7%) remained stunted in early adolescence, while of 125 individuals who were not stunted at 12 months, only nine (7.2%) were stunted at follow-up.
Figure 3.
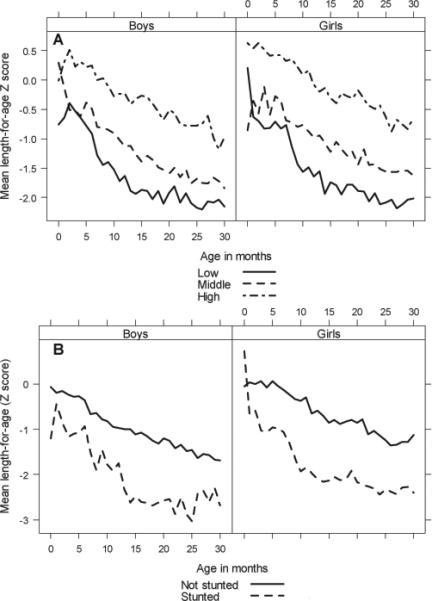
(a) Mean LAZ of subjects in early childhood by adolescent HAZ tertile. (b) Mean LAZ of subjects in early childhood by stunting adolescent status. Both stratified by sex.
Effects of growth during early childhood on adolescent weight and body composition
The effects of growth during early childhood on adolescent weight and body composition were more complex to understand. Both LAZ at birth and change in LAZ during early childhood were positively associated with fat free mass index in boys but not in girls (Table 4). In girls, each SD increase in LAZ at birth was associated with an increase of 0.7 kg/m2 in fat mass index (p=0.03) and an increase of 2.1% (p=0.02) in percent body fat.
Table 4.
Adolescent body composition outcomes by estimated length-for-age and weight-for-length values at birth, and slope of change in length-for-age and weight-for-length during early childhood; 2008-2009, Lima, Peru.
| Fat mass index (95% CI) | p | Fat free mass index (95% CI) | p | % Body Fat (95% CI) | p | |
|---|---|---|---|---|---|---|
| Boys | ||||||
| Length-for-age at birth (per SD) | 0.5 (−0.0 to 1.0) | 0.06 | 0.5 (0.1 to 0.9) | 0.01 | 1.3 (−0.3 to 2.9) | 0.11 |
| Change in length-for-age 0-29 mo (per SD/30 mo) | 0.3 (−0.0 to 0.7) | 0.07 | 0.4 (0.1 to 0.7) | <0.01 | 0.7 (−0.4 to 1.8) | 0.19 |
| Weight-for-length at birth (per SD) | 1.0 (0.2 to 1.7) | <0.01 | 1.0 (0.5 to 1.6) | <0.001 | 3.0 (0.9 to 5.1) | <0.01 |
| Change in weight-for-length 0-5 months (per SD/6 mo) | 1.2 (0.6 to 1.9) | <0.001 | 1.1 (0.6 to 1.5) | <0.001 | 3.8 (1.9 to 5.7) | <0.001 |
| Change in weight-for-length 6-30 months (per SD/24 mo) | 0.3 (−0.4 to 1.0) | 0.42 | 0.6 (0.1 to 1.1) | 0.03 | 0.7 (−1.4 to 2.8) | 0.51 |
| Girls | ||||||
| Length-for-age at birth (per SD) | 0.7 (0.1 to 1.3) | 0.03 | 0.1 (−0.2 to 0.4) | 0.58 | 2.1 (0.4 to 3.8) | 0.02 |
| Change in length-for-age 0-29 mo (per SD/30 mo) | 0.5 (−0.1 to 1.1) | 0.13 | 0.1 (−0.2 to 0.5) | 0.50 | 1.4 (−0.3 to 3.2) | 0.11 |
| Weight-for-length at birth (per SD) | 1.0 (0.1 to 1.9) | 0.03 | 0.7 (0.3 to 1.2) | <0.01 | 2.8 (0.3 to 5.3) | 0.03 |
| Change in weight-for-length 0-5 months (per SD/6 mo) | 1.1 (0.3 to 1.9) | 0.01 | 0.7 (0.2 to 1.1) | <0.01 | 3.2 (1.0 to 5.5) | 0.01 |
| Change in weight-for-length 6-30 months (per SD/24 mo) | 1.0 (0.1 to 2.0) | 0.04 | 0.5 (−0.0 to 1.0) | 0.06 | 3.2 (0.4 to 5.9) | 0.03 |
The influence of WLZ at birth and during early childhood on BAZ in early adolescence was less clear (Figure 4). First, we found that the effect of changes in WLZ on adolescent body composition differed by sex. While the rate of change in WLZ from 0 to five months was a significant predictor of adolescent BAZ in both sexes, it was also an important predictor of adiposity in girls and fat free mass index in boys (Table 2). Increased rate of change in WLZ from six to 30 months was associated with increased BAZ and percent body fat in females, and greater fat free mass index in males (Table 2). This effect, however, was of a lower magnitude than that observed with the change in WLZ from birth to five months of age.
Figure 4.
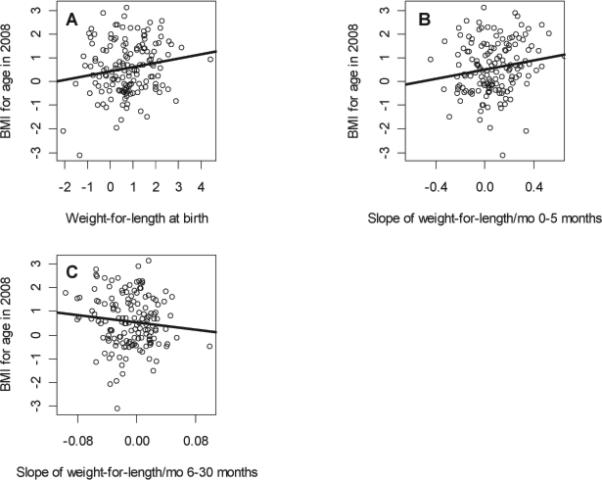
Scatter plot of (a) weight-for-length intercept, (b) weight-for-length slope 0-5 months during early childhood, and (c) weight-for-length slope 6-30 months during early childhood, by adolescent body mass index for age in 2008; 2008-2009.
In Figure 5, we summarize the relationship between WLZ in infancy by BAZ tertiles and overweight status in early adolescence. Individuals who were overweight or in the highest tertile of BAZ in early adolescence had markedly higher WLZ values during early childhood compared to adolescents who were not overweight or were in the lowest tertile of BAZ at follow-up. These differences show sex-based variability and appear to be more pronounced in males. In Figure 6, we show weight parameters in early childhood stratified by fat free mass index, fat mass index and percent body fat tertiles in early adolescence. Higher fat free mass levels in early adolescence appear to be associated with more rapid growth in both weight and height during early childhood in boys but not in girls. Conversely, greater adolescent fat mass levels appeared to be associated with more rapid weight gain in girls but not in boys. This indicates that more rapid weight gain in infancy in boys leads to higher muscle mass in early adolescence, whereas this association appears to be absent in girls. In logistic regression analysis, we found that the rate of change in WLZ from 0 to 5 months of age was the strongest predictor of adolescent overweight status in both boys (OR=5.6, 95% CI 1.8 to 17.7) and girls (OR=3.6, 95% CI 1.4 to 9.7).
Figure 5.
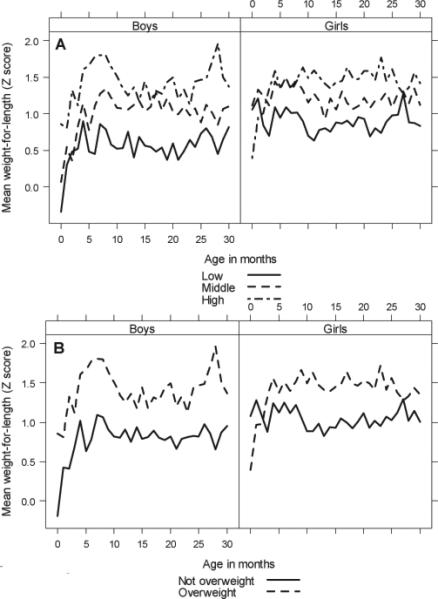
(a) Mean WLZ in infancy by adolescent BAZ tertiles. (b) Mean WLZ in during early childhood by adolescent overweight status. Both stratified by sex.
Figure 6.
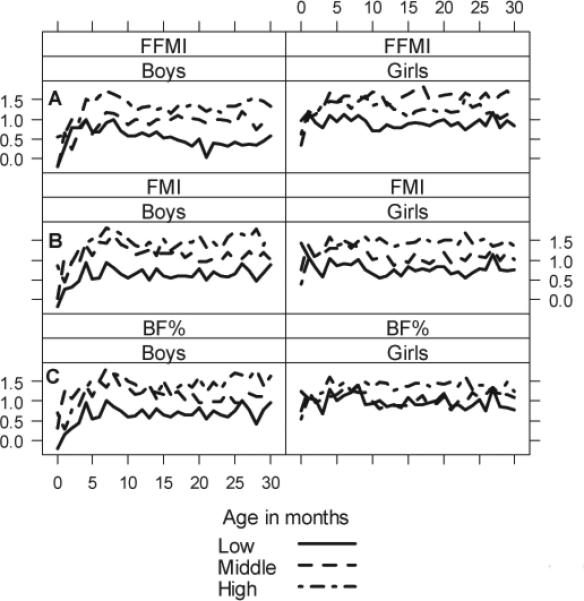
Mean WLZ in early childhood by (a) percent body fat tertiles, (b) Fat mass index tertiles, (c) Fat free mass index tertiles. Stratified by sex.
DISCUSSION
In this longitudinal study of a birth cohort of Peruvian children, we found that length-for-age at birth and the rate of change in length-for-age in early childhood were strong predictors of adolescent stature. Our data indicates that linear growth retardation early in life is a relatively fixed process throughout early adolescence in this population. It further suggests that, in developing countries, children who are short at birth do not catch-up in length as children and have a shorter stature in early adolescence. This effect was consistent in both boys and girls. Furthermore, there was no single window of time during early childhood in which growth in length had a greater impact on height at early adolescence. Therefore, in developing countries, growth in length early in childhood is critical in propelling an individual to reach their full height potential and any linear growth retardation in early childhood manifests in near permanent deficits in height in early adolescence.
We used a novel analytical approach that combined linear splines and random-effects to model the separate contributions of growth in height and weight during early childhood on adolescent stature and body composition. Our finding of a positive association between linear growth retardation in early childhood and stunted stature in early adolescence is consistent with observations in other developing countries (Coly et al., 2006; Billewicz et al., 1982; Sachdev et al., 2005; Martorell et al., 1995). On the other hand, we found that the effect of weight gain during early childhood on weight in early adolescence was more complex to understand. Specifically, rate of change in weight-for-length in early childhood were positively associated with age- and sex-adjusted body mass index and a greater risk of being overweight in early adolescence. Our findings are consistent with data from other studies of populations in both developing and developed countries (Sachdev et al., 2005; Victora et al., 2008; Taveras et al., 2009; Adair et al., 2009; Corvalan et al., 2007; Law et al., 2002; Victora et al., 2007; Baird et al., 2005). This increased risk of being overweight in early adolescence, in turn increases the risk of becoming overweight and obese in adulthood (Guo et al., 2002).
However, body mass index is a poor measure of body composition as it overestimates fatness in muscular individuals (Rothman, 2008). When we assessed the independent contributions of fat free mass and adiposity to total adolescent body composition, we found that an increased rate of change in weight-for-length during early childhood predicted a greater relative accumulation of lean mass in males and adiposity in females. These findings are consistent with those observed in Brazil and Guatemala (Corvalan et al., 2007; Victora et al., 2007; Gigante et al., 2007; Li et al., 2003; Wells et al., 2005) and may indicate sex-dependent variation in the developmental determinants of body composition.
Boys are known to grow more rapidly in utero than girls (Lubchenco et al., 1963; Pedersen, 1980), leaving less reserve energy in the placenta and increasing the risk of intrauterine undernutrition and subsequent compensatory catch-up growth should the mother not access sufficient nutrients during gestation (Lampl et al., 2010; Eriksson et al., 2010). This may help explain the increased prevalence of hypertension and other conditions associated with early undernutrition in males (Eriksson et al., 2010). If this hypothesis is correct we would expect males to benefit more than females from catch-up growth early in development. Our finding of increased rate of weight gain in early childhood associating with increased lean mass in early adolescence, in males but not females, support this hypothesis. In Guatemala male children who were stunted in early childhood had less lean mass in adulthood, while early childhood stunting in females associated with decreases in both lean and fat mass (Li et al., 2003). In addition to sex-based variability in response to growth insults during gestation and infancy, these growth patterns indicate differences in how best to interpret growth markers in populations with a high versus low prevalence of undernutrition, differences which need to be carefully explored given the potential intergenerational consequences of stunting in females (Kuzawa, 2007). Furthermore, among stunted infants, distinction must be made between growth indicating regression to the mean and true catch-up growth (Cameron et al., 2005).
We considered various potential shortcomings in our analysis. While other studies that have examined the developmental origins of disease have had access to larger samples (Sachdev et al., 2005; Monteiro et al., Barker et al., 2005; Adair and Cole, 2003), few other longitudinal studies of early growth outcomes have had an equivalent frequency of serial measurement follow-up early in life, which afforded us enough power to find effects of early childhood growth on adolescent height using the methodology described above. A similar methodology was used in the Barry Caerphilly Growth Study conducted in Wales, United Kingdom (McCarthy et al., 2007); however, they relied on three-month long intervals between measurements. Additionally, emerging evidence indicates infant growth patterns and associated adult health outcomes may differ between populations in developed and developing countries due to nutrition, environmental exposures and physical activity (Popkin, 1998, 2001), limiting the generalizability of their findings to developing populations.
Another limiting factor was the timing of follow-up occurred near the onset of puberty. Unfortunately we did not collect information on pubertal staging which may temper our conclusions, limiting them to the early-adolescent period. During early adolescence, males and females are known to experience significant increases in fat free and fat mass, respectively (Loomba-Albrecht and Styne, 2009). Differences in the timing of the growth spurt could be a major potential confounding factor in interpreting results; however, while it is likely some portion of our sample was undergoing their natural pubertal development, it is possible that some individuals advanced their timing of menarche (Vink et al., 2010) following increased compensatory growth velocity in early childhood, and thus the onset of puberty and fat accumulation in females,. Therefore, the degree to which increased rate of growth in early childhood may predict fat mass may be exaggerated by measuring fat mass at puberty. Stunting has been shown to be associated with delayed maturation in several developing country populations. Thus, that children with low length-for-age in early childhood that are shorter than other children at puberty onset might be due to either a permanent deficit in stature or to a greater pubertal delay than other children and thus to a delayed growth spurt in height. Pubertal maturation also has important impacts on fat mass and fat free mass. Finally, while the algorithms used to calculate bioimpedance were not validated for this population, Wells et al. showed aggregate calculations to be a robust substitute. It should be noted that a recently published reference curve for adiposity in a Caucasian population places both genders mean body fat percentage above the 85th percentile in adiposity (McCarthy et al. 2006). Thus it is possible that the bioimpedance calculation overestimated adiposity, although just as likely that the reference curve underestimated normal levels of adiposity in this particular population.
In developing countries undergoing rapid economic development, the prevalence of overweight and obesity is increasing dramatically while infectious disease and malnutrition remain as major health burdens (Monteiro et al., 2003; Popkin, 2001; Uuay et al., 2001). Within these societies, addressing malnutrition early in development is proving increasingly complex, as the potentially deleterious effects of rapid weight gain in infancy must be weighed against the short (Pelletier et al., 1993) and long term (Martorell et al., 2010) benefits of nutritional supplementation in underweight infants. In this study we found the risks associated with rapid growth early in development to be unclear. The dangerous consequence of limiting rapid compensatory growth was diminished growth early in life, which was strongly associated with short adolescent height. Our findings are consistent with those of a recent multi-country study (Stein et al., 2010), and contribute to the field of evolutionary biology. Decreased adult stature has been associated with a host of adverse outcomes, including: increased risk of respiratory morbidity (Leon et al., 1995), cardiovascular morbidity (Davey Smith et al., 2000; Rosenberg et al., 1995), decreased income (Victora et al., 2008), increased offspring morbidity, underweight, and stunting (Ozaltin et al., 2010; Subramanian et al., 2009). Our finding that poor growth early in life contributes to later phenotypic variation, indicates an evolutionary stress response on the organism level with apparent intergenerational effects (Frisancho, 2009).
In summary, our data lends support to the notion that linear growth retardation in early childhood is a fixed process and that height deficits accumulated during childhood appear very difficult to recover and persist in early adolescence as stunted stature. Therefore, interventions aiming to reduce the risk of linear growth retardation and related health outcomes must focus on the prenatal period and the first three years of life with programs such as nutritional supplementation, infectious disease control, improved access to water and sanitation, and micronutrient interventions.
Acknowledgments
Grant support: The original study was funded by an International Centers for Tropical Disease Research (ICTDR) grant from the National Institutes of Allergy and Infectious Diseases awarded to the Johns Hopkins Bloomberg School of Public Health (U01-A135894). Jaime Miranda, Robert Gilman and William Checkley were supported by a contract (HHSN268200900033C) with the National Heart, Lung and Blood Institute, National Institutes of Health. William Checkley was further supported by a Clinician Scientist Award from the Johns Hopkins University and a K99/R00 Pathway to Independence Award (K99HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health. Robie Sterling was supported by a pre-doctoral T35 Training Grant (T35AI065385) of the National Institutes of Health.
Footnotes
Statement of Disclosure: We have no conflicts of interest to disclose.
Author contributions: R.S., J.M, R.G and W.C designed research; R.S., J.M and W.C. conducted research; R.S. and W.C. analyzed data; all authors participated in writing the manuscript; and, W.C. had primary responsibility for final content. All authors read and approved the final manuscript.
LITERATURE CITED
- Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41:451–456. doi: 10.1161/01.HYP.0000054212.23528.B2. [DOI] [PubMed] [Google Scholar]
- Adair LS, Martorell R, Stein AD. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: When does weight gain matter? Am J Clin Nutr. 2009;89:1383–1392. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- Billewicz WZ, McGregor IA. A birth-to-maturity longitudinal study of heights and weights in two West African (Gambian) villages, 1951-1975. Ann Hum Biol. 1982;9:309–320. doi: 10.1080/03014468200005811. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Bloem M. The 2006 WHO child growth standards. BMJ. 2007;334:705–706. doi: 10.1136/bmj.39155.658843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Preece MA, Cole TJ. Catch-up growth or regression to the mean? Recovery from stunting revisited. Am J Hum Biol. 2005;17:412–417. doi: 10.1002/ajhb.20408. [DOI] [PubMed] [Google Scholar]
- Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–175. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- Checkley W, Gilman RH, Black RE, Lescano AG, Cabrera L, Taylor DN, Moulton LH. Effects of nutritional status on diarrhea in Peruvian children. J Pediatr. 2002;140:210–218. doi: 10.1067/mpd.2002.121820. [DOI] [PubMed] [Google Scholar]
- Checkley W, Gilman RH, Black RE, Epstein LD, Cabrera L, Sterling CR, Moulton LH. Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet. 2004;363:112–118. doi: 10.1016/S0140-6736(03)15261-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou LA. The long-term health and economic consequences of the 1959-1961 famine in China. J Health Econ. 2007;26:659–681. doi: 10.1016/j.jhealeco.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Coly AN, Milet J, Diallo A, et al. Preschool stunting, adolescent migration, catch-up growth, and adult height in young Senegalese men and women of rural origin. J Nutr. 2006;136:2412–2420. doi: 10.1093/jn/136.9.2412. [DOI] [PubMed] [Google Scholar]
- Corvalan C, Gregory CO, Ramirez-Zea M, Martorell R, Stein AD. Size at birth, infant, early and later childhood growth and adult body composition: a prospective study in a stunted population. Int J Epidemiol. 2007;36:550–557. doi: 10.1093/ije/dym010. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Hart C, Upton M, Hole D, Gillis C, Watt G, Hawthorne V. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Commun Health. 2000;54:97–103. doi: 10.1136/jech.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1992;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsén T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–5. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- Forsen T, Osmond C, Eriksson JG, Barker DJ. Growth of girls who later develop coronary heart disease. Heart. 2004;90:20–24. doi: 10.1136/heart.90.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho AR. Reduced rate of fat oxidation: a metabolic pathway to obesity in the developing nations. Am J Hum Biol. 2003;15:522–532. doi: 10.1002/ajhb.10191. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Developmental adaptation: where we go from here. Am J Hum Biol. 2009;21:694–703. doi: 10.1002/ajhb.20891. [DOI] [PubMed] [Google Scholar]
- Gigante DP, Victora CG, Horta BL, Lima RC. Undernutrition in early life and body composition of adolescent males from a birth cohort study. Br J Nutr. 2007;97:949–954. doi: 10.1017/S0007114507433025. [DOI] [PubMed] [Google Scholar]
- Gilman RH, Marquis GS, Ventura G, Campos M, Spira W, Diaz F. Water cost and availability: key determinants of family hygiene in a Peruvian shantytown. Am J Public Health. 1993;83:1554–1558. doi: 10.2105/ajph.83.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Sawaya AL, Coward WA, Wright A, Martins PA, de Nascimento C, Tucker KL, Roberts SB. Energy expenditure of stunted and nonstunted boys and girls living in the shantytowns of Sao Paulo, Brazil. Am J Clin Nutr. 2000a;72:1025–1031. doi: 10.1093/ajcn/72.4.1025. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2000b;72:702–707. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- Houtkooper LB, Going SB, Lohman TG, Roche AF, Van Loan M. Bioelectrical impedance estimation of fat-free body mass in children and youth: a cross-validation study. J Appl Physiol. 1992;72:366–373. doi: 10.1152/jappl.1992.72.1.366. [DOI] [PubMed] [Google Scholar]
- Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–1324. doi: 10.1016/j.jclinepi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. Developmental origins of life history: growth, productivity, and reproduction. Am J Hum Biol. 2007;19:654–661. doi: 10.1002/ajhb.20659. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Pike IL. Introduction. Fetal origins of developmental plasticity. Am J Hum Biol. 2005;17:1–4. doi: 10.1002/ajhb.20090. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lampl M, Gotsch F, Kusanovic JP, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22:431–43. doi: 10.1002/ajhb.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Commun Health. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CM, Shiell AW, Newsome CA, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- Leon DA, Smith GD, Shipley M, Strachan D. Adult height and mortality in London: early life, socioeconomic confounding, or shrinkage? J Epidemiol Commun Health. 1995;49:5–9. doi: 10.1136/jech.49.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr. 2003;77:1498–1505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. BMJ. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- Martorell R, Schroeder DG, Rivera JA, Kaplowitz HJ. Patterns of linear growth in rural Guatemalan adolescents and children. J Nutr. 1995;125(Suppl 4):1060S–1067S. doi: 10.1093/jn/125.suppl_4.1060S. [DOI] [PubMed] [Google Scholar]
- Martorell R, Melgar P, Maluccio JA, Stein AD, Rivera JA. The nutrition intervention improved adult human capital and economic productivity. J Nutr. 2010;140:411–414. doi: 10.3945/jn.109.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86:907–13. doi: 10.1093/ajcn/86.4.907. [DOI] [PubMed] [Google Scholar]
- McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes (Lond) 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- Mellits ED, Cheek DB. The assessment of body water and fatness from infancy to adulthood. Monogr Soc Res Child Dev. 1970;35:12–26. [PubMed] [Google Scholar]
- Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27:1274–1282. doi: 10.1038/sj.ijo.0802409. [DOI] [PubMed] [Google Scholar]
- Niehaus MD, Moore SR, Patrick PD, et al. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- Ozaltin E, Hill K, Subramanian SV. Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA. 2010;303:1507–1516. doi: 10.1001/jama.2010.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JF. Ultrasound evidence of sexual difference in fetal size in first trimester. Br Med J. 1980;281:1253. doi: 10.1136/bmj.281.6250.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier DL, Frongillo EA, Jr., Habicht JP. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am J Public Health. 1993;83:1130–1133. doi: 10.2105/ajph.83.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobelli A, Andreoli A, Cervelli V, Carbonelli MG, Peroni DG, De Lorenzo A. Predicting fat-free mass in children using bioimpedance analysis. Acta Diabetol 40 Suppl. 2003;1:S212–215. doi: 10.1007/s00592-003-0069-z. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Richards MK, Montiero CA. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr. 1996;126:3009–3016. doi: 10.1093/jn/126.12.3009. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998;1:5–21. doi: 10.1079/phn19980004. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- Raum E, Arabin B, Schlaud M, Walter U, Schwartz FW. The impact of maternal education on intrauterine growth: a comparison of former West and East Germany. Int J Epidemiol. 2001;30:81–87. doi: 10.1093/ije/30.1.81. [DOI] [PubMed] [Google Scholar]
- Rosenberg CR, Shore RE, Pasternack BS. Height and mortality after myocardial infarction. J Commun Health. 1995;20:335–343. doi: 10.1007/BF02283058. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes 32 Suppl. 2008;3:S56–59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- Sawaya AL, Martins P, Hoffman D, Roberts SB. The link between childhood undernutrition and risk of chronic diseases in adulthood: a case study of Brazil. Nutr Rev. 2003;61(5 Pt 1):168–175. doi: 10.1301/nr.2003.may.168-175. [DOI] [PubMed] [Google Scholar]
- Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long-term cardiovascular health? Circulation. 2004;109:1108–1113. doi: 10.1161/01.CIR.0000118500.23649.DF. [DOI] [PubMed] [Google Scholar]
- Stein AD, Wang M, Martorell R, Norris SA, Adair LS, Bas I, Sachdev HS, Bhargava SK, Fall CH, Gigante DP, Victora CG, Cohorts Group Growth patterns in early childhood and final attained stature: data from five birth cohorts from low- and middle-income countries. Am J Hum Biol. 2010;22:353–9. doi: 10.1002/ajhb.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SV, Ackerson LK, Davey Smith G, John NA. Association of maternal height with child mortality, anthropometric failure, and anemia in India. JAMA. 2009;301:1691–1701. doi: 10.1001/jama.2009.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R, Albala C, Kain J. Obesity trends in Latin America: transiting from under- to overweight. J Nutr. 2001;131:893S–899S. doi: 10.1093/jn/131.3.893S. [DOI] [PubMed] [Google Scholar]
- United Nations United Nations Millennium Declaration. 8th Plenary Meeting.; United Nations, New York. 2000. A/55/49. [Google Scholar]
- Victora CG, Sibbritt D, Horta BL, Lima RC, Cole T, Wells J. Weight gain in childhood and body composition at 18 years of age in Brazilian males. Acta Paediatr. 2007;96:296–300. doi: 10.1111/j.1651-2227.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink EE, van Coeverden SC, van Mil EG, Felius BA, van Leerdam FJ, Delemarre-van de Waal HA. Changes and tracking of fat mass in pubertal girls. Obesity (Silver Spring) 2010;18:1247–1251. doi: 10.1038/oby.2009.366. [DOI] [PubMed] [Google Scholar]
- Wells JC, Cole TJ, ALSPAC study team Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- Wells JC, Hallal PC, Wright A, Singhal A, Victora CG. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes. 2005;29:1192–1198. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- Wells JC, Williams JE, Haroun D, Fewtrell MS, Colantuoni A, Siervo M. Aggregate predictions improve accuracy when calculating metabolic variables used to guide treatment. Am J Clin Nutr. 2009;89:491–499. doi: 10.3945/ajcn.2008.26629. [DOI] [PubMed] [Google Scholar]


