Abstract
Ablation of Mig-6 in the murine uterus leads to the development of endometrial hyperplasia and estrogen-induced endometrial cancer. An additional endometrial cancer mouse model is generated by ablation of Pten (either as heterozygotes or by conditional uterine ablation). To determine the interplay between Mig-6 and the PTEN/PI3K signaling pathway during endometrial tumorigenesis, we have generated mice with Mig-6 and Pten conditionally ablated in progesterone receptor positive cells (PRcre/+Mig-6f/fPtenf/f ; Mig-6d/dPtend/d). The ablation of both Mig-6 and Pten dramatically accelerated the development of endometrial cancer compared to single ablation of either gene. The epithelium of Mig-6d/dPtend/d mice showed a significant decrease in the number of apoptotic cells compared to Ptend/d mice. The expression of the estrogen-induced apoptotic inhibitors Birc1 was significantly increased in the Mig-6d/dPtend/d mice. We identified ERK2 as a MIG-6 interacting protein by co-immunoprecipitation and demonstrated that the level of ERK2 phosphorylation was increased upon Mig-6 ablation either singly or in combination with Pten ablation. These results suggest that Mig-6 exerts a tumor suppressor function in endometrial cancer by promoting epithelial cell apoptosis through the down-regulation of the estrogen-induced apoptosis inhibitors Birc1 and the inhibition of ERK2 phosphorylation.
Keywords: Mig-6, Pten, Uterus, Endometrial cancer
Introduction
Endometrial cancer is the most frequently diagnosed malignancy of the female genital tract. According to the National Cancer Institute (NCI), endometrial cancer is the most common type of gynecological cancer. In the United States, approximately 41,200 cases are diagnosed and about 7,350 women die from the disease each year (Jemal et al., 2006). The majority of endometrial cancers (~90%) are adenocarcinomas, which originate in uterine epithelial cells. All endometrial cancers can be further delineated into two types (Deligdisch and Holinka, 1987; Di Cristofano and Ellenson, 2007). Type I endometrial cancers are estrogen (E2)-dependent and appear mostly in pre- and peri-menopausal women. Frequently, these cancers show mutations in DNA-mismatch repair genes (MLH1, MSH2, MSH6), PTEN, K-ras and β-catenin (Di Cristofano and Ellenson, 2007). In contrast, Type II endometrial cancers are E2-independent and are diagnosed mostly in post-menopausal women, thin and fertile women, or women with normal menstrual cycles.
PTEN (phosphatase and tensin homologue deleted from chromosome 10) is one of the most frequently mutated tumor suppressor genes in human cancers (Steck et al., 1997). PTEN is completely lost or mutated in >50% of primary endometrioid endometrial cancers (Sun et al., 2001) and in at least 20% of endometrial hyperplasias, the precancerous lesions of the endometrium (Levine et al., 1998; Sun et al., 2001). Thus, loss of PTEN is a very early event in the multi-step process leading to endometrioid endometrial cancer. PTEN acts as a negative regulator of phosphoinositide 3-kinases (PI3K) signaling which regulates a number of cellular functions through the activation of Akt (Jiang and Liu, 2008). Previously, loss of Pten (either as a heterozygote or by uterine specific ablation) has been shown to induce endometrial cancer in mice highlighting its important role in endometrial cancer development (Daikoku et al., 2008; Vilgelm et al., 2006). This mutation and subsequent Akt activation resulted in the activation of ERα-dependent pathways which play an important role in endometrial cancer tumorigenesis (Vilgelm et al., 2006). Interestingly, the PI3K signaling pathway can also be activated by E2 suggesting a complex interaction exists between these two signaling pathways (Chambliss et al., 2002).
Mig-6 is an immediate early response gene that can be induced by various mitogens and commonly occurring chronic stress stimuli (Makkinje et al., 2000; Saarikoski et al., 2002). It is an adaptor molecule containing a CRIB domain, a src homology 3 (SH3) binding domain, a 14-3-3 binding domain and an EGFR binding domain, but no domain with enzymatic activity (Burbelo et al., 1995; Makkinje et al., 2000). Previously, the interaction between MIG-6 and the 14-3-3 proteins has been demonstrated (Makkinje et al., 2000). Ablation of Mig-6 in mice leads to the development of animals with epithelial hyperplasia, adenoma, and adenocarcinomas in organs, such as the uterus, lung, gallbladder, and bile duct (Anastasi et al., 2005; Ferby et al., 2006; Jeong et al., 2009; Jin et al., 2007; Zhang et al., 2006). Decreased expression of Mig-6 is observed in human breast carcinomas which correlate with reduced overall survival of breast cancer patients (Amatschek et al., 2004; Anastasi et al., 2005). These data point to Mig-6 as a tumor suppressor gene in both mice and humans. Previously, we demonstrated that the absence of Mig-6 in mice results in the inability of P4 to inhibit E2-induced uterine weight gain and expression of E2-responsive target genes (Jeong et al., 2009). PRcre/+ Mig-6f/f (Mig-6d/d) mice develop hyperplasia and endometrial cancer in a hormone-dependent manner. Additionally, the observation that endometrial carcinomas from women have a significant reduction in MIG-6 expression provides compelling support for an important growth regulatory role for Mig-6 in the uterus of both humans and mice (Jeong et al., 2009). This demonstrates that Mig-6 is a critical regulator of the tumorigenesis of endometrial cancer. However, the mechanism of Mig-6 action in endometrial cancer remains unknown.
In this study, we utilized conditional Pten and Mig-6 ablation in the uteri of mice to demonstrate a synergistic effect of dysregulation of the Pten and Mig-6 signaling pathways during endometrial tumorigenesis. Ablation of both genes dramatically accelerated the development of endometrial cancer compared to single mutation of either gene. Thus, these results demonstrate the importance of Pten and Mig-6 regulation in the tumorigenesis of endometrial cancer by promoting epithelial cell apoptosis.
Results
Generation of mice with Pten and Mig-6 ablation in the murine uterus
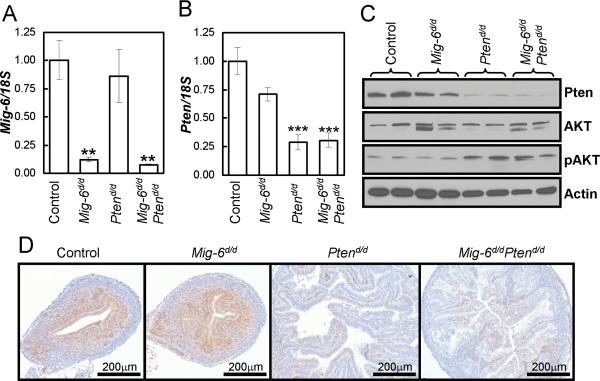
The most common genetic mutations in human endometrioid carcinoma are found in the Pten gene (Di Cristofano and Ellenson, 2007; Podsypanina et al., 1999). Pten+/- and mice with Pten conditionally ablated in the uterus (PRcre/+Ptenf/f; Ptend/d) develop endometrioid endometrial adenocarcinoma (Daikoku et al., 2008; Lian et al., 2006). In order to investigate the effects of the MIG-6 and the PTEN/PI3K/AKT signaling pathways on uterine tumorigenesis, mice with Pten floxed (Ptenf/f) (Lesche et al., 2002) and Mig-6 floxed (Mig-6f/f) (Jin et al., 2007) were bred to the PRCre mouse model (Soyal et al., 2005) to generate ablation of Pten and Mig-6 in the uterus. Ablation of Pten and Mig-6 (Mig-6d/dPtend/d) was assayed by real-time RT-PCR, Western blot and immunohistochemical analysis (n=3). Mig-6 mRNA expression was detected in the control (WT, PRcre/+, Mig-6f/f, and Ptenf/f) uteri while not in the Mig-6d/d and Mig-6d/dPtend/d uteri (Fig. 1A). There was no effect on Mig-6 expression by Pten ablation. The expression of Pten mRNA was detected in the control, but not in the Ptend/d and Mig-6d/dPtend/d uteri (Fig. 1B). While there was a slight decrease in Pten expression in the Mig-6d/d uteri, it was not significant. The decrease in Pten expression correlated with a decrease in protein expression as observed both by western blot and immunohistochemical analysis (Fig. 1 C and D). Ablation of Pten also resulted in increased activation of AKT as expected (Fig. 1C). These results suggest that PRcre efficiently ablated Pten and Mig-6 in the mouse uterus.
Figure 1.
Analysis of conditionally ablated Pten and Mig-6 in the murine uterus. (A and B) Real-time RT-PCR analysis of Mig-6 (A) and Pten (B) in whole uterine extracts from control, Mig-6d/d, Ptend/d and Mig-6d/d Ptend/d 2 week old mice. **, p<0.01; ***, p<0.001 (C and D) Western blot analysis (C) and Immunohistochemical analysis (D) for PTEN in control, Mig-6d/d, Ptend/d and Mig-6d/d Ptend/d 2 week old mice.
Endometrial cancer development in mice with Pten and Mig-6 ablation in PR-expressing cells
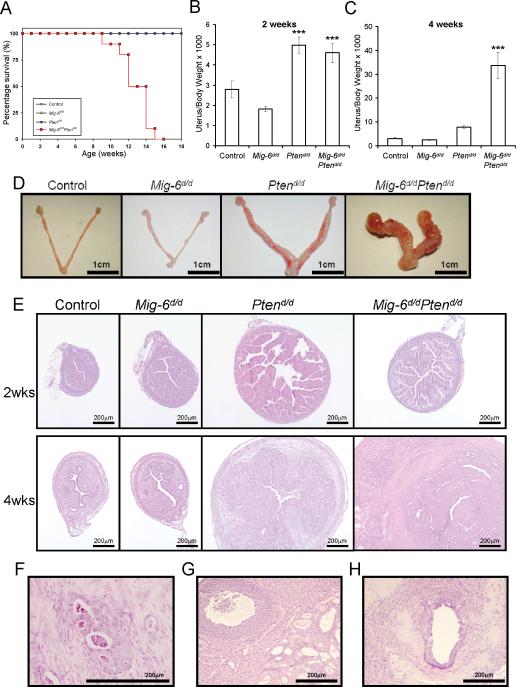
Previously, ablation of Pten in the uterus was shown to decrease survival due to the development of endometrial cancer (Daikoku et al., 2008). Therefore, we first examined the lifespan of control, Mig-6d/d, Ptend/d and Mig-6d/d Ptend/d mice. The survival time of Mig-6d/d Ptend/d mice was significantly shorter compared with control, Mig-6d/d and Ptend/d mice (p < 0.0001; Fig. 2A). To investigate the impact of Pten and Mig-6 ablation on endometrial cancer development and progression, control, Mig-6d/d, Ptend/d and Mig-6d/d Ptend/d mice were sacrificed at 2 and 4 weeks of age and uterine weight, gross and histological morphology were examined (n=8 per genotype per age). Ptend/d and Mig-6d/d Ptend/d mice showed a significant increase in uterine weight at 2 weeks of age compared to control and Mig-6d/d mice (Fig. 2B). The uterine weight of Mig-6d/d Ptend/d mice was significantly increased compared to other mice including Ptend/d mice at 4 weeks of age (Fig. 2B and C). Gross morphology at 4 weeks of age showed that the ablation of Mig-6 and Pten dramatically accelerated the development of endometrial cancer compared to single ablation of either gene (Fig. 2C). Histological analysis demonstrated that the uteri of Ptend/d and Mig-6d/dPtend/d mice exhibit a similar endometrial hyperplastic phenotype at 2 weeks of age (Fig. 2D). The Mig-6d/dPtend/d mice developed endometrial adenocarcinoma at 4 weeks of age characterized by neoplastic endometrial glands invading through the myometrium (Fig. 2F). However, the Ptend/d mice still exhibited endometrial hyperplasia at 4 weeks of age (Fig. 2D). Endometrial adenocarcinoma with invasion into the myometrium was observed in the Ptend/d mice at 2 months of age (Daikoku et al., 2008). While endometrial hyperplasia and adenocarcinoma were observed, myometrial hyperplasia was not observed in the uteri of Mig-6d/dPtend/d mice. The Mig-6d/dPtend/d mice displayed distant metastases into the ovary (Fig. 2G), diaphragmatic skeletal muscle (Fig. 2H), lymph node, colon, and pancreas. These results suggest that Mig-6 plays an important role as a suppressor of the development of endometrial cancer caused by Pten ablation.
Figure 2.
Development of endometrial cancer in Mig-6d/dPtend/d mice. (A) Survival curve in control (PRcre/+, Mig-6f/f, Ptenf/f, and Mig-6f/fPtenf/f), Mig-6d/d, Ptend/d, and Mig-6d/dPtend/d mice. p < 0.0001, logrank test. (B and C) The ratio of uterine weight to body weight in control, Mig-6d/d, Ptend/d, and Mig-6d/dPtend/d mice at 2 (B) and 4 (C) weeks of age. ***, p < 0.001, one-way ANOVA followed by Tukey's post hoc multiple range test. (D) Gross anatomy of control, Mig-6d/d, Ptend/d, and Mig-6d/dPtend/d mice at 4 weeks of age. (E) Histology of uteri from mice with Pten and Mig-6 ablation. H&E staining of control, Mig-6d/d, Ptend/d, and Mig-6d/dPtend/d mice at 2 and 4 weeks of age. (F) Endometrial cancer that has invaded through the myometrium. (G and H) Endometrial cancer that has metastasized into the ovary (G) and skeletal muscle (H).
Mig-6 exerts a propaoptotic effect as an endometrial cancer tumor suppressor
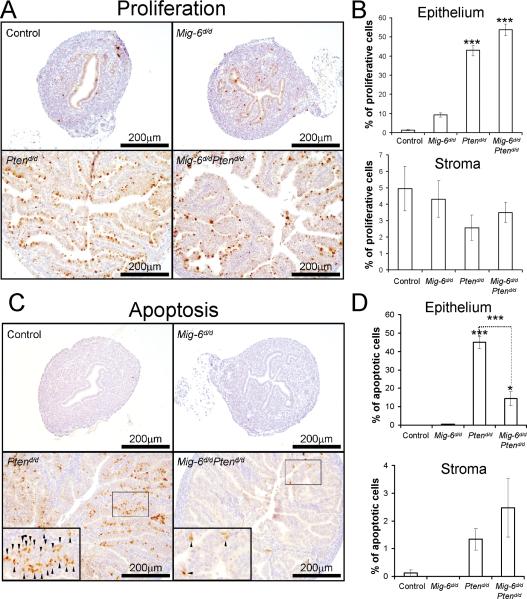
In order to determine if the endometrial hyperplasia and cancer in the Ptend/d and Mig-6d/d Ptend/d mice is caused by an alteration in cell proliferation and/or apoptosis, we performed immunohistochemical staining for phospho-histone H3, a mitotic marker, and cleaved caspase 3, an apoptotic marker, in mice at 2 weeks of age. Immunohistochemical staining of phosphohistone H3 demonstrated that proliferation was significantly increased in the epithelium of Ptend/d and Mig-6d/dPtend/d compared to control and Mig-6d/d mice (Fig. 3 A and B) . However, no significant difference was observed between the Ptend/d and Mig-6d/dPtend/d mice. Immunohistochemical staining of cleaved caspase 3 showed a significant decrease in the number of apoptotic cells in the epithelium of Mig-6d/dPtend/d mice compared to Ptend/d mice (Fig. 3 C and D). There was also no difference in stromal cell proliferation and apoptosis. These results suggest that Mig-6 acts as a tumor suppressor to induce apoptosis when Pten is mutated.
Figure 3.
The regulation of proliferation and apoptosis by Mig-6. (A) Immunohistochemical analysis of phospho-histone H3 as a proliferation marker in uteri of control, Mig-6d/d, Ptend/d, and Mig-6d/d Ptend/d mice at 2 weeks of age. (B) Quantification of phospho-histone H3 positive cells in epithelial and stromal cells (C) Immunohistochemical analysis of cleaved caspase 3 as an apoptotic cell marker. Small arrows indicate apoptotic cells. (D) Quantification of cleaved caspase 3 positive cells in epithelial and stromal cells. *, p<0.05; ***, p<0.001
Mig-6 represses Birc1 expression in the murine uterus
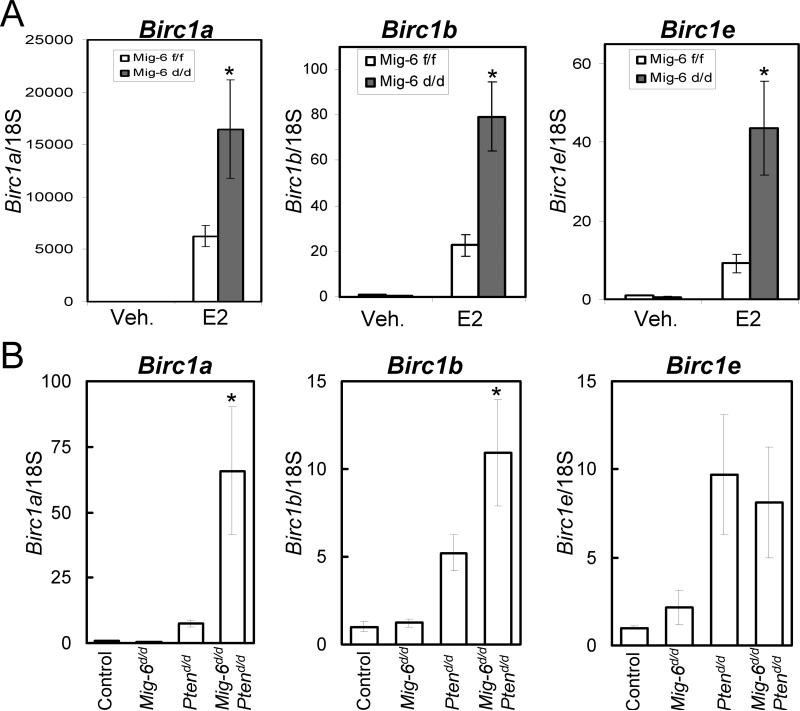
The decision as to whether or not a cell undergoes apoptosis is determined by the opposing actions of pro- and anti-apoptotic effectors (Song and Santen, 2003). It is known that E2 can tip this balance toward cell survival in uterine epithelial cells by inducing the expression of baculoviral inhibitors of apoptosis repeat-containing 1 (Birc1), a family of anti-apoptotic proteins (Yin et al., 2008). To determine if Mig-6 promotes uterine epithelial apoptosis by suppressing Birc1 expression, the expression of Birc1a, Birc1b, and Birc1e was determined in the Mig-6f/f and Mig-6d/d mice treated with E2 for 3 days by real-time RT-PCR (n=3). Interestingly, the expression of Birc1 genes was significantly increased in the Mig-6d/d mice treated with E2 compared to Mig-6f/f mice (Fig. 4A). These results suggest that Mig-6 induces uterine epithelial apoptosis via down-regulation of Birc1 expression. We also examined the expression of Birc1a, Birc1b, and Birc1e in control, Mig-6d/d, Ptend/d, and Mig-6d/dPtend/d mice at 2 weeks of age (n=3) (Fig. 4B). The expression of Birc1a and Birc1b but not Birc1e was significantly increased in Mig-6d/dPtend/d mice compared to the other groups. While the expression of Birc1a, Birc1b, and Birc1e was slightly increased in Ptend/d mice compare to control and Mig-6d/d mice, the increase was not significant. These results suggest that Mig-6 exerts a tumor suppressor function by inducing uterine epithelial apoptosis through the suppression of Birc1 expression in tumorigenic conditions such as unopposed E2 action or Pten ablatxion.
Figure 4.
The regulation of Birc1 genes in the uteri of Mig-6 ablation. (A) Real-time RT-PCR analysis of Birc1a, Birc1b, and Birc1e was performed on uteri of Mig-6d/d and Mig-6f/f mice treated with E2 for 3 days. (B) Real-time RT-PCR analysis of Birc1a, Birc1b, and Birc1e was performed on uteri of control, Mig-6d/d, Ptend/d, and Mig-6d/d Ptend/d mice at 2 weeks of age. *, p < 0.05
The Interaction of MIG-6 with ERK2 and its regulation of ERK2 phosphorylation
Although these results have established Mig-6’s role in steroid hormone regulation and tumorigenesis, the molecular mechanism of Mig-6 action is not clear. As MIG-6 is an adaptor molecule, we have turned to a biochemical and proteomics approach to identify MIG-6 associating proteins in order to gain insight into its mechanism of action. One of the hallmarks of endometrial cancer is the loss of ovarian steroid hormone (E2 and P4) control over uterine epithelial cell proliferation and apoptosis (Franco et al., 2008; Ito et al., 2007). E2 promotes endometrial cancer by stimulating proliferation and inhibiting apoptosis while P4 antagonizes these actions of E2 in the uterus. Our previous results showed that the absence of Mig-6 in mice results in the inability of P4 to inhibit E2-induced uterine weight gain and E2-responsive target genes expression (Jeong et al., 2009). These results suggest that Mig-6 suppresses E2 signaling in the presence of P4. Therefore, we isolated endogenous MIG-6 protein complexes using an anti-MIG-6 antibody (Sigma-Aldrich, St. Louis, MO) from the uteri of Mig-6f/f and Mig-6d/d mice treated with E2+P4 for 3 days and identified associated proteins using mass spectrometry. Proteins that were identified from lysates prepared by immunoprecipitation in the Mig-6f/f mice but not in the Mig-6d/d mice were considered true interacting proteins. The use of the Mig-6d/d tissue serves as the control for non-specific and cross-reacting proteins. Using this criterion, the identified MIG-6 interacting proteins are listed in Table 1.
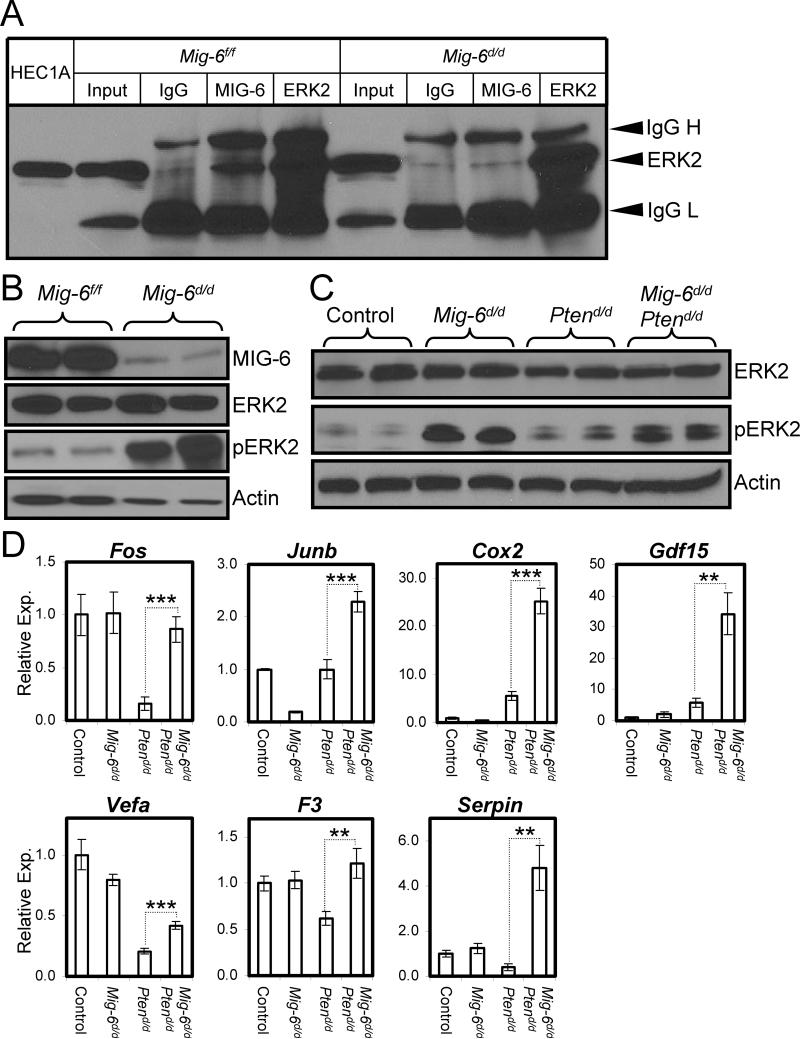
14-3-3 proteins are known MIG-6-associating proteins (Zhang and Vande Woude, 2007) that regulate the phosphorylation of proteins involved in PTEN/PI3K/AKT signaling (Kakinuma et al., 2008; Slaets et al., 2008). We also found novel MIG-6 associated molecules such as signal transducer and activator of transcription 3 (STAT3), extracellular signal-regulated kinase 2 (ERK2), and growth factor receptor bound protein 2 (GRB2). ERK2 is a classical MAPK that is activated mainly by growth factors or mitogenic stimuli. It is activated by phosphorylation which results in its translocation into the nucleus where it phosphorylates transcription factors (Eldredge et al., 1994; Fukuda et al., 1997). It has been reported that ERK affects apoptosis by promoting the expression of inhibitor of apoptosis proteins (IAPs) (Erhardt et al., 1999; Tashker et al., 2002; Xia et al., 1995). To validate the interaction between MIG-6 and ERK2 proteins, we performed coimmunoprecipitation experiments using lysates from the uteri of Mig-6f/f and Mig- 6d/d mice treated with E2+P4 for 3 days. Co-immunoprecipiatation was performed with anti-IgG, anti-MIG-6 and anti-ERK2 antibodies, and analyzed by Western blot analysis using anti-ERK2 antibodies to detect ERK2. ERK2 protein could be detected from immunoprecipitates with the anti-MIG-6 and anti-ERK2 antibodes confirming the interaction between MIG-6 and ERK2 (Fig. 5A). These results indicate that the interaction between MIG-6 and ERK2 may play an important role in the regulation of the phosphorylation of ERK2 and subsequent regulation of apoptosis.
Figure 5.
Interaction between MIG-6 and ERK2 in the murine uterus. (A) Validation of the MIG-6 interaction with ERK2 by co-immunoprecipitation and Western blot analysis. (B) Western blot analysis of ERK2 and phospho-ERK2 in the uteri of 5 month old Mig-6f/f and Mig- 6d/d mice. (C) Western blot analysis of ERK2 and phospho-ERK2 in the uteri of 2 weeks old control, Mig-6d/d, Ptend/d, and Mig-6d/d Ptend/d mice. (D) The expression of ERK2 target genes. Real-time RT-PCR analysis of Fos, Junb, Ptgs2, Gdf15, Vegfa, F3, and Serpin1 was performed on uteri of control, Mig-6d/d, Ptend/d, and Mig-6d/d Ptend/d mice. **, p<0.01; ***, p<0.001
To determine if the hyperplasia phenotypes observed may be due to altered ERK2 phosphorylation in the Mig-6f/f uteri, we examined the expression of ERK2 by Western blot analysis in the Mig-6f/f uteri at 5 months of age. The level of phospho-ERK2 but not total ERK2 was increased in Mig-6d/d uteri compared to Mig-6f/f uteri (Fig. 5B). We also observed an increased level of phospho-ERK2 but not total ERK2 in Mig-6d/d and Mig-6d/d Ptend/d mice compared to control and Ptend/d mice (Fig. 5C).
In order to determine if the uteri of Mig-6d/d Ptend/d exhibited altered ERK2 signaling, control, Mig-6d/d, Ptend/d and Mig-6d/d Ptend/d mice were sacrificed at 2 weeks of age (n = 6) and the expression of ERK2 target genes, Fos (FBJ osteosarcoma oncogene) (Kyriakis and Avruch, 2001), Junb (Jun-B oncogene) (Gesty-Palmer et al., 2005), Ptgs2 (prostaglandin-endoperoxide synthase 2; Cox2) (Smith et al., 2000), Gdf15 (growth differentiation factor 15) (Malathi et al., 2005), Vegfa (vascular endothelial growth factor A) (Milanini-Mongiat et al., 2002), F3 (coagulation factor III) (Gesty-Palmer et al., 2005) and Serpine1 (serine (or cysteine) peptidase inhibitor, clade E, member 1) (Gesty-Palmer et al., 2005), was examined. The expression of these ERK2 target genes was not altered in Mig-6d/d mice compared to control mice. Interestingly, Mig-6d/d Ptend/d uteri showed significantly increased expression of these ERK2 target genes compared to Ptend/d uteri (Fig. 5D). These results suggest that Mig-6 plays a tumor suppressor function in the context of Pten ablation by promoting epithelial cell apoptosis and by inhibiting ERK2 phosphorylation.
Discussion
Endometrial cancer is the most common gynecological cancer and has been shown to be associated with mutations in the tumor suppressor gene Pten among others (Di Cristofano and Ellenson, 2007). Loss of PTEN is an early event in the multi-step process leading to endometrioid endometrial cancer. Pten+/- and mice with Pten conditionally ablated in the uterus (Ptend/d) develop endometrioid endometrial adenocarcinoma (Daikoku et al., 2008; Lian et al., 2006). This mutation and subsequent Akt activation play an important role in the tumorigenesis of endometrial cancer (Vilgelm et al., 2006). The expression of MIG-6 is decreased in human endometrial cancer patients and Mig-6d/d mice develop invasive endometrioid-type endometrial adenocarcinoma with unopposed estrogen treatment (Jeong et al., 2009). Introduction of Mig-6 ablation into the Ptend/d mice accelerated the tumorigenesis of endometrial cancer as compared to Pten ablation alone (Fig. 2). The neoplastic endometrial glands in the double mutant mice invaded through the uterine muscle wall and, with age, led to the development of distant metastases. These results suggest that the tumor suppressor function of Mig-6 is important to prevent the development of endometrial hyperplasia or endometrial cancer in the tumorigenic conditions of unopposed estrogen or Pten loss.
It is well known that endometrioid endometrial cancer is an estrogen-dependent disease and progestin hormone therapy has been used to slow the growth of endometrial cancer due to its inhibitory effects on E2 action. The impact of unopposed E2 treatment on the development of endometrial cancer in the context of Pten ablation remains unknown. However, ovariectomized Ptend/d mice develop endometrial hyperplasia albeit at a slower rate than intact Ptend/d mice suggesting that the tumorigenesis of the Ptend/d mice is partially steroid hormone dependent (unpublished data). Thus, further investigations into the impact of E2 and E2 plus P4 treatment on the development of endometrial cancer in the Ptend/d and Mig-6d/d Ptend/d mice need to be performed in order to further elucidate the effect of steroid hormone signaling on endometrial cancer development.
Most endometrial cancers are characterized by actively proliferating glands, increased Akt signaling and decreased apoptosis (Ejskjaer et al., 2007; Khalifa et al., 1994; Sivridis and Giatromanolaki, 2004). Loss of Pten in the uteri of mice and humans results in increased epithelial proliferation (Fig. 3A and 3B), increased phosphorylation of Akt (Fig. 1C), and altered E2 signaling (Daikoku et al., 2008; Lian et al., 2006; Vilgelm et al., 2006) as well as the development of endometrioid endometrial cancer (Daikoku et al., 2008; Kanamori et al., 2001). Mig-6d/d mice exhibit altered E2 signaling (Jeong et al., 2009) and a slight but not significant increase in epithelial proliferation (Fig. 3A and 3B) with no effect on Akt signaling or epithelial apoptosis. The double mutant mice also have comparable levels of epithelial proliferation (Fig. 3A and 3B) when compared to the Ptend/d mice. However, in contrast to the Ptend/d mice, these mice exhibit dramatically decreased epithelial apoptosis (Fig. 3C and 3D). As these mice develop endometrial cancer earlier than the Ptend/d mice, these data suggest that the decreased epithelial apoptosis may contribute to the accelerated tumorigenesis.
As E2 signaling is altered by both Pten and Mig-6 ablation, it suggests that E2 signaling may play a role in the enhanced tumorigenesis of the double mutant mice. Estrogen suppresses uterine epithelial apoptosis by inducing BIRC1 expression (Yin et al., 2008). The Birc1 genes encode a family of antiapoptotic proteins which can physically interact with active caspase-3 and -7 and with active caspase-9 in the presence of ATP (Davoodi et al., 2004; Maier et al., 2002). The expression of Birc1a, Birc1b, and Birc1e is significantly increased in Mig-6d/d mice treated with E2 (Fig. 4). Also, the expression of Birc1a and Birc1b was significantly increased in the Mig-6d/d Ptend/d mice compared to controls (Fig. 4). As these genes are well-known apoptosis inhibitors (Endrizzi et al., 2000; Roy et al., 1995), their increased expression may contribute to the decreased apoptosis of the double mutant mice. Thus, Mig-6 may act as a tumor suppressor in the context of Pten ablation by promoting apoptosis through the expression of the Birc1 family of proteins.
The estrogen receptors (ERs) mediate the effect of E2 under physiological and pathological conditions either by the activation of E2-target gene transcription (Acconcia and Kumar, 2006) or by nongenomic mechanisms which result in the rapid activation of several signal transduction pathways to regulate different cellular processes, such as proliferation, apoptosis, and differentiation. The nongenomic action of E2 has been linked to numerous pathways (EGFR, IGF-IR, c-MET) resulting in the activation of two key signaling cascades, the PTEN/PI3K/AKT and the MAPK pathways (Bhat-Nakshatri et al., 2008; Cheskis et al., 2008; Freeman et al., 2006; Thomas et al., 2008; Vilgelm et al., 2006). One consequence of this nongenomic E2 action is an inhibition of cellular apoptosis which has been observed in various cell types, such as vascular endothelial, smooth and skeletal muscles, and breast cancer cells (Bjornstrom and Sjoberg, 2005; Boland et al., 2008; Song and Santen, 2003; Spyridopoulos et al., 1997). MAPK/ERK-kinases (MEKs) trigger the activation of ERKs by phosphorylating a threonine and a tyrosine in their activation loop. Specificity in the signaling between these modules is achieved by protein-protein interactions and scaffolding molecules (Kolch, 2005). Here, we have identified a novel interaction of MIG-6 with ERK2 and demonstrated that ablation of Mig-6 leads to increased phosphorylation of ERK2 and expression of its target genes (Fig. 5). Abnormal or constitutive phosphorylation of ERK2 leads to tumorigenesis through inappropriate suppression of apoptosis (Evan and Vousden, 2001; Lowe et al., 2004). Thus, these data suggest the Mig-6 may also exert its tumor suppressor function in the context of Pten ablation by regulating the phosphorylation status of ERK2. This increase in phosphorylation may also contribute to the decreased apoptosis observed either by regulating Birc1 expression or by an unknown mechanism. These results suggest that Mig-6 may exert its tumor suppressor function in endometrial cancer by inhibiting ERK2 phosphorylation. Further studies need to be conducted to determine the precise mechanism by which these various pathways are integrated by Mig-6 to regulate epithelial apoptosis during endometrial tumorigenesis which may lead to the development of additional diagnostics or therapeutics for endometrial cancer.
In conclusion, our results demonstrate the synergistic effect of conditional Pten and Mig-6 loss on endometrial cancer development. This accelerated tumorigenesis is likely due to decreased epithelial apoptosis partly through increased expression of the Birc1 family of apoptotic inhibitors and increased phosphorylation of ERK2. This study has established an endometrial cancer mouse model which replicates common characteristics of the human disease providing a model system to further investigate the genetic and molecular events involved in the transition from normal to hyperplastic/neoplastic endometrium. These and future results will contribute to the understanding of the molecular mechanism of tumorigenesis and to the development of therapeutic approaches for endometrial cancer.
Materials and methods
Animals and Tissue Collection
Mice were maintained in the designated animal care facility at Baylor College of Medicine according to the institutional guidelines for the care and use of laboratory animals. PRCre/+ mice were previously generated (Soyal et al., 2005). The Ptenf/f were acquired from Dr. Hong Wu (University of California, Los Angeles, Los Angeles, CA) (Lesche et al., 2002). Mice of various genotypes were sacrificed at 2 and 4 weeks of age. At the time of dissection, uterine tissues were placed in the appropriate fixative or flash frozen and stored at −80°C. Statistical analysis for the survival curve was performed using the logrank test.
Western Blot Analysis
Samples containing 15 μg proteins were applied to SDS-PAGE. The separated proteins were transferred onto a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membranes were blocked overnight with 0.5% casein (wt/vol) in PBS with 0.1% Tween 20 (vol/vol) (Sigma–Aldrich, St. Louis, MO) and probed with anti-MIG-6 (Sigma–Aldrich, St. Louis, MO; PE-16 ), anti-PTEN (Cell Signaling Technology, Inc., Danvers, MA; #9559), anti-ERK2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; SC-1647), anti-phospho-ERK2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; SC-7883), anti-AKT (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; SC-55523), or anti-phospho-AKT (Cell Signaling Technology, Inc., Danvers, MA; #9275S) antibodies. Immunoreactivity was visualized by incubation with a horseradish peroxidase-linked secondary antibody and treatment with ECL reagents. To control for loading, the membrane was stripped and probed with anti-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; SC-1615) and developed again.
Immunohistochemistry
Uterine sections from paraffin-embedded tissue were preincubated with 10% normal serum in PBS (pH 7.5) and then incubated with anti-PTEN (Cell Signaling Technology, Inc., Danvers, MA; #9559), anti-phospho-Histone H3 (Upstate Biotechnology, Lake Placid, NY; 06-570) or anti-cleaved caspase 3 (Cell Signaling Technology, Inc., Danvers, MA; #9661L) antibody in 10% normal serum in PBS (pH 7.5). On the following day, sections were washed in PBS and incubated with a secondary antibody (5 μl/ml; Vector Laboratories, Burlingame, CA) for 1 hr. at room temperature. Immunoreacitivity was detected using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR analysis was conducted on isolated RNA. Expression levels of Mig-6, Pten, Birc1a, Birc1b, and Birc1e were measured by Real-time RT-PCR TaqMan analysis (Applied Biosystems, Foster City, CA). cDNA was made from 1 μg of total RNA using random hexamers and M-MLV Reverse Transcriptase (Invitrogen Corp., Carlsbad, CA). RTPCR was performed using RT-PCR Universal Master Mix reagent (Applied Biosystems, Foster City, CA). All real-time RT-PCR results were normalized against 18S RNA using ABI rRNA control reagents. Statistical analyses were performed using one-way ANOVA followed by Tukey's post hoc multiple range test with the Instat package from GraphPad (San Diego, CA, USA).
Immuno-affinity purification
We isolated endogenous MIG-6 protein complexes using an anti-MIG-6 antibody (Sigma-Aldrich, St. Louis, MO) from lysate prepared from uteri of Mig-6f/f and Mig-6d/d mice treated with E2+P4 for 3 days as previously reported (Jung et al., 2005). The immunoprecipitates was washed 3 times with NETN (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% NP- 40) and boiled with Laemmli buffer and subjected to SDS-PAGE (4-20% Tris/Glycine NOVEX Gel, Invitrogen, Carlsbad, CA). The Coomassie brilliant blue-stained protein bands were excised and in gel digested with trypsin.
Protein Identification
Nano-HPLC/MS/MS for peptide identification was carried out as described before (Jung et al., 2008). An 50 mm × 75 um, C18 column (BioBasic C18, 5 μm, 300 Å pore diameter, PicoFritTM, New objective) was used on-line with a LTQ mass spectrometer (Finnigan LTQTM, ThermoFinnigan, San Jose, CA). The LTQ were operated in the data-dependant mode acquiring fragmentation spectra of the top 20 strongest ions. Obtained MS/MS spectra were analyzed against modified NCBI-ref protein sequence database using BioWorks database search engine (BioWorksBrowser ver 3.2, Thermo Electron, San Jose, CA). All peptide identification with stringent BioWorksBrowser filtering criteria - peptide probability > 1 X 10-6 and Xcorr score > 2.0 - was manually examined and all peptides have to be identified by consecutive b- or y- ions so that false identifications were eliminated.
Acknowledgements
We thank Francesco J. DeMayo for fruitful discussion; Jinghua Li for technical assistance; Cory A. Rubel, M.S. and Michael J. Large for manuscript preparation. We thank Dr. Hong Wu for the floxed Pten mice. This work was supported by the Reproductive Biology Training Grant T32HD007165 and a scholarship from Baylor Research Advocates for Student Scientists (to H.L.F.), NIH R01CA77530 (to J.P.L.), NIH P50CA098258 (to R.R.B.), and NIH R01HD057873 (to J.W.J.). We thank the support of the pathway discovery core of the Dan Duncan Cancer Center in Baylor College of Medicine for proteomic work.
References
- Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238:1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Amatschek S, Koenig U, Auer H, Steinlein P, Pacher M, Gruenfelder A, et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res. 2004;64:844–56. doi: 10.1158/0008-5472.can-03-2361. [DOI] [PubMed] [Google Scholar]
- Anastasi S, Sala G, Huiping C, Caprini E, Russo G, Iacovelli S, et al. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene. 2005;24:4540–8. doi: 10.1038/sj.onc.1208658. [DOI] [PubMed] [Google Scholar]
- Bhat-Nakshatri P, Wang G, Appaiah H, Luktuke N, Carroll JS, Geistlinger TR, et al. AKT alters genome-wide estrogen receptor alpha binding and impacts estrogen signaling in breast cancer. Mol Cell Biol. 2008;28:7487–503. doi: 10.1128/MCB.00799-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–42. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Boland R, Vasconsuelo A, Milanesi L, Ronda AC, de Boland AR. 17beta-estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids. 2008;73:859–63. doi: 10.1016/j.steroids.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–4. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–46. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, et al. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–5. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–27. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi J, Lin L, Kelly J, Liston P, MacKenzie AE. Neuronal apoptosis-inhibitory protein does not interact with Smac and requires ATP to bind caspase-9. J Biol Chem. 2004;279:40622–8. doi: 10.1074/jbc.M405963200. [DOI] [PubMed] [Google Scholar]
- Deligdisch L, Holinka CF. Endometrial carcinoma: two diseases? Cancer Detect Prev. 1987;10:237–46. [PubMed] [Google Scholar]
- Di Cristofano A, Ellenson LH. Endometrial Carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- Ejskjaer K, Sorensen BS, Poulsen SS, Forman A, Nexo E, Mogensen O. Expression of the epidermal growth factor system in endometrioid endometrial cancer. Gynecol Oncol. 2007;104:158–67. doi: 10.1016/j.ygyno.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Eldredge ER, Korf GM, Christensen TA, Connolly DC, Getz MJ, Maihle NJ. Activation of c-fos gene expression by a kinase-deficient epidermal growth factor receptor. Mol Cell Biol. 1994;14:7527–34. doi: 10.1128/mcb.14.11.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrizzi MG, Hadinoto V, Growney JD, Miller W, Dietrich WF. Genomic sequence analysis of the mouse Naip gene array. Genome Res. 2000;10:1095–102. doi: 10.1101/gr.10.8.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–15. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006;12:568–73. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19:178–86. doi: 10.1016/j.semcdb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Freeman D, Lesche R, Kertesz N, Wang S, Li G, Gao J, et al. Genetic background controls tumor development in PTEN-deficient mice. Cancer Res. 2006;66:6492–6. doi: 10.1158/0008-5472.CAN-05-4143. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997;272:32642–8. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, El Shewy H, Kohout TA, Luttrell LM. beta-Arrestin 2 expression determines the transcriptional response to lysophosphatidic acid stimulation in murine embryo fibroblasts. J Biol Chem. 2005;280:32157–67. doi: 10.1074/jbc.M507460200. [DOI] [PubMed] [Google Scholar]
- Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma--new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54:667–79. doi: 10.1507/endocrj.kr-114. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci U S A. 2009;106:8677–82. doi: 10.1073/pnas.0903632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–8. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Jin N, Gilbert JL, Broaddus RR, Demayo FJ, Jeong JW. Generation of a Mig-6 conditional null allele. Genesis. 2007;45:716–21. doi: 10.1002/dvg.20348. [DOI] [PubMed] [Google Scholar]
- Jung SY, Li Y, Wang Y, Chen Y, Zhao Y, Qin J. Complications in the assignment of 14 and 28 Da mass shift detected by mass spectrometry as in vivo methylation from endogenous proteins. Anal Chem. 2008;80:1721–9. doi: 10.1021/ac7021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SY, Malovannaya A, Wei J, O'Malley BW, Qin J. Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Mol Endocrinol. 2005;19:2451–65. doi: 10.1210/me.2004-0476. [DOI] [PubMed] [Google Scholar]
- Kakinuma N, Roy BC, Zhu Y, Wang Y, Kiyama R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J Cell Biol. 2008;181:537–49. doi: 10.1083/jcb.200707022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y, Kigawa J, Itamochi H, Shimada M, Takahashi M, Kamazawa S, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001;7:892–5. [PubMed] [Google Scholar]
- Khalifa MA, Mannel RS, Haraway SD, Walker J, Min KW. Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol. 1994;53:84–92. doi: 10.1006/gyno.1994.1092. [DOI] [PubMed] [Google Scholar]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–37. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–9. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–8. [PubMed] [Google Scholar]
- Lian Z, De Luca P, Di Cristofano A. Gene expression analysis reveals a signature of estrogen receptor activation upon loss of Pten in a mouse model of endometrial cancer. J Cell Physiol. 2006;208:255–66. doi: 10.1002/jcp.20681. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Maier JK, Lahoua Z, Gendron NH, Fetni R, Johnston A, Davoodi J, et al. The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J Neurosci. 2002;22:2035–43. doi: 10.1523/JNEUROSCI.22-06-02035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkinje A, Quinn DA, Chen A, Cadilla CL, Force T, Bonventre JV, et al. Gene 33/Mig-6, a transcriptionally inducible adapter protein that binds GTP-Cdc42 and activates SAPK/JNK. A potential marker transcript for chronic pathologic conditions, such as diabetic nephropathy. Possible role in the response to persistent stress. J Biol Chem. 2000;275:17838–47. doi: 10.1074/jbc.M909735199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K, Paranjape JM, Bulanova E, Shim M, Guenther-Johnson JM, Faber PW, et al. A transcriptional signaling pathway in the IFN system mediated by 2′-5′-oligoadenylate activation of RNase L. Proc Natl Acad Sci U S A. 2005;102:14533–8. doi: 10.1073/pnas.0507551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanini-Mongiat J, Pouyssegur J, Pages G. Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002;277:20631–9. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96:1563–8. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–78. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- Saarikoski ST, Rivera SP, Hankinson O. Mitogen-inducible gene 6 (MIG-6), adipophilin and tuftelin are inducible by hypoxia. FEBS Lett. 2002;530:186–90. doi: 10.1016/s0014-5793(02)03475-0. [DOI] [PubMed] [Google Scholar]
- Sivridis E, Giatromanolaki A. Endometrial adenocarcinoma: beliefs and scepticism. Int J Surg Pathol. 2004;12:99–105. doi: 10.1177/106689690401200202. [DOI] [PubMed] [Google Scholar]
- Slaets H, Dumont D, Vanderlocht J, Noben JP, Leprince P, Robben J, et al. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Akt-phosphorylation and up-regulation of 14-3-3. Proteomics. 2008;8:1237–47. doi: 10.1002/pmic.200700641. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- Spyridopoulos I, Sullivan AB, Kearney M, Isner JM, Losordo DW. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis. Estradiol as a survival factor. Circulation. 1997;95:1505–14. doi: 10.1161/01.cir.95.6.1505. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Sun H, Enomoto T, Fujita M, Wada H, Yoshino K, Ozaki K, et al. Mutational analysis of the PTEN gene in endometrial carcinoma and hyperplasia. Am J Clin Pathol. 2001;115:32–8. doi: 10.1309/7JX6-B9U9-3P0R-EQNY. [DOI] [PubMed] [Google Scholar]
- Tashker JS, Olson M, Kornbluth S. Post-cytochrome C protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol Biol Cell. 2002;13:393–401. doi: 10.1091/mbc.01-06-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RS, Sarwar N, Phoenix F, Coombes RC, Ali S. Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity. J Mol Endocrinol. 2008;40:173–84. doi: 10.1677/JME-07-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert MJ, Steensma HY, van Heusden GP. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 2001;23:936–46. doi: 10.1002/bies.1134. [DOI] [PubMed] [Google Scholar]
- Vilgelm A, Lian Z, Wang H, Beauparlant SL, Klein-Szanto A, Ellenson LH, et al. Akt-mediated phosphorylation and activation of estrogen receptor alpha is required for endometrial neoplastic transformation in Pten+/− mice. Cancer Res. 2006;66:3375–80. doi: 10.1158/0008-5472.CAN-05-4019. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yin Y, Huang WW, Lin C, Chen H, MacKenzie A, Ma L. Estrogen suppresses uterine epithelial apoptosis by inducing birc1 expression. Mol Endocrinol. 2008;22:113–25. doi: 10.1210/me.2007-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B, et al. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene. 2006 doi: 10.1038/sj.onc.1209790. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Vande Woude GF. Mig-6, signal transduction, stress response and cancer. Cell Cycle. 2007;6:507–13. doi: 10.4161/cc.6.5.3928. [DOI] [PubMed] [Google Scholar]