Abstract
Both attractiveness judgements and mate preferences vary considerably cross-culturally. We investigated whether men's preference for femininity in women's faces varies between 28 countries with diverse health conditions by analysing responses of 1972 heterosexual participants. Although men in all countries preferred feminized over masculinized female faces, we found substantial differences between countries in the magnitude of men's preferences. Using an average femininity preference for each country, we found men's facial femininity preferences correlated positively with the health of the nation, which explained 50.4% of the variation among countries. The weakest preferences for femininity were found in Nepal and strongest in Japan. As high femininity in women is associated with lower success in competition for resources and lower dominance, it is possible that in harsher environments, men prefer cues to resource holding potential over high fecundity.
Keywords: facial preferences, femininity, national health, other-race effect
1. Introduction
Cultures vary considerably in the characteristics they consider most attractive [1,2]. Individuals within one group (restricted by nationality or geographical location) may show a common pattern of preferences towards a variety of stimuli. In this paper, we investigate cross-cultural variation in perceptions of attractiveness and test the hypothesis that persistently variable exposure to pathogens across geographical regions leads to cross-cultural differences in sexual preferences.
In humans, pubertal hormones promote the development of sexual dimorphism in craniofacial traits [3,4]. In women, oestrogen promotes feminine traits (e.g. larger eyes and fuller lips [5])—features that men prefer over more masculine features [6,7]. Accordingly, oestrogen-related traits may signal female reproductive value [8], such as underlying fertility [9] and indirect genetic benefits that enhance offspring fitness [6].
Men's preferences could vary cross-culturally as facial femininity preferences change with pathogen prevalence. There are several reasons to expect such preferences to be stronger in places where health is better and pathogens are fewer. Firstly, preferences for facial femininity are stronger for short-term than long-term relationships ([10] but see also [11]) and vary positively with men's testosterone levels [12] and sexual motivation [13]. In countries with lower mortality risk and better healthcare, men have less restricted sexual attitudes towards short-term relationships [14]. Hence, we might expect greater preferences for femininity in countries with better health conditions. Secondly, female facial femininity is associated with greater perceived maternal tendencies and actual maternal investment and parental qualities [15,16]. Thus, preferences for facial femininity might reflect a prioritization of cues indicative of maternal investment.
However, if facial femininity signals reproductive value and heritable benefits that might enhance offspring survival [16], we could expect stronger preferences for femininity in countries with worse healthcare. This hypothesis is in line with recent findings [17–19] that in cultures with lower versus higher standards of healthcare, women prefer more masculine men. While women may face stronger trade-offs than men in mate selection [20], male preferences for femininity may also be stronger in countries with lower healthcare.
To test these two competing hypotheses and shed light on cross-cultural variation in preferences for femininity in female faces, we investigated facial preferences across 28 countries. Specifically, we aimed to determine (i) whether men from different countries differ in their preferences for sexual dimorphism in women and (ii) whether this variation is associated with countrywide health of the nation.
2. Material and methods
Pictures of Caucasian women's faces, aged 18–24 (chosen randomly from photographs gathered in previous research [21]), were transformed with PsychoMorph [22] on a femininity–masculinity scale (following [6]), by adding or subtracting 50% of the linear difference between a 40 adult-male composite and a 40 adult-female composite (age matched). Importantly, the two stimuli pictures (examples in the electronic supplementary material, S1) in each pair differed only in sexually dimorphic cues of face shape [23].
As online and laboratory studies of variation in preference for masculinity produce equivalent patterns of results [2], we used web-based surveys available in 16 languages. Participants selected via forced choice, the more attractive of two stimuli. Individual preferences for femininity were calculated as the proportion of feminized pictures selected among 20 pairs of pictures. We obtained responses from 1972 heterosexual males aged 18–45 years from 28 countries.
We used national health index (NHI) as a measure of the health of the nation [17], so that high NHI scores reflect better health. Other explanatory variables were gross national income (GNI) and sex ratio. For data analysis, see the electronic supplementary material, S2.
3. Results
Within countries, preferences for our stimuli did not differ between Caucasian and non-Caucasian respondents (Wilcoxon signed-rank test (Wt): Z = 32.5, n = 17, p = 0.13). In all countries, men had significantly higher preferences for feminized over masculinized faces (M = 0.683, n = 28, s.d. = 0.054; Wt: Z = −18.7 to −2.0, p ≤ 0.05). However, preferences for femininity differed among countries (χ2 = 297.4, d.f. = 27, p < 0.0001), ranging from 0.525 in Nepal to 0.778 in Japan (electronic supplementary material, S2). Preferences for femininity were positively correlated with NHI, which explained 50.4% of the variation among countries (figure 1). This result was confirmed by hierarchic linear regression analysis (electronic supplementary material, S2) and accounting for unequal sample sizes did not change this conclusion (weighted Pearson correlation coefficient: r = 0.80, n = 28, p < 0.0001). Mean age of participants, sex ratio and GNI did not meet the 0.15 significance level for entry into the model. We found no significant relationships between femininity preference index and sociosexual orientation inventory (SOI) (r = −0.30, n = 19, p = 0.20).
Figure 1.
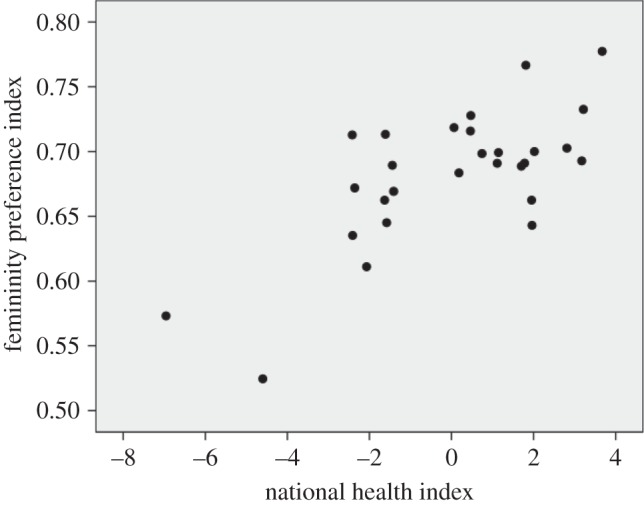
Relationship between femininity preference index and NHI. Average men's preference for femininity plotted against NHI of their country (r = 0.71, n = 28, p < 0.0001).
4. Discussion
To our knowledge, our study is the first to quantify geographical variation in men's preferences for facial femininity. Men across all 28 countries preferred feminine over masculine women's faces; however, the strength of these preferences varied significantly. As NHI increased, so did preferences for facial femininity. Although our respondents represent different ethnic groups, and all stimulus female's faces were Caucasian, we did not find the other-race effect (as defined by Meissner & Brigham [24]).
Interestingly, we did not find a relationship between men's socio-sexuality and facial femininity preferences, i.e. NHI is a better predictor of men's facial femininity preferences cross-culturally than socio-sexuality. Men's preferences for facial femininity are greater for short-term than long-term relationships [25] and preferences for attractive mates are greater among men with less restricted socio-sexualities [26]. However, in this study, we did not measure preferences for long- and short-term relationships separately, which could explain the lack of association between socio-sexuality and facial femininity preferences between the countries we examined.
As femininity is associated with lower ratings of dominance in women [15], it is possible that in harsh environments men have stronger preferences for cues to effective resource acquisition and holding potential rather than high fecundity. Indeed, previous studies in rural Jamaica and rural Bangladesh found that men in harsher environments show unusually weak (or absent) femininity preferences [2,27]. Further in non-industrialized populations and in countries with poor health where men are exposed to more pathogens, testosterone levels are, on average, lower [28] especially among men under 45 years old [29]. As all the participants in our sample were under 45 and circulating testosterone levels correlate positively with preferences for femininity [12], this mechanism might also explain our results. However, future studies incorporating broader variation in national levels of industrialization, which also predicts lower circulating testosterone, would be beneficial to fully test this hypothesis.
Our findings do not support the hypothesis of increased preference for putative ‘good genes’ in harsh environments [14]. Little et al. [30] suggested that exposure to visual cues of pathogens increases preferences for sexual dimorphism because it can be adaptive to attune preferences in favour of ‘good gene’ markers. This variation in preferences can also be interpreted as intra-individual change depending on a recent visual stimulus (i.e. pathogen cues presented immediately before judging facial stimuli), rather than variation depending on living conditions (the cited study was conducted in the single country). Thus, the differences between our findings and those of previous studies may reflect a difference between preferences formed under the influences of varied life histories between nations and an individual's attuning to immediate visual cues of mate quality. However, as pathogen disgust was suggested to be rather stable over time [31], future research should focus on the distinction between regional and facultative variation.
Our results also differ from Gangestad et al. [32], who found that men and women living in countries with higher pathogen loads stated stronger preferences for attractive mates. Likewise, Lee et al. [33] found that men with higher concerns regarding pathogens preferred more feminine faces. In our study, men judged the attractiveness of faces that differed in masculinity and femininity. It may be that craniofacial shape is a stable cue of long-term health and that other facial features, like skin complexion, may be more cross-culturally variable with regards to pathogen prevalence as a signal of current health and disease resistance [34]. Thus, we cannot rule out that pathogens affect female attractiveness cross-culturally and future studies testing male preferences for other facial traits would be valuable.
Women's preferences for facial masculinity in men's faces are stronger in countries with lower NHI [17]. While national income inequality, a proxy for male–male competition, was found to be a stronger predictor of women's preferences for masculinity than NHI [35], these findings were not replicated in subsequent regional and cross-cultural samples, showing that national health was a better predictor of masculinity preferences [18,19]. We also found that NHI is a better predictor of cross-cultural differences in men's preferences than GNI.
To conclude, we demonstrated systematic cross-cultural variation in men's preference for femininity, which was lower in countries with worse health conditions. The suggested explanations for this pattern are: (i) stronger preference for cues to resource holding potential than for fecundity in harsh environments and (ii) lowered testosterone levels in countries with lower national health.
Data accessibility
Data available from the Dryad Digital Repository: doi:10.5061/dryad.32610.
Funding statement
The study was supported by Academy of Finland to M.J.R., Turku University Foundation to U.M.M, strategic research grant of University of Turku to M.V.K, Romanian National Authority for Scientific Research, CNCS—UEFISCDI to O.A.G. (PN-II-RU-PD-2011-3-0131) and CONACyT to J.C.G. (152666).
References
- 1.Buss DM. 1989. Sex differences in human mate preferences: evolutionary hypotheses tested in 37 cultures. Behav. Brain Sci. 12, 1–49. ( 10.1017/S0140525X00023992) [DOI] [Google Scholar]
- 2.Penton-Voak IS, Jacobson A, Trivers R. 2004. Population differences in attractiveness judgements of male and female faces: comparing British and Jamaican samples. Evol. Hum. Behav. 25, 355–370. ( 10.1016/j.evolhumbehav.2004.06.002) [DOI] [Google Scholar]
- 3.Puts AP, Jones BC, DeBruine LM. 2012. Sexual selection on human faces and voices. J. Sex Res. 49, 227–243. ( 10.1080/00224499.2012.658924) [DOI] [PubMed] [Google Scholar]
- 4.Srael H. 1969. Pubertal influence upon the growth and sexual differentiation of the human mandible. Arch. Oral. Biol. 14, 583–590. ( 10.1016/0003-9969(69)90181-2) [DOI] [PubMed] [Google Scholar]
- 5.Jones D. 1995. Sexual selection, physical attractiveness, and facial neoteny. Curr. Anthropol. 36, 723–748. ( 10.1086/204427) [DOI] [Google Scholar]
- 6.Little AC, Connely J, Feinberg DR, Jones BC, Roberts SC. 2011. Human preference for masculinity differs according to context in faces, bodies, voices, and smell. Behav. Ecol. 22, 862–868. ( 10.1093/beheco/arr061) [DOI] [Google Scholar]
- 7.Little AC, Jones BC, DeBruine LM, Feinberg DR. 2008. Symmetry and sexual dimorphism in human faces: interrelated preferences suggest both signal quality. Behav. Ecol. 19, 902–908. ( 10.1093/beheco/arn049) [DOI] [Google Scholar]
- 8.Thornhill R, Gangestad SW. 2006. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evol. Hum. Behav. 27, 131–144. ( 10.1016/j.evolhumbehav.2005.06.001) [DOI] [Google Scholar]
- 9.Moore FR, Law Smith MJ, Taylor V, Perrett DI. 2011. Sexual dimorphism in the female face is a cue to health and social status but not age. Pers. Individ. Differ. 50, 1068–1073. ( 10.1016/j.paid.2011.01.026) [DOI] [Google Scholar]
- 10.Confer JC, Perilloux C, Buss DM. 2010. More than just a pretty face: men's priority shifts toward bodily attractiveness in short-term versus long-term mating contexts. Evol. Hum. Behav. 31, 348–353. ( 10.1016/j.evolhumbehav.2010.04.002) [DOI] [Google Scholar]
- 11.Schmitt DP. 2005. Sociosexuality from Argentina to Zimbabwe: a 48-nation study of sex, culture, and strategies of human mating. Behav. Brain Sci. 28, 247–275. ( 10.1017/S0140525X05000051) [DOI] [PubMed] [Google Scholar]
- 12.Welling LLM, Jones BC, DeBruine LM, Smith FG, Feinberg DR, Little AC, Al-Dujaili EAS. 2008. Men report stronger attraction to femininity in women's faces when their testosterone levels are high. Horm. Behav. 54, 703–708. ( 10.1016/j.yhbeh.2008.07.012) [DOI] [PubMed] [Google Scholar]
- 13.Jones BC, Little AC, Watkins CD, DeBruine LM. 2011. Reported sexual desire predicts men's preferences for sexually dimorphic cues in women's faces. Arch. Sex. Behav. 40, 1281–1285. ( 10.1007/s10508-010-9721-1) [DOI] [PubMed] [Google Scholar]
- 14.Schaller M, Murray DR. 2008. Pathogens, personality and culture: disease prevalence predicts worldwide variability in sociosexuality, extraversion and openness to experience. J. Pers. Soc. Psychol. 95, 212–222. ( 10.1037/0022-3514.95.1.212) [DOI] [PubMed] [Google Scholar]
- 15.Perrett DI, Lee KJ, Penton-Voak IS, Rowland DR, Yoshikawa S, Burt DM, Akamatsu S. 1998. Effects of sexual dimorphism on facial attractiveness. Nature 394, 884–887. ( 10.1038/29772) [DOI] [PubMed] [Google Scholar]
- 16.Law Smith MJ, Deady DK, Moore FR, Jones BC, Cornwell RE, Stirrat M, Lawson JF, Feinberg DR, Perrett DI. 2012. Maternal tendencies in women are associated with estrogen levels and facial femininity. Horm. Behav 61, 12–16. ( 10.1016/j.yhbeh.2011.09.005) [DOI] [PubMed] [Google Scholar]
- 17.DeBruine LM, Jones BC, Crawford JR, Welling LLM, Little AC. 2010. The health of a nation predicts their mate preferences: cross-cultural variation in women's preferences for masculinized male faces. Proc. R. Soc. B 277, 2405–2410. ( 10.1098/rspb.2009.2184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore FR, et al. 2013. Cross-cultural variation in women's preferences for cues to sex- and stress-hormones in the male face. Biol. Lett. 9, 20130050 ( 10.1098/rsbl.2013.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBruine LM, Jones BC, Little AC, Crawford JR, Welling LM. 2011. Further evidence for regional variation in women's masculinity preferences. Proc. R. Soc. B 278, 813–814. ( 10.1098/rspb.2010.2200) [DOI] [Google Scholar]
- 20.Gangestad SW, Simpson JA. 2000. The evolution of human mating: trade-offs and strategic pluralism. Behav. Brain Sci. 23, 573–644. ( 10.1017/S0140525X0000337X) [DOI] [PubMed] [Google Scholar]
- 21.Rantala MJ, Coetzee V, Moore FR, Skrinda I, Kecko S, Krama T, Kivleniece I, Krams I. 2013. Facial attractiveness is related to women's cortisol and body fat, but not with immune responsiveness. Biol. Lett. 9, 20130255 ( 10.1098/rsbl.2013.0255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiddeman B, Burt DM, Perrett DI. 2001. Computer graphics in facial perception research. IEEE Comp. Graph. Appl. 21, 42–50. ( 10.1109/38.946630) [DOI] [Google Scholar]
- 23.DeBruine LM, Jones BC, Little AC, Finlay GS. 2010. Are attractive men's faces masculine or feminine? The importance of controlling confounds in face stimuli. J. Exp. Psychol. Hum. Percept. Perform. 36, 751–758. ( 10.1037/a0016457) [DOI] [PubMed] [Google Scholar]
- 24.Meissner CA, Brigham JC. 2001. Thirty years of investigating the own-race bias in face memory for faces: a meta-analytic review. Psychol. Public Policy Law 7, 1–35. ( 10.1037/1076-8971.7.1.3) [DOI] [Google Scholar]
- 25.Little AC, Jones BC, Feinberg DR, Perrett DI. 2013. Men's strategic preferences for femininity in female faces. Br. J. Psychol . ( 10.1111/bjop.12043) [DOI] [PubMed] [Google Scholar]
- 26.Simpson JA, Gangestad SW. 1992. Sociosexuality and romantic partner choice. J. Pers. 60, 31–51. ( 10.1111/j.1467-6494.1992.tb00264.x) [DOI] [Google Scholar]
- 27.de Barra M, DeBruine LM, Jones BC, Mahmud ZH, Curtis VA. 2013. Illness in childhood predicts face preferences in adulthood. Evol. Hum. Behav. 34, 384–389. ( 10.1016/j.evolhumbehav.2013.07.001) [DOI] [Google Scholar]
- 28.Muehlenbein MP, Bribiescas RG. 2005. Testosterone mediated immune functions and male life histories. Am. J. Hum. Biol. 17, 527–558. ( 10.1002/ajhb.20419) [DOI] [PubMed] [Google Scholar]
- 29.Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. 2002. Population variation in age-related decline in male salivary testosterone. Hum. Reprod. 17, 3251–3253. ( 10.1093/humrep/17.12.3251) [DOI] [PubMed] [Google Scholar]
- 30.Little AC, DeBruine LM, Jones BC. 2010. Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proc. R. Soc. B 278, 2032–2039. ( 10.1098/rspb.2010.1925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olatunji BO, Tomarken A, Puncochar BK. 2013. Disgust propensity potentiates evaluative learning of aversion. Emotion 13, 881–890. ( 10.1037/a0032743) [DOI] [PubMed] [Google Scholar]
- 32.Gangestad SW, Haselton MG, Buss DM. 2006. Evolutionary foundations of cultural variation: evoked culture and mate preferences. Psychol. Inq. 17, 75–95. ( 10.1207/s15327965pli1702_1) [DOI] [Google Scholar]
- 33.Lee AJ, Dubbs SL, Kelly AJ, von Hippel W, Brooks RC, Zietsch BP. 2013. Human facial attributes, but not perceived intelligence, are used as cues of health and resource provision potential. Behav. Ecol. 24, 779–787. ( 10.1093/beheco/ars199) [DOI] [Google Scholar]
- 34.Stephen ID, Scott IM, Coetzee V, Pound N, Perrett DI, Penton-Voak IS. 2012. Cross-cultural effects of color, but not morphological masculinity, on perceived attractiveness of men's faces. Evol. Hum. Behav 33, 260–267. ( 10.1016/j.evolhumbehav.2011.10.003) [DOI] [Google Scholar]
- 35.Brooks R, Scott IM, Maklakov AA, Kasumovic MM, Clark AP, Penton-Voak IS. 2011. National income inequality predicts women's preferences for masculinized faces better than health does. Proc. R. Soc. B 278, 810–812. ( 10.1098/rspb.2010.0964) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: doi:10.5061/dryad.32610.


