Abstract
The traps of many carnivorous plants are red in colour. This has been widely hypothesized to serve a prey attraction function; colour has also been hypothesized to function as camouflage, preventing prey avoidance. We tested these two hypotheses in situ for the carnivorous plant Drosera rotundifolia. We conducted three separate studies: (i) prey attraction to artificial traps to isolate the influence of colour; (ii) prey attraction to artificial traps on artificial backgrounds to control the degree of contrast and (iii) observation of prey capture by D. rotundifolia to determine the effects of colour on prey capture. Prey were not attracted to green traps and were deterred from red traps. There was no evidence that camouflaged traps caught more prey. For D. rotundifolia, there was a relationship between trap colour and prey capture. However, trap colour may be confounded with other leaf traits. Thus, we conclude that for D. rotundifolia, red trap colour does not serve a prey attraction or camouflage function.
Keywords: plant–insect interactions, leaf colour, carnivorous plants, Drosera rotundifolia, prey attraction
1. Introduction
Carnivorous plants attract, trap and digest animal prey, using the nutrients gained to enhance fitness [1,2]. To attract prey, carnivorous plants use a variety of mechanisms such as olfactory [3], nectar [4,5] and visual cues [6]. Red coloration has been widely hypothesized as a visual cue used to lure potential prey, by increasing contrast with the background [7–10]. However, experimental evidence is limited and hampered by methodological issues such as a lack of ecological relevance [5,10] or confounding of attraction and capture mechanisms (for example in [11,12]). As a result, prey attraction to red carnivorous plant traps has not yet been conclusively demonstrated for any species.
In this study, we investigated in situ the role of red colour in attracting prey onto the adhesive traps of the carnivorous plant Drosera rotundifolia. Drosera rotundifolia grows on Sphagnum hummocks on ombrotrophic bogs. Their leaves form small rosettes (ca 5 cm in diameter) and catch prey (predominantly Diptera and Collembola [13]) using sticky mucilage secreted on the end of stalked glands on the leaf. Attraction of prey to these traps has not yet been demonstrated, but their leaves are a distinctive red colour, which has been hypothesized to serve a prey capture function [7,8]. This might be through direct attraction of prey or possibly by camouflaging the trap, as suggested by Jürgens et al. [3]; this might be of benefit if prey capture is a passive process where a conspicuous trap might deter prey. We tested the hypotheses that: (i) potential prey are attracted to red coloured traps and (ii) that traps that are more cryptic will be more successful at trapping prey. We also evaluated prey capture of differently coloured D. rotundifolia leaves to establish whether any differences are observed naturally.
2. Material and methods
Over a three week period during August and September 2012, we conducted three separate studies on an ombrotrophic (rain-fed) raised bog at Thorne Moors, England (53°37′55″ N, 0°54′21″ W). We used an area of approximately 100 m2 with abundant D. rotundifolia growing in a Sphagnum substrate. The three experiments were interspersed across the study area, but separated by at least 5 m to avoid interference. We constructed artificial traps by printing red and green D. rotundifolia-shaped images (four 1.7 × 1.2 cm leaves per trap) onto photopaper (electronic supplementary material, figure S1). Traps were laminated and covered with non-setting adhesive (OecoTak A5, Oecos, Hertfordshire, UK). Neutral coloured traps were prepared by excluding the coloured images (i.e. only laminate and glue), these traps were translucent rather than transparent. The colour of artificial traps mostly fell within the natural range of D. rotundifolia at the same site (table 1). After 7 days, the traps were removed and replaced with new traps in the same locations. This was repeated three times for all artificial and natural traps. Captured prey items were identified to order level. The length of each prey item was measured under a binocular microscope to determine treatment impacts on prey size as well as number.
Table 1.
Colour characteristics of artificial traps, artificial traps on artificial backgrounds and D. rotundifolia leaves.
| colour (CIELAB a*)a |
colour difference (ΔE76)b |
||||||
|---|---|---|---|---|---|---|---|
| min. | max. | mean | min. | max. | mean | ||
| D. rotundifolia traps | −12.1 | 47.5 | 22.7 | 10.2 | 56.2 | 32.9 | |
| D. rotundifolia background | −12.2 | 24.6 | −1.0 | — | — | — | |
| artificial traps on natural background | red | 14.7 | 45.1 | 27.2 | 34.6 | 67.4 | 46.6 |
| green | −15.1 | −6.4 | −10.4 | 18.0 | 73.8 | 56.2 | |
| clear | −3.2 | 5.8 | 0.6 | 18.6 | 60.5 | 46.7 | |
| artificial traps on artificial background | red on green | −11.5 (background colour) | 43.8 | 53.8 | 48.8 | ||
| red on red | 43.1 (background colour) | 10.1 | 12.9 | 11.7 | |||
| green on green | −11.5 (background colour) | 7.6 | 11.6 | 9.2 | |||
| green on red | 43.1 (background colour) | 65.5 | 71.3 | 69.0 | |||
anegative values indicate a green coloration, and positive a red coloration.
blarger values indicate greater colour contrasts.
(a). Experiment 1: are prey attracted to green or red artificial traps?
Red, green and neutral artificial traps were placed together (one of each) in random locations (n = 12 in total) on hummocks throughout the study area (see the electronic supplementary material, figure S1).
(b). Experiment 2: is prey attraction a result of trap contrast against its background?
Artificial backgrounds were constructed using the same red and green coloration as the artificial traps, printed onto A4 sized standard photo paper and then laminated. Three red and three green artificial traps were stapled onto each red or green background. Six red and six green backgrounds were randomly distributed in pairs (one red and one green) throughout the study area resulting in a 2 × 2 split-plot design.
(c). Natural observations: does Drosera rotundifolia leaf colour influence prey capture?
Sixty plants were randomly selected and labelled, before removing all captured arthropods. Plants were photographed to determine the colour of each leaf and the immediate background (a 5 × 5 cm2 centred on the plant). All the leaves on each plant were measured and prey capture recorded; data were pooled to give mean values for each plant. Leaf area was estimated based on measurements of length and width, assuming an ellipsoidal shape. After seven days, the plants were revisited and all captured arthropods removed with tweezers, this was repeated twice more
(d). Colour analysis
Leaf colour in CIE 1976 (L*a*b*) colour space (CIELAB) was determined to enable assessment of colour difference and how red or green a leaf is (based on a*). Colour differences between leaves and background (conspicuousness) were determined by calculating the delta-E 1976 (ΔE76) (details of colour analysis are in the electronic supplementary material).
(e). Statistical analysis
Prey count and length data were pooled within plants and analysed in IBM SPSS Statistics for Windows v. 20.0 [14]. Initial exploration using repeated measures ANOVA showed no statistically significant interaction between sampling date and any of the experimental treatments. Therefore, data from the three sampling dates were pooled for all analyses. Experiment 1 was analysed using a one-way ANOVA, and experiment 2 was analysed as a split-plot design. Differences between treatments were assessed with Fisher's LSD. Homoscedasticity was tested using plots of residuals, and normality was tested using normal probability plots. The observational study was analysed using multiple logistic regression to determine the impact of the measured leaf variables on the probability of prey capture, and correlation to determine the relationships between other measured variables. Differences in the taxa of captured prey were analysed using Pearson's χ2 test.
3. Results
For all trap types, Diptera were the most common arthropod order caught (57–84% of total), followed by Hymenoptera (7.5–17.4%) then Collembola (5.8–16.5%) (figure 1, χ2 = 1273.84, n = 705, d.f. = 4, p < 0.001). Capture rates differed between trap types (χ2 = 173.58, n = 70, d.f. = 7, p < 0.001): traps in experiment 1 (103–139 arthropods/7 days) and natural traps (121 arthropods/7 days) caught more than traps in experiment 2 (32–53 arthropods/7 days). There was a significant interaction between trap type and the distribution of prey taxa (figure 1, χ2 = 50.10, n = 705, d.f. = 28, p = 0.01), owing to small differences in capture by natural compared with artificial traps (i.e. Diptera being a smaller component and Hymenoptera and Collembola being a larger component of natural traps).
Figure 1.
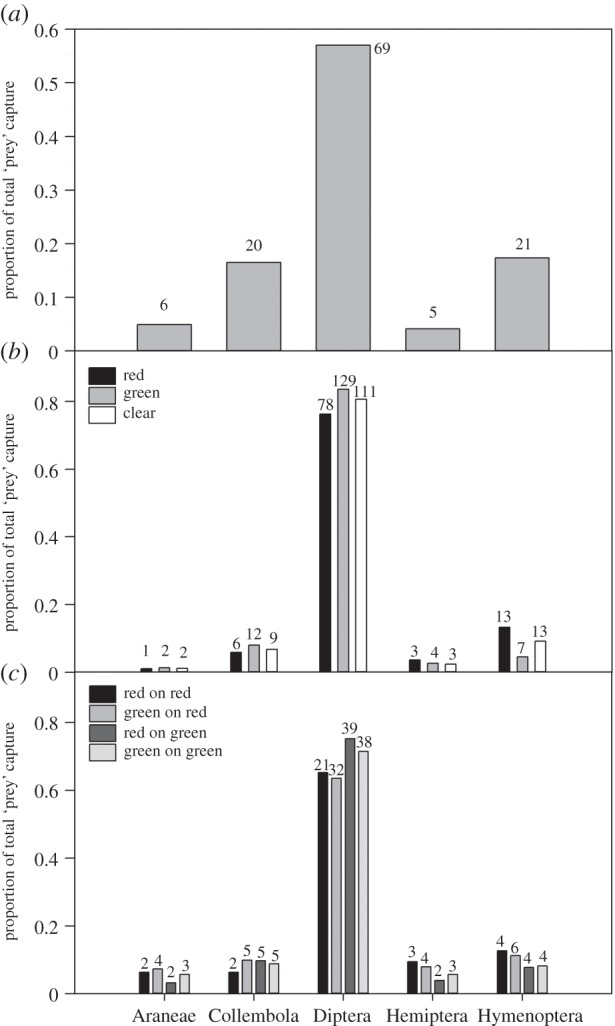
Proportion of total arthropod capture in each taxa for (a) D. rotundifolia leaves, (b) red, green and clear artificial traps, and (c) artificial red and green traps on red and green artificial backgrounds. Numbers above the bars indicate the number of prey in each group.
(a). Experiment 1: artificial traps
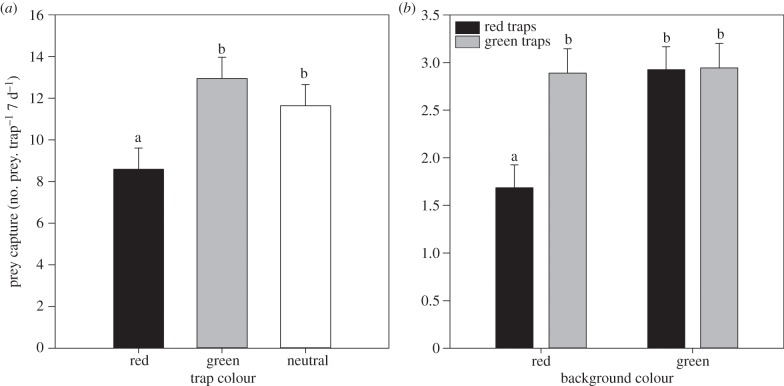
Green and clear artificial traps caught significantly more prey than red traps (F2,33 = 4.807, p < 0.001; figure 2a) with no significant difference in the length of the captured invertebrates apparent (F2,33 = 0.676, p = 0.516).
Figure 2.
Numbers of arthropods captured by (a) artificial traps on artificial backgrounds and (b) red, green and clear artificial traps. Bars show mean ± s.e. Different letters above bars indicate significant differences (Fisher's LSD).
(b). Experiment 2: artificial background
Trap and background colour significantly affected prey capture rates, and the interaction between the two was statistically significant (trap colour—F1,5 = 12.707, p = 0.005; background colour—F1,5 = 7.709, p = 0.020; interaction—F1,10 = 11.949, p = 0.006). Red traps on red backgrounds attracted fewest prey; other combinations did not differ (figure 2b). There was no significant impact of trap or background colour on the length of prey captured (trap colour—F1,34 = 0.599, p = 0.457; background colour—F1,10 = 0.019, p = 0.892; interaction—F1,10 = 3.792, p = 0.080).
(c). Experiment 3: Drosera rotundifolia
Plants with redder leaves had a higher probability of prey capture success (logistic regression: Wald(1) = 7.052, p = 0.008, B ± s.e. = 0.059 ± 0.022), as did plants with more leaves (logistic regression: Wald(1) = 3.880, p = 0.49, B ± s.e. = 0.249 ± 0.127). There was no impact of leaf size (logistic regression: Wald(1) = 1.803, p = 0.179) or conspicuousness (ΔE76) (Wald(1) = =1.429,
p = 0.232). (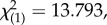 p = 0.008
p = 0.008 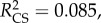
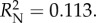 )
)
There were significant positive correlations between leaf colour (a*), ΔE76 and leaf area, and a significant negative correlation between leaf area and the number of leaves on a plant (table 2). There was no correlation between the number of leaves and trap colour or ΔE76. For those leaves that captured prey, plants with fewer and larger leaves caught more and larger prey, but leaf area and number were both negatively correlated with prey capture efficiency (table 2). For these plants, there was no correlation between trap colour or conspicuousness and any of the measures of prey capture, with the exception that more conspicuous traps were less efficient at catching prey.
Table 2.
Results of Pearson's correlation between leaf characteristics for all plants, and between leaf characteristics and prey capture for those plants that captured prey. Colour, trap colour (a*); leaves, number of leaves; area, leaf area; prey number, number of prey captured per plant; prey length, mean length of captured prey; capture efficiency, number of prey captured per unit leaf/trap area. Values shown in italics are statistically significant (p < 0.05).
| area | colour | leaves | ΔE76 | ||
|---|---|---|---|---|---|
| all plants | |||||
| area | Pearson's correlation | 0.175 | −0.177 | 0.216 | |
| p | 0.029 | 0.027 | 0.007 | ||
| colour | Pearson's correlation | 0.027 | 0.652 | ||
| p | 0.742 | 0.000 | |||
| leaves | Pearson's correlation | 0.095 | |||
| p | 0.240 | ||||
| only plants that captured prey | |||||
| prey number | Pearson's correlation | 0.267 | 0.180 | −0.501 | −0.018 |
| p | 0.016 | 0.108 | <0.001 | 0.874 | |
| prey length | Pearson's correlation | 0.212 | 0.145 | −0.283 | 0.004 |
| p | 0.058 | 0.195 | 0.010 | 0.973 | |
| capture efficiency | Pearson's correlation | −0.375 | 0.020 | −0.397 | −0.271 |
| p | 0.001 | 0.862 | <0.001 | 0.015 | |
4. Discussion
In our study, a red coloration did not fulfil a prey attraction function or serve as advantageous camouflage in D. rotundifolia, as has been previously assumed or suggested [3,7,8]. Instead, red coloration might deter potential prey. This is not entirely surprising. Red detection is more difficult for species without red receptors than for those with red receptors [15]. It is unlikely that the red coloration of carnivorous plant traps would evolve as a visual cue to attract prey, unless they capture ecologically significant numbers of these ‘red-sensitive’ prey. Diptera do not possess red receptors [16] and were the most abundant prey species for the D. rotundifolia studied here. There would therefore be no likely ecological advantage of attempting to attract these prey using red traps.
The covariation among the various trap characteristics and prey capture suggests trade-offs in terms of investment in traps. Leaf size–number trade-offs have been demonstrated both between and within species [17,18]. We found a leaf size–area trade-off for D. rotundifolia. In addition, having fewer, smaller leaves appears to be the most efficient (i.e. prey capture per unit trap area) way to capture prey, though having more leaves increases the probability of any prey capture at all. This benefit will be balanced against the other benefits of small or large leaves, so in D. rotundifolia and maybe other carnivorous plants we might expect this trade-off to alter in relation to resource availability.
Artificial and natural trap colour was coincident and they caught similar prey, supporting the use of artificial traps as a surrogate for natural traps. Interestingly, redder natural D. rotundifolia traps had a greater likelihood of prey capture than greener traps. The artificial traps measured true colour attraction, but prey presence on the traps of D. rotundifolia is a measure of both prey attraction (not limited to colour attraction) and capture, with potential for confounding of factors. Reduced prey attraction for red traps on red backgrounds is likely to be a consequence of potential prey being deterred from red (owing to the large area of red present in these experimental units) rather than an explicit effect of the degree of crypsis. Additionally, we did not explicitly test the role of UV reflection which might play a role in prey attraction [6]. The prey captured by D. rotundifolia in our study are able to perceive UV [16] and there may be variation in UV reflectance of red and green traps not accounted for with the artificial traps. Thus, UV pigmentation might have a prey attraction function that we could not detect.
Acknowledgements
We thank Julian Small of Natural England for site access and Lucy Kitcher for fieldwork assistance. Many thanks to Aaron Ellison and two anonymous reviewers for their useful and constructive advice.
Data accessibility
Data deposited in dryad digital repository: http://dx.doi.org/10.5061/dryad.h7s03 [19].
References
- 1.Juniper BBE, Robins RJ, Joel DM. 1989. The carnivorous plants. New York, NY: Academic Press. [Google Scholar]
- 2.Ellison AM, Gotelli NJ. 2001. Evolutionary ecology of carnivorous plants. Trends Ecol. Evol. 16, 623–629. ( 10.1016/S0169-5347(01)02269-8) [DOI] [Google Scholar]
- 3.Jürgens A, El-Sayed AM, Suckling DM. 2009. Do carnivorous plants use volatiles for attracting prey insects? Funct. Ecol. 23, 875–887. ( 10.1111/j.1365-2435.2009.01626.x) [DOI] [Google Scholar]
- 4.Bauer U, Willmes C, Federle W. 2009. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Ann. Bot. 103, 1219–1226. ( 10.1093/aob/mcp065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett KF, Ellison AM. 2009. Nectar, not colour, may lure insects to their death. Biol. Lett. 5, 469–472. ( 10.1098/rsbl.2009.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joel D, Juniper B, Dafni A. 1985. Ultraviolet patterns in the traps of carnivorous plants. New Phytol. 101, 585–593. ( 10.1111/j.1469-8137.1985.tb02864.x) [DOI] [Google Scholar]
- 7.Lloyd FE. 1942. The carnivorous plants. Waltham, MA: The Chronica Botanica Company. [Google Scholar]
- 8.Ichiishi S, Nagamitsu T, Kondo Y, Iwashina T, Kondo K, Tagashira N. 1999. Effects of macro-components and sucrose in the medium on in vitro red-colour pigmentation in Dionea muscipula Ellis and Drosera spathulata Labill. Plant Biotechnol. 16, 235–238. ( 10.5511/plantbiotechnology.16.235) [DOI] [Google Scholar]
- 9.Jaffe K, Michelangeli F, Gonzalez JM, Miras B, Ruiz MC. 1992. Carnivory in pitcher plants of the genus Heliamphora (Sarraceniaceae). New Phytol. 122, 733–744. ( 10.1111/j.1469-8137.1992.tb00102.x) [DOI] [Google Scholar]
- 10.Schaefer HM, Ruxton GD. 2008. Fatal attraction: carnivorous plants roll out the red carpet to lure insects. Biol. Lett. 4, 153–155. ( 10.1098/rsbl.2007.0607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlovic A, Krausko M, Libiaková M, Adamec L. 2014. Feeding on prey increases photosynthetic efficiency in the carnivorous sundew Drosera capensis. Ann. Bot. 113, 69–78. ( 10.1093/aob/mct254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cresswell JE. 1993. The morphological correlates of prey capture and resource parasitism in pitchers of the carnivorous plant Sarracenia purpurea. Am. Midl. Nat. 129, 35–41. ( 10.2307/2426433) [DOI] [Google Scholar]
- 13.Ellison AM, Gotelli NJ. 2009. Energetics and the evolution of carnivorous plants: Darwin's ‘most wonderful plants in the world’. J. Exp. Bot. 60, 19–42. ( 10.1093/jxb/ern179) [DOI] [PubMed] [Google Scholar]
- 14.IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
- 15.Chittka L, Spaethe J, Schmidt A, Hickelsberger A. 2001. Adaptation, constraint, and chance in the evolution of flower color and pollinator color vision. In Cognitive ecology of pollination (eds Chittka L, Thompson J.), pp. 106–126. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510. ( 10.1146/annurev.ento.46.1.471) [DOI] [PubMed] [Google Scholar]
- 17.Whitman T, Aarssen LW. 2009. The leaf size/number trade-off in herbaceous angiosperms. J. Plant Ecol. 3, 49–58. ( 10.1093/jpe/rtp018) [DOI] [Google Scholar]
- 18.Kleiman D, Aarsen LW. 2007. The leaf size/number trade-off in trees. J. Ecol. 95, 376–382. ( 10.1111/j.1365-2745.2006.01205.x) [DOI] [Google Scholar]
- 19.Foot G, Rice S, Millett J. 2014. Data from: trap colour of the carnivorous plant Drosera rotundifolia does not serve a prey attraction or camouflage function. Dryad Digital Repository. ( 10.5061/dryad.h7s03) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposited in dryad digital repository: http://dx.doi.org/10.5061/dryad.h7s03 [19].