Abstract
Few studies have examined how landmarks affect territories' fundamental characteristics. In this field study, we investigated effects of landmarks on territory size, shape and location in a cichlid fish (Amatitlania siquia). We provided cans as breeding sites and used plastic plants as landmarks. During 10 min trials, we recorded locations where residents chased intruders and used those locations to outline and measure the territory. In two experiments, we observed pairs without landmarks and with either a point landmark (one plant) or linear landmark (four plants) placed near the nest can. We alternated which trial occurred first and performed the second trial 24 h after the first. Territories were approximately round without landmarks or with a point landmark but were significantly more elongated when we added a linear landmark. Without landmarks, nests were centrally located; however, with any landmark, pairs set territory boundaries closer to the landmark and thus the nest. Territory size was significantly reduced in the presence of any landmark. This reduction suggests that a smaller territory with well-defined boundaries has greater benefits than a larger territory with less well-defined borders.
Keywords: landmark, cichlid, defensibility territory boundary
1. Introduction
Intraspecific variation in territory size and shape can affect individual fitness and the social interactions of territorial species [1,2]. In addition, such differences among territories can have strong influences on the structure and dynamics of territorial populations [3]. Most theoretical and empirical work has focused on optimization of territory size, identifying resource distribution and defensive costs as primary determinants of size [2]. By contrast, there have been few studies explicitly investigating territory shape (but see [4–7]). Theoretical work on territory shape predicts that territories should be round and, in nature, many territories do approximate a circle [1,3,8,9]. The idea that a round territory is optimal is based on the assumptions that defence costs are minimized in a round territory and that resources are distributed uniformly within the territory [6].
However, in nature these conditions are not always met, and some studies suggest that landmarks [10] and uneven topography [6] can affect territory shape and size by altering defensibility. Although we do not know how malleable territorial boundaries are, responsiveness to changes in the landscape could benefit territorial residents by reducing their defensive costs. Such responsiveness would also enable researchers to manipulate territories to test theoretical predictions about territoriality. For this field study, we used Amatitlania siquia, a species in the convict cichlid group native to Costa Rica and Nicaragua. It is a substrate-brooding cichlid that provides bi-parental care for offspring, and pairs defend breeding territories around their nests. We placed point and linear landmarks near pairs' nests to determine whether and how these fish might change the shape, size and boundaries of their territories in response.
2. Material and methods
We conducted this study in Lake Xiloá, Nicaragua, in December 2012 using SCUBA. The study area was 85 × 30 m, ranged from 8 to 15 m in depth and had an even silt/sand substrate without rocks or benthic macrophytes. Owing to the intense competition for breeding sites among cichlids in Lake Xiloá, A. siquia tend to occupy any suitable site for spawning. This feature increased the likelihood of success for the experimental manipulation. We provided empty beer cans (12 cm in height and 6 cm in diameter) with their tops removed as artificial breeding sites and allowed 7 days for breeding pairs to nest. As landmarks, we used open-branched plastic plants that were 6 cm wide and similar in form to the naturally occurring Chara sp. in the lake. Fish were readily visible through the plants, which we set 6 cm from the nest cans, and residents commonly swam and chased intruders that they saw through the plants. We compared territories' shape, size and boundary location in trials without landmarks to trials with point landmarks (one plant; N = 24) or linear landmarks (four plants in a line and immediately adjacent to one another; N = 24). We observed each focal pair with and without landmarks, alternating which trial occurred first and performing the second trial 24 h after the first. Focal territories were at least 2 m distant from any other territory and did not share any boundaries.
In trials, we observed the residents' behaviour for 10 min. Breeding pairs typically remain near their nest and sally out to chase intruders. Each time a resident chased an intruder, we recorded the chase's location, which we defined as the intruder's position when the resident initiated the chase [11]. We used these locations to outline territories' boundaries using the minimum convex polygon method [12]. We analysed a scaled digital image of each territory using ImageJ software to compare territory sizes. To assess territory shape, we measured territory length, defined as the greatest linear distance between territory boundaries, and territory width, the greatest distance between boundaries perpendicular to the length. We used the ratio of length to width as a measure of territory shape. To measure boundary shifts in response to landmarks, we determined before trials where we would place landmarks. We then measured distance from nest to boundary in that direction in both trials, whether or not landmarks were present. We performed Wilcoxon signed-rank tests to analyse differences in territory shapes and sizes. To analyse distances from nest to boundary, we used Wilcoxon signed-rank tests for non-normal data and paired t-tests for normal data.
3. Results
Territory size was significantly reduced in the presence of both point and linear landmarks. Median territory size was 0.27 m2 when there was no landmark, but when we added a point landmark it was 0.19 m2 (Wilcoxon: W = 61.0, n = 24, p = 0.02). Similarly, median territory size was 0.19 m2 with no landmark but 0.15 m2 with a linear landmark (Wilcoxon: W = 55.0, n = 24, p = 0.01). In the point landmark experiment, the median number of chases was 27 with no landmark (range 6–71) and 26.5 with a point landmark (range 9–74). Similar numbers of chases were observed in the linear landmark experiment (no landmark: median = 22, range 8–76; linear landmark: median = 21, range 4–55).
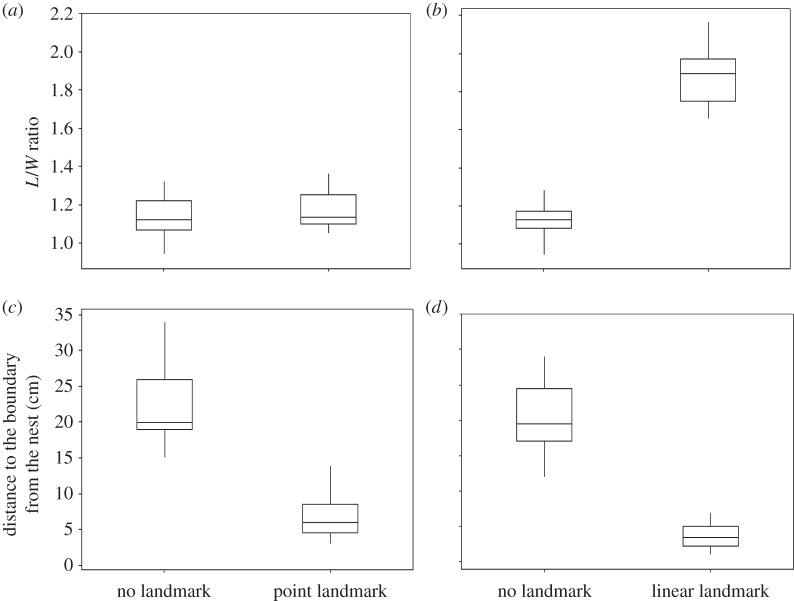
In the point landmark experiment, territory shape was always approximately circular and thus did not change across trials (figure 1a; Wilcoxon: W = 124.0, n = 24, p = 0.31). However, boundary location did change. With no landmark, the nest was near the centre of the territory. When we added a point landmark, fish moved their territory boundary near the landmark and thus the nest (figure 1c; Wilcoxon: W = 306.5, n = 24, p < 0.05). However, pairs did not compensate for that loss of area by significantly changing the distance from the nest to the boundary opposite the landmark (t-test: t = 1.13, d.f. = 22, p = 0.27).
Figure 1.
Shape of the territory (a) in the point landmark treatment, and (b) in the linear landmark treatment. The presence of point landmarks did not alter territory shape, but convicts modified the shape from round to elongated when linear landmarks were present. Distance to the boundary from the nest (c) in the point landmark treatment, and (d) in the linear landmark treatment. Fish moved the boundary closer to the landmark and thus the nest when they were present.
A similar boundary shift occurred in linear landmark trials. Again, with no landmark nests were near the centres of territories, but when we added a linear landmark fish set their boundary near the landmark and thus the nest (figure 1d; Wilcoxon: W = 240.0, n = 24, p < 0.05). As before, the location of the boundary opposite the landmark did not change significantly across trials (t-test: t = 1.72, d.f. = 22, p = 0.10). However, in contrast to the point landmark experiment, a significant change in shape also occurred. With a linear landmark, territories were more elongated than when landmarks were absent (figure 1b; Wilcoxon: W = 300.0, n = 24, p < 0.05). Territories were always elongated parallel to the linear landmark (Wilcoxon: W = 0, n = 24, p < 0.05). The change in shape resulted from the territories becoming more narrow, as there was no significant increase in length parallel to the landmark (Wilcoxon: W = 98.0, n = 24, p = 0.11).
4. Discussion
Landmarks affected territory shape, size and boundary location relative to nests. Amatitlania siquia pairs adjusted their territorial boundaries within 24 h in response to the presence or absence of a landmark. Territories without landmarks were nearly circular, in accordance with the generally accepted idea of the optimal territory shape [1,3,8,9], with the nest approximately in the centre. A centrally located nest is likely to facilitate brood defence against predators. When either point or linear landmarks were present, fish set their territorial boundary near the landmark and thus the nest. The willingness of fish to move the boundary closer to the nest suggests that landmarked boundaries provide substantial benefits, given that having the boundary near the nest may increase the risk of predation of fry. In other species, setting boundaries at landmarks is strongly linked with a reduction in defensive costs [13–15]. Clearly defined boundaries can allow residents to determine easily when another individual has intruded and can reduce time spent defending against neighbours [13]. Although landmarks could lower defensive costs, adjusting territory boundaries may be cost-effective only when territories are not contiguous [16,17], as in this study, or when individuals are not highly territorial [18,19]. A strong response to landmarks may be most likely in species defending territories in simple, homogeneous habitats, such as the fish we observed in this study.
Despite not being constrained by neighbours, fish did not alter their boundaries on the side of their territory away from the landmark. Accordingly, their use of landmarks as boundaries resulted in a 29.6% reduction in territory size in the presence of a point landmark and a 21.1% reduction with a linear landmark. These reductions suggest that it may be beneficial to defend a smaller territory with well-defined boundaries compared with a larger area with poorly defined boundaries. Given that pairs mostly stay near their nests, it may be too energetically costly to chase distant intruders in order to maintain a set territory size, particularly if such intruders are relatively unthreatening to fry or resident pairs. In addition, if residents extended their territory farther from the nest, they might increase their risk of losing fry because it would take longer to return to the nest after chasing more distant intruders.
Territories were approximately round when no landmarks or a simple point landmark was present, but territories with linear landmarks were significantly more elongated. Similarly elongated territories in other species have often been observed in the field, suggesting that this may be a common phenomenon. Such territories may occur due to landmarks (e.g. [10,20]) and can also result from habitat or topographic constraints [10,21,22] and linear resource patches [23,24].
This is the first field experiment to demonstrate that landmarks can affect both the size and shape of territories. Past research examining the theoretical foundations of territoriality, such as the effects of territory size and shape on residents' fitness, has been confounded by individual differences among territorial residents. Our study suggests a new technique, the use of simple landmarks to manipulate territories, that will enable researchers to easily address these questions. Future studies should also address the costs and benefits associated with landmarks over extended periods of time in order to fully understand their effects on territory boundaries and defence.
Acknowledgements
We thank K. McKaye, E. van den Bergh and L. Canda for facilities and field assistance at Lake Xiloá.
Funding statement
This research was partially funded by a Guy Jordan award from the American Cichlid Association and an Ideawild equipment grant to P.S.S.
References
- 1.Hixon M. 1980. Food production and competitor density as the determinants of feeding territory size. Am. Nat. 115, 510–530. ( 10.1086/283577) [DOI] [Google Scholar]
- 2.Schoener TW. 1983. Simple models of optimal feeding territory size: a reconciliation. Am. Nat. 121, 608–629. ( 10.1086/284090) [DOI] [Google Scholar]
- 3.Adams ES. 2001. Approaches to the study of territory size and shape. Annu. Rev. Ecol. Evol. Syst. 32, 277–303. ( 10.1146/annurev.ecolsys.32.081501.114034) [DOI] [Google Scholar]
- 4.Getty T. 1981. Analysis of central-place space use patterns: the elastic disc revisited. Ecology 62, 907–914. ( 10.2307/1936988) [DOI] [Google Scholar]
- 5.Ford R. 1983. Home range in a patchy environment: optimal foraging predictions. Am. Zool. 23, 315–326. [Google Scholar]
- 6.Eason PK. 1992. Optimization of territory shape in heterogeneous habitats: a field study of the red-capped cardinal (Paroaria gularis). J. Anim. Ecol. 61, 411–424. [Google Scholar]
- 7.Eason PK, Stamps JA. 1992. The effect of visibility on territory size and shape. Behav. Ecol. 3, 166–172. ( 10.1093/beheco/3.2.166) [DOI] [Google Scholar]
- 8.Andersson M. 1978. Optimal foraging area: size and allocation of search effort. Theor. Popul. Biol. 13, 397–409. ( 10.1016/0040-5809(78)90054-0) [DOI] [PubMed] [Google Scholar]
- 9.Dill L. 1978. An energy-based model of optimal feeding territory size. Theor. Popul. Biol. 14, 396–429. ( 10.1016/0040-5809(78)90016-3) [DOI] [PubMed] [Google Scholar]
- 10.Watson A, Miller GR. 1971. Territory size and aggression in a fluctuating red grouse population. J. Anim. Ecol. 40, 367–383. ( 10.2307/3251) [DOI] [Google Scholar]
- 11.Breau C, Grant JWA. 2002. Manipulating territory size via vegetation structure: optimal size of area guarded by the convict cichlid (Pisces, Cichlidae). Can. J. Zool. 80, 376–380. ( 10.1139/z02-002) [DOI] [Google Scholar]
- 12.Mohr CO. 1947. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 37, 223–249. ( 10.2307/2421652) [DOI] [Google Scholar]
- 13.Eason PK, Cobbs GA, Trinca KG. 1999. The use of landmarks to define territorial boundaries. Anim. Behav. 58, 85–91. ( 10.1006/anbe.1999.1133) [DOI] [PubMed] [Google Scholar]
- 14.LaManna JR, Eason PK. 2003. Effects of landmarks on territorial establishment. Anim. Behav. 65, 471–478. ( 10.1006/anbe.2003.2095) [DOI] [Google Scholar]
- 15.Heap S, Byrne P, Stuart-Fox D. 2012. The adoption of landmarks for territorial boundaries. Anim. Behav. 83, 871–878. ( 10.1016/j.anbehav.2012.01.016) [DOI] [Google Scholar]
- 16.Eberhard JR, Ewald PW. 1994. Food availability, intrusion pressure, and territory size: an experimental study of Anna's Hummingbirds (Calypte anna). Behav. Ecol. Sociobiol. 34, 11–18. [Google Scholar]
- 17.Sherman PT, Eason PK. 1998. Size determinants in territories with inflexible boundaries: manipulation experiments on white-winged trumpeters’ territories. Ecology 79, 1147–1159. [Google Scholar]
- 18.Mares MA, Lacher TE, Willig MR, Bitar NA, Adams R, Klinger A, Tazik D. 1982. An experimental analysis of social spacing in Tamias striatus . Ecology 63, 267–273. ( 10.2307/1938940) [DOI] [Google Scholar]
- 19.Sullivan TP, Klenner W. 1992. Response to Koford: red squirrels and supplemental feeding. J. Mammal. 73, 933–936. ( 10.2307/1382219) [DOI] [Google Scholar]
- 20.Lack D. 1943. The life of the Robin, 1st edn London, UK. [Google Scholar]
- 21.Welsh DA. 1975. Savannah sparrow breeding and territoriality on a Nova Scotia dune beach. Auk 92, 235–251. ( 10.2307/4084553) [DOI] [Google Scholar]
- 22.Reid ML, Weatherhead PJ. 1988. Topographical constraints on competition for territories. Oikos 51, 115–117. ( 10.2307/3565819) [DOI] [Google Scholar]
- 23.Kesler DC. 2012. Foraging habitat distributions affect territory size and shape in the Tuamotu kingfisher. Int. J. Zool. 2012, 1–7. ( 10.1155/2012/632969) [DOI] [Google Scholar]
- 24.Davies NB. 1976. Food, flocking and territorial behaviour of the pied wagtail (Motacilla alba yarrellii) in winter. J. Anim. Ecol. 45, 235–253. ( 10.2307/3777) [DOI] [Google Scholar]