Abstract
We investigated the neuroanatomy of the macroglomerular complex (MGC), which is involved in sex pheromone processing, in five species in the subfamily Bombycinae, including Ernolatia moorei, Trilocha varians, Rondotia menciana, Bombyx mandarina and Bombyx mori. The glomerulus located at the dorsal-most part of the olfactory centre shows the largest volume in moth species examined to date. Such normal glomerular organization has been observed in E. moorei and T. varians, which use a two-component mixture and includes the compound bombykal as a mating signal. By contrast, the other three species, which use another component as a single attractant, exhibited a modified arrangement of the MGC. This correlation between pheromone usage and neural organization may be useful for understanding the process of speciation.
Keywords: antennal lobe, Bombycidae, neurophylogeny, pheromone
1. Introduction
Sex pheromones are widely used for communication in Lepidoptera [1]. Pheromone information is processed by a group of dorsally located sexually dimorphic glomeruli in the macroglomerular complex (MGC), which functions to discriminate sex pheromones. Acquisition of such dimorphic regions may have occurred at multiple times during evolution [2]. In the MGC, individual glomeruli process specific pheromone components [3]. Organization of the MGC is diverse among moth species [4]. Morphological features conserved across lepidopteran species include the following: (i) the glomerulus, with the largest volume located in the dorsal-most region, at the entry site of the antennal nerve and (ii) smaller glomeruli (also known as satellite glomeruli in [4]), which are located ventrally to the largest glomerulus.
The modification of pheromone components drives speciation [5], which may cause changes in neural circuits for pheromone recognition and are often associated with structural changes in the MGC [6]. Because the diversity in glomerular organization in the MGC may reflect the different pheromone components used, comparative neuroanatomy among closely related moth species may be useful for studying their phylogenetic relationships [7].
In this study, we analysed the organization of the primary olfactory centre, the antennal lobe (AL) in five closely related species of the subfamily Bombycinae, in which sex pheromone compounds have been systematically examined [8]. These species use similar compounds that differ only in their terminal functional groups (i.e. alcohol, aldehyde or acetate), with different combinations. Trilocha varians uses a combination of (E,Z)-10,12-hexadecadienal (bombykal) and (E,Z)-10,12-hexadecadienyl acetate (bombykyl acetate), an acetate derivative of (E,Z)-10,12-hexadecadienol (bombykol) as an attractive component mixture [8], which is also widely used in the closely related families, Sphingidae and Saturnidae. Ernolatia moorei uses the same compounds as sex pheromones (T. Daimon, T. Fujii, T. Shimada & Y. Ishikawa, unpublished observation). Three other species, Bombyx mori, Bombyx mandarina and Rondotia menciana, use different individual components as single attractive compounds. Bombyx mori and B. mandarina use bombykol as an attractive component [9], whereas R. menciana uses bombykyl acetate [10]. Although bombykal has been reported to be a minor pheromone component in B. mori, this compound has an inhibitory effect [9,11]. We examined the possible neuroanatomical correlations to explain the differences observed in pheromone components among species.
2. Material and methods
Bombyx mori (Lepidoptera: Bombycidae: Bombycinae) larvae were reared on an artificial diet (Silk Mate 2S and PS; Nosan Bio Department, Japan) at 26°C and 60% relative humidity under a long-day photoperiod regime (16 L : 8D). Trilocha varians and E. moorei larvae were reared on the leaves of Ficus microcarpa as described previously [12]. Bombyx mandarina and R. menciana larvae were reared on mulberry leaves at 26°C under a 12 L : 12 D photoperiod. Animals were used within 3–7 days of eclosion.
The brain was dissected in saline solution containing 140 mM NaCl, 5 mM KCl, 7 mM CaCl2, 1 mM MgCl2, 4 mM NaHCO3, 5 mM trehalose, 5 mM N-tris (hydroxymethyl) methyl-2-aminoethanesulfonic acid and 100 mM sucrose (pH 7.3). The sample was then incubated with 0.1% Lucifer yellow CH (Sigma-Aldrich, St Louis, MO, USA) in saline solution for staining. The brain was fixed for 4–10 h at room temperature using 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3) containing 10% sucrose, dehydrated in a series of ethanol solutions and cleared in methyl salicylate (Wako, Osaka, Japan). Each stained tissue was frontally imaged using a confocal imaging system (LSM510; Carl Zeiss, Jena, Germany) with a Plan Apochromat 40× (numerical aperture, 1.0) objective. The tissues were examined at an excitation wavelength of 458 nm using a long-pass emission filter (more than 475 nm) in whole mounts. Serial optical sections were acquired at 0.7 µm intervals. We counted the number of glomeruli in the AL in confocal images. Manual tracing and volume measurement were carried out using AVIZO v. 6.0 (Visage Imaging, San Diego, CA, USA).
3. Results
According to the terminology used in previous studies, we referred to the glomeruli in alphabetical order [4]. The dorsal-most glomerulus was termed ‘a’, with other glomeruli named from the medial to the lateral side. For Bombycinae, the glomeruli in the MGC were aligned at the dorsoventral axis and were therefore we named the remaining glomeruli in the alignment sequence on this axis. ‘a’ and ‘b’ glomeruli correspond to the toroid and cumulus in bombycid moths. To verify that these glomeruli are male-specific, we also evaluated female ALs in four species (see the electronic supplementary material, figure S1).
Ernolatia moorei was found to have a total of 60.4 ± 1.7 glomeruli (n = 6), two of which were male-specific (figure 1a). Trilocha varians contained a total of 60.7 ± 3.0 glomeruli (n = 7), two of which were male-specific (figure 1b). In these two species, the cumulus and toroid volumes were similar. Rondotia menciana had a total of 60.8 ± 2.5 glomeruli (n = 5), four glomeruli of which were in the MGC (figure 1c). Two dorsally located glomeruli occupied a large volume. The two additional smaller glomeruli were located ventrally. The shape of these glomeruli was highly elongated, which was similar to the ‘horseshoe’ glomerulus described in B. mori [13]. Bombyx mandarina had a total of 66 ± 1.2 glomeruli (n = 5), four of which were male-specific (figure 1d). Bombyx mori had 64 ± 2 glomeruli in total [14], with four glomeruli present in the MGC (figure 1e). For these three species, the volume of the toroid was approximately two times larger than the volume of the cumulus. In all species, glomeruli in the MGC were distorted compared with most ordinary glomeruli. This feature was clear, particularly for the toroid. Some ordinary glomeruli located in the posterior region of the AL were also distorted.
Figure. 1.
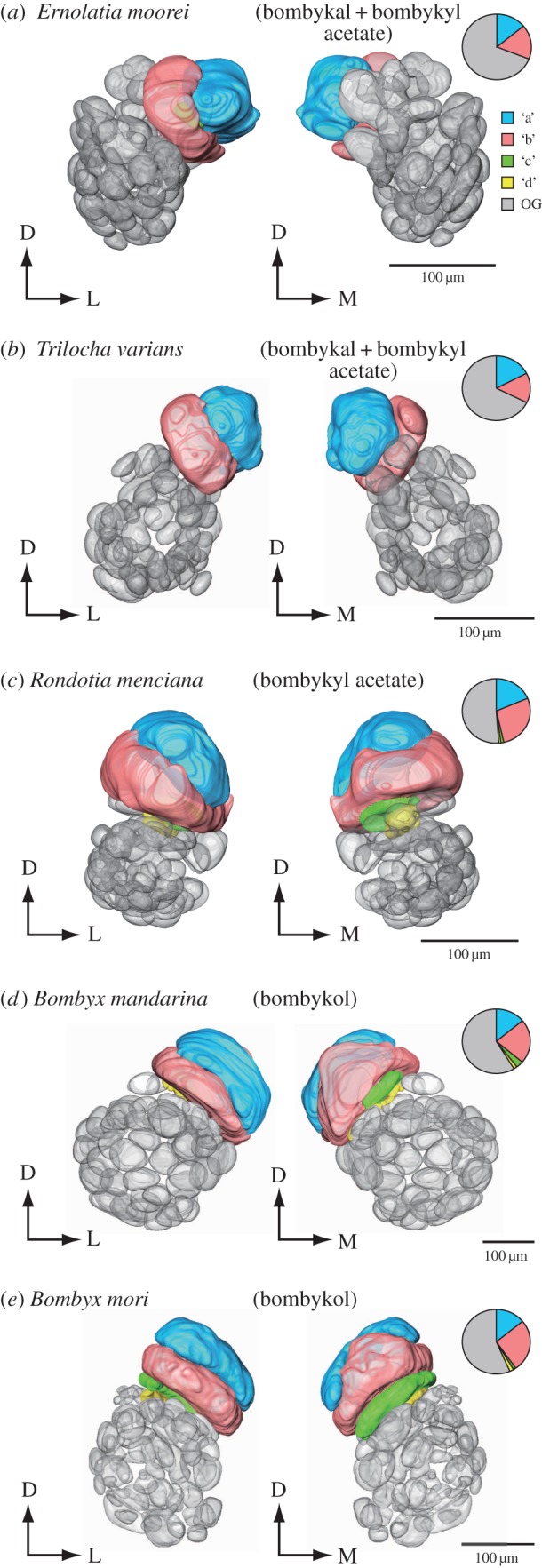
Structure of the AL of five moth species. AL of (a) male E. moorei, (b) T. varians, (c) R. menciana, (d) B. mandarina and (e) B. mori. Anterior (left) and posterior views (right) are shown. Relative volume for MGC glomeruli and ordinary glomeruli for individual data are shown in the pie chart. Attractive pheromone components for each species are shown in parentheses. Male-specific sexually dimorphic glomeruli are shown in colour. Glomeruli were named according to the nomenclature raised by Hansson [4]. ‘a’, ‘b’, ‘c’ and ‘d’ are coloured with blue, red, green and yellow, respectively. Ordinary glomeruli are shown in grey. D, dorsal; L, lateral; M, medial.
In most previously studied cases, the glomerulus located in the dorsal-most region occupies the largest volume of the AL [4]. All Bombycinae species showed similar organization (i.e. a combination of two large glomeruli: cumulus and toroid, and additional smaller glomeruli). To compare the organization, we calculated the volume ratio of the cumulus to the toroid (figure 2). The volume ratios were 1.169 ± 0.161 for E. moorei (n = 10), and 1.220 ± 0.077 for T. varians (n = 8) and were significantly larger than values for the other three species (0.628 ± 0.027 for R. menciana (n = 5), 0.545 ± 0.067 for B. mandarina (n = 7), 0.542 ± 0.072 for B. mori (n = 16)) (*p < 10−15, analysis of variance followed by Scheffe's F-test).
Figure 2.
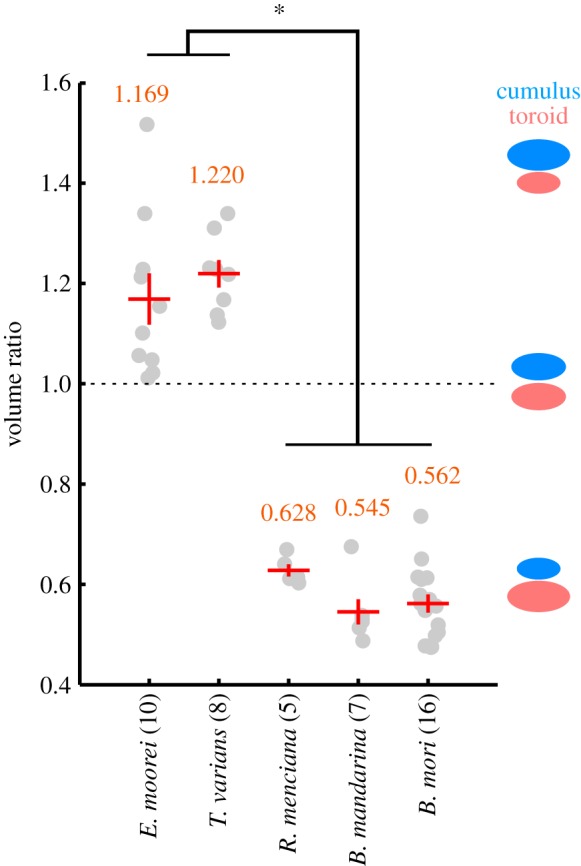
Comparison of cumulus–toroid volume ratio. Relative volume values were obtained as the absolute volume of the cumulus divided by that of the toroid. Grey circles represent the value for individual samples. Red symbols represent mean ± s.e.m. Average values are indicated in orange. Volume ratio larger than 1 means a cumulus that is larger than the toroid and vice versa. Bombyx mori, B. mandarina and R. menciana showed significantly lower value than E. moorei and T. varians. *p < 10−15, analysis of variance followed by Scheffe's F-test.
4. Discussion
Although all species of Bombycinae contained a pair of large and horizontally elongated glomeruli (‘a’ and ‘b’; figure 1), the glomerular organization in the five species investigated in this study can be clearly divided into two groups. Trilocha varians and E. moorei have similar-sized toroid and cumulus structures, whereas R. menciana, B. mandarina and B. mori have extensively enlarged toroid structures (figure 2). The organization in the former group is similar to that in Manduca sexta, in which the mixture of bombykal and an additional component is required to initiate orientation behaviour [15]. In T. varians and E. moorei, the MGC contained a pair of enlarged glomeruli with nearly equal volume as that in M. sexta [16]. It has been reported that the dorsal-most glomerulus has the largest volume in moth species [4]. Three species in the latter group, R. menciana, B. mandarina and B. mori, are counterexamples of this morphological feature.
We describe a possible evolutionary scenario for morphological changes in the MGC based on changes in sex pheromone components as related to speciation in the Bombycinae. Changes in pheromone component use from a blend of two components to a single component may drive changes in glomerular organization. The first group, T. varians and E. moorei, uses the blend of the two pheromone components; the males show behavioural responses only when both components are present [8] (T. Daimon, T. Fujii, T. Shimada & Y. Ishikawa, unpublished observation). By contrast, in the second group, R. menciana, B. mandarina and B. mori, a single component is sufficient to trigger mating behaviour. Therefore, one possibility is that recognition of the blend of two pheromone components requires two similar-sized glomeruli, and once a moth switches to use just one of the two components, either glomerulus may undergo volume enlargement to increase the sensitivity to a single attractant. This may explain why the toroid of the latter group is enlarged when a single attractant is processed (see the electronic supplementary material). Further investigation of outgroups of other Lepidoptera possessing a single pheromone component is required to address this issue.
Notably, bombykal strongly inhibits the mating behaviour of B. mori and B. mandarina [9]. This indicates that the role of bombykal has been completely changed from an attractant to a behavioural antagonist in Bombyx species. Because the bombycid moths investigated in this study have modified glomerular organization but retain the overall structure of the AL, this shift can be attributed to changes in the expression of odorant receptors, targeting of axonal projection of odorant receptor neurons or rewiring the connection between pheromonal glomeruli and the downstream circuit.
In this study, we report neuroanatomical changes correlated with sex pheromone usage among bombycid moths, which may have resulted in speciation by facilitating species-specific chemical communication.
Data accessibility
Movie of reconstructed glomeruli can be accessed at the Invertebrate Brain Platform (IVBPF; https://invbrain.neuroinf.jp/modules/newdb13/). Confocal stack for male AL can be accessed from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.n8s6t.
Funding statement
Financial support was obtained from the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (B) 21370029 to R.K., Grant-in-Aid for Young Scientist (A) 23688008 to T.D. and Grant-in-Aid for Scientific Research on Innovative Areas 22128004 to T.S.
References
- 1.Byers JA. 2006. Pheromone component patterns of moth evolution revealed by computer analysis of the Pherolist. J. Anim. Ecol. 75, 399–407. ( 10.1111/j.1365-2656.2006.01060.x) [DOI] [PubMed] [Google Scholar]
- 2.Strausfeld N, Reisenman CE. 2009. Dimorphic olfactory lobes in the arthropoda. Ann. NY Acad. Sci. 1170, 487–496. ( 10.1111/j.1749-6632.2009.04020.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson BS, Christensen TA, Hildebrand JG. 1991. Functionally distinct subdivisions of the macroglomerular complex in the antennal lobe of the male sphinx moth Manduca sexta. J. Comp. Neurol. 312, 264–278. ( 10.1002/cne.903120209) [DOI] [PubMed] [Google Scholar]
- 4.Hansson B. 1996. Antennal lobe projection patterns of pheromone-specific olfactory receptor neurons in moths. In Insect pheromone research: new directions (eds Carde RT, Minks A.), pp. 64–184. Chapman and Hall. [Google Scholar]
- 5.Symonds MRE, Elgar MA. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228. ( 10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 6.Kárpáti Z, Olsson S, Hansson BS, Dekker T. 2010. Inheritance of central neuroanatomy and physiology related to pheromone preference in the male European corn borer. BMC Evol. Biol. 10, 286 ( 10.1186/1471-2148-10-286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harzsch S. 2006. Neurophylogeny: architecture of the nervous system and a fresh view on arthropod phyologeny. Integr. Comp. Biol. 46, 162–194. ( 10.1093/icb/icj011) [DOI] [PubMed] [Google Scholar]
- 8.Daimon T, Fujii T, Yago M, Hsu Y-F, Nakajima Y, Fujii T, Katsuma S, Ishikawa Y, Shimada T. 2012. Female sex pheromone and male behavioral responses of the bombycid moth Trilocha varians: comparison with those of the domesticated silkmoth Bombyx mori. Naturwissenschaften 99, 207–215. ( 10.1007/s00114-012-0887-3) [DOI] [PubMed] [Google Scholar]
- 9.Daimon T, Fujii T, Fujii T, Yokoyama T, Katsuma S, Shinoda T, Shimada T, Ishikawa Y. 2012. Reinvestigation of the sex pheromone of the wild silkmoth Bombyx mandarina: the effects of bombykal and bombykyl acetate. J. Chem. Ecol. 38, 1031–1035. ( 10.1007/s10886-012-0164-0) [DOI] [PubMed] [Google Scholar]
- 10.Dai XJ, Xu SF, Wang MZ, Zhu YX, Tang XH, Zhu JW, Du JW, Dong TXDM. 1988. E-10, Z-12-Hexadecedienyl acetate sex pheromone of the mulberry white caterpillar Rondotia menciana Moore (Lepidoptera, Bombycidae). Kexue Tongbao 33, 1575–1576. [Google Scholar]
- 11.Kaissling KE, Kasang G, Bestmann HJ, Stransky W, Vostrowsky O. 1978. A new pheromone of the silkworm moth Bombyx mori. Naturwissenschaften 65, 382–384. ( 10.1007/BF00439702) [DOI] [Google Scholar]
- 12.Daimon T, Yago M, Hsu Y-F, Fujii T, Nakajima Y, Kokusho R, Abe H, Katsuma S, Shimada T. 2012. Molecular phylogeny, laboratory rearing, and karyotype of the bombycid moth, Trilocha varians. J. Insect Sci. 12, 1–17. ( 10.1673/031.012.4901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanzaki R, Soo K, Seki Y, Wada S. 2003. Projections to higher olfactory centers from subdivisions of the antennal lobe macroglomerular complex of the male silkmoth. Chem. Senses 28, 113–130. ( 10.1093/chemse/28.2.113) [DOI] [PubMed] [Google Scholar]
- 14.Kazawa T, Namiki S, Fukushima R, Terada M, Soo K, Kanzaki R. 2009. Constancy and variability of glomerular organization in the antennal lobe of the silkmoth. Cell Tissue Res. 336, 119–136. ( 10.1007/s00441-009-0756-3) [DOI] [PubMed] [Google Scholar]
- 15.Tumlinson JH, Brennan MM, Doolittle RE, Mitchell ER, Brabham A, Mazomenos BE, Baumhover AH, Jackson DM. 1989. Identification of a pheromone blend attractive to Manduca sexta (L.) males in a wind tunnel. Arch. Insect Biochem. Physiol. 10, 255–271. ( 10.1002/arch.940100402) [DOI] [Google Scholar]
- 16.Huetteroth W, Schachtner J. 2005. Standard three-dimensional glomeruli of the Manduca sexta antennal lobe: a tool to study both developmental and adult neuronal plasticity. Cell Tissue Res. 319, 513–524. ( 10.1007/s00441-004-1016-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Movie of reconstructed glomeruli can be accessed at the Invertebrate Brain Platform (IVBPF; https://invbrain.neuroinf.jp/modules/newdb13/). Confocal stack for male AL can be accessed from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.n8s6t.


