Abstract
In captivity, migratory birds show increased activity during the time that they would normally migrate. The phenology and intensity of such ‘migratory restlessness’ has been shown to mirror species- and population-specific migration patterns observed in the wild and has consequently been used as a proxy for the motivation to migrate. Many studies doing so, however, were aiming to explain among-individual variation in migratory behaviour or traits, and not species- or population-specific traits. These studies thus assumed that, also at the level of the individual, migratory restlessness is an accurate proxy for the motivation to migrate. We tested this assumption for the first time and found that it holds; individuals showing very little migratory restlessness remained at stopover for longer than one night, whereas most individuals showing more restlessness departed sooner. This finding validates the use of migratory restlessness as a proxy for the motivation to migrate, thereby justifying the conclusions made in a large body of research on avian migration.
Keywords: migratory restlessness, proxy, Zugunruhe, stopover, radio-telemetry
1. Introduction
Research on bird migration has leaned heavily on measuring migratory restlessness in captive individuals. Migratory restlessness mainly consists of wing fluttering while perched [1] and is expressed as the duration or amount of activity within a given time period, e.g. the dark phase of a diurnal cycle. The occurrence and intensity of migratory restlessness in captive birds mirrors the migration patterns of wild conspecifics [2,3]. For example, species- or population-specific migration distances observed in the wild match the seasonal duration of migratory restlessness shown by captive conspecifics [4,5]. Similarly, population-specific onset of autumnal migration matches the start of migratory restlessness in captive conspecifics [6]. Such observations suggest that migratory restlessness signals the motivation to migrate.
Optimal migration theory commonly assumes that birds minimize the overall time of migration [7,8]. Migrants spend most of their time at stopovers, during which they rest and replenish the fuel (in the form of fat) used during the preceding flights [9,10]. Consequently, explaining variation in stopover duration has been the focus of numerous studies, many of which have used migratory restlessness in captive birds as a proxy for the motivation to migrate. For example, the decision to terminate stopover and resume migration, assessed by migratory restlessness, has been linked to food availability [11–17], food quality [18], size and accumulation of fat reserves [11,19–23], weather [24] and circulating hormone levels [16,25]. However, the decision to depart from stopover is made by individuals, whereas studies indicating that migratory restlessness reflects the motivation to migrate were done at the species or population level [2–5]. In other words, the assumption that, at the level of the individual, migratory restlessness is an accurate proxy for the motivation to migrate remains to be tested. For this purpose, we measured migratory restlessness in 20 northern wheatears (Oenanthe oenanthe) at stopover during a single night. The following afternoon all birds were released, carrying a radio-transmitter to allow exact determination of the night of departure. We found that the amount of migratory restlessness shown by individual birds matched the number of nights they stayed at the stopover site after release. This result justifies the use of migratory restlessness as a proxy for departure likelihood from stopover and, more generally, as a proxy for the motivation to migrate at the level of the individual.
2. Material and methods
The study was conducted on Helgoland (54°11′ N, 07°55′ E), a small (1 km2) island some 50 km off the German North Sea coastline. Northern wheatears are small (ca 25 g) insectivorous nocturnal long-distance migrants. From 17 to 20 August 2013, 20 migrating northern wheatears were caught using mealworm-baited spring traps. Upon capture, C.E. measured wing length (maximum chord) to the nearest 0.5 mm, and body mass to the nearest 0.1 g. Wing length was used to calculate lean body mass, employing a linear regression based on 220 ‘lean’ northern wheatears caught on Helgoland in previous years: lean body mass (g) = 0.29 g mm−1 × wing length (mm) – 6.85 g (linear regression: n = 220, F1,218 = 95.07, adj-R2 = 0.30, p < 0.0001, after [23]). Fat reserves were then calculated as: (body mass (g) – lean body mass (g))/lean body mass (g). Mean and s.d. fat reserves of the 20 birds used in this study was 0.089 ± 0.086. After trapping, birds were put in individual cages (40 × 40 × 30 cm) set up in a single indoor room with artificial lighting. The lights in the room went on at 7.00 and off at 21.00 CET, approximately 30 min after natural sunset. The temperature was held constant at 21°C, and birds had ad libitum access to food (mealworms) and water. Food trays were removed at lights off. Migratory restlessness was recorded automatically with motion-sensitive microphones, attached to the right wall of each cage. Each time a bird moved, this generated an impulse that was transmitted to a recording device. To avoid the recording of occasional non-migratory activity, we set a threshold of three impulses per second before it was recorded as an activity count [26,27]. Another device created a CSV file summarizing the activity counts over 15 min periods. Individual tracking of radio-tagged birds revealed that northern wheatears leave Helgoland exclusively at night, between 1 and 5.5 h after sunset [28]. Therefore, migratory restlessness, measured in the night of 20 August, was defined as the number of 15 min periods between 1 and 5.5 h after lights off, during which a bird showed at least five activity counts. The following afternoon, all birds were fitted with a 0.42 g Holohil BD-2N radio-transmitter and released between 15.00 and 16.00 that day. Using a Yaesu FT-290RII receiver and a Yagi hand-held antenna, we searched for all tagged birds from 16 fixed points distributed to cover the whole island (following [29]). Daily searches, starting the day after release, lasted from approx. 10.00 to 12.00. To exclude temporary misses, each bird was searched for, for at least two days after the radio-signal was last heard.
Statistical analyses were performed with program MARK [30] and SPSS 21.0 (IBM, New York, NY, USA). Because the s.d. was larger than the mean, we used generalized linear models (GLM) with a negative binomial distribution and log link function in SPSS. Radio-telemetry data were analysed with the known-fate model for radio-tagged animals implemented in MARK. Use of the known-fate model was justified because encounter probability of radio-tagged birds in our study was 1.0. Migratory restlessness was included as an individual covariate. Data from 20 birds over 12 tracking occasions (daily searches) were used. We constructed both constant and time-dependent models for ‘survival’ (here actually measuring duration of stay after release), including migratory restlessness both as linear and interaction terms (table 1). In total, we ran five models. The highest ranking model (with lowest AICc) was considered the best fit to the data.
Table 1.
Model results from the known-fate model. Ф: apparent survival probability (here actually measuring duration of stay after release); mr: migratory restlessness; t: parameter varying over time; (.) denotes constant parameter.
| model | AICc | ΔAICc | AICc weight | model likelihood | parameters | deviance |
|---|---|---|---|---|---|---|
| Ф (t × mr) | 48.45 | 0.00 | 0.86 | 1.00 | 7 | 31.42 |
| Ф (mr) | 52.68 | 4.20 | 0.10 | 0.12 | 2 | 48.39 |
| Ф (t + mr) | 54.66 | 6.21 | 0.04 | 0.05 | 6 | 40.45 |
| Ф (.) | 63.92 | 15.47 | 0 | 0 | 1 | 61.83 |
| Ф (t) | 77.45 | 29.00 | 0 | 0 | 10 | 50.98 |
3. Results and discussion
Individuals showing very little migratory restlessness remained at the stopover site for longer than one night after being released, whereas most individuals showing more restlessness departed on the first night after release (GLM: β ± s.e. = −0.25 ± 0.08, Wald = 9.12, d.f. = 1, p = 0.003, figure 1). The observed relationship between migratory restlessness and departure latency was driven mainly by the birds with low restlessness (scores of 0–4, figure 1). These (seven) birds were, however, not exhausted or otherwise abnormal; their mean fat reserves (0.07) and restlessness scores (2.29) were similar to those of 72 other northern wheatears caught on Helgoland during the same autumn migration season (fat = 0.03; restlessness score = 2.67).
Figure 1.
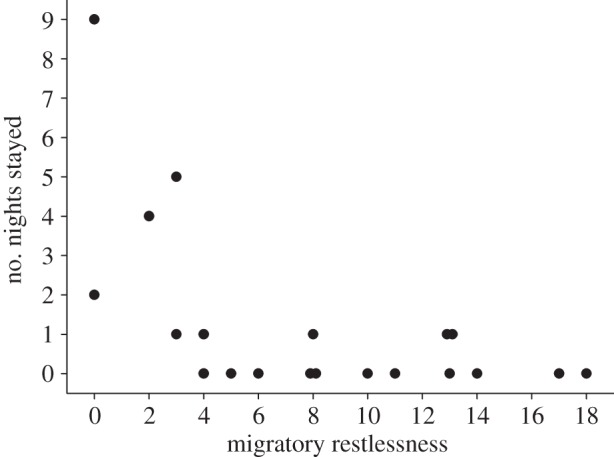
The relationship between nocturnal migratory restlessness and the number of nights birds stayed on Helgoland after release. Migratory restlessness was defined as the number of 15 min periods between 1 and 5.5 h after lights off, during which a bird showed at least five activity counts (n = 20).
A more comprehensive known-fate survival analysis in program MARK confirmed the result of the GLM. Including migratory restlessness as covariate improved the model fit considerably (table 1), indicating that migratory restlessness explained variation in departure likelihood. The model receiving the highest support was the full model, including a varying probability of birds remaining at stopover per tracking occasion (t) and an interaction with migratory restlessness (*mr). As migratory restlessness has also been defined as the activity recorded in a given time period, usually (part of) the night [14,16,20–22,25], we repeated the analyses with migratory restlessness defined as the total number of activity counts recorded between 1 and 5.5 h after lights off. This did not change the results qualitatively (GLM: β ± s.e. = −0.004 ± 0.0016, Wald = 6.26, d.f. = 1, p = 0.012). Again, inclusion of migratory restlessness improved the model ranking considerably, with the full model receiving the highest support (see the electronic supplementary material, table S1).
Levels of migratory restlessness were lower than those observed previously in northern wheatears on Helgoland during spring migration [23]. The most likely explanation for this seasonal difference is that in spring migrants generally travel faster, and make shorter stopovers, than in autumn [31].
Our results, confirmed, for the first time, that migratory restlessness can be used as an accurate proxy for the motivation to migrate at the level of the individual. Migratory restlessness is a key measure in avian migration research, and many studies have used inter-individual variation in migratory restlessness to explain how environmental and intrinsic factors temporally affect migration [11–25]. Our findings therefore justify the conclusions of a large body of research on avian migration.
Acknowledgements
Oscar Vedder, Karen Bouwman and two anonymous referees provided useful comments to earlier versions of the manuscript.
All procedures were approved by the Ministry for Agriculture, the Environment and rural Areas, Schleswig-Holstein, Germany.
Data accessibility
Data are available from the electronic supplementary material associated with this paper.
References
- 1.Berthold P, Fiedler W, Querner U. 2000. Migratory restlessness or Zugunruhe in birds: a description based on video recordings under infrared illumination. J. Ornithol. 141, 285–299. ( 10.1007/BF02462238) [DOI] [Google Scholar]
- 2.Gwinner E. 1986. Circannual rhythms. Berlin, Germany: Springer. [Google Scholar]
- 3.Berthold P. 1996. Control of bird migration. Berlin, Germany: Springer. [Google Scholar]
- 4.Gwinner E. 1968. Artspezifische Muster der Zugunruhe bei Laubsängern und ihre mögliche Bedeutung für die Beendigung des Zuges im Winterquartier. Z. Tierpsychol. 25, 843–853. ( 10.1111/j.1439-0310.1968.tb00046.x) [DOI] [Google Scholar]
- 5.Berthold P. 1973. Relationships between migratory restlessness and migration distance in six Sylvia species. Ibis 115, 594–599. ( 10.1111/j.1474-919X.1973.tb01998.x) [DOI] [Google Scholar]
- 6.Berthold P. 1988. Wegzugbeginn und Einsetzen der Zugunruhe bei 19 Vogelpopulationen - eine vergleichende Untersuchung. J. Ornithol. 131, 217–222. [Google Scholar]
- 7.Alerstam T, Lindström A. 1990. Optimal bird migration: the relative importance of time, energy and safety. In Bird migration (ed. Gwinner E.), pp. 331–351. Berlin, Germany: Springer. [Google Scholar]
- 8.Hedenström A, Alerstam T. 1997. Optimal fuel loads in migratory birds: distinguishing between time and energy minimization. J. Theor. Biol. 189, 227–234. ( 10.1006/jtbi.1997.0505) [DOI] [PubMed] [Google Scholar]
- 9.Green M, Alerstam T, Clausen P, Drent R, Ebbinge BS. 2002. Dark-bellied brent geese Branta bernicla bernicla, as recorded by satellite telemetry, do not minimize flight distance during spring migration. Ibis 144, 106–121. ( 10.1046/j.0019-1019.2001.00017.x) [DOI] [Google Scholar]
- 10.Schmaljohann H, Fox JW, Bairlein F. 2012. Phenotypic response to environmental cues, orientation and migration costs in songbirds flying halfway around the world. Anim. Behav. 84, 623–640. ( 10.1016/j.anbehav.2012.06.018) [DOI] [Google Scholar]
- 11.Biebach H. 1985. Sahara stopover in migratory flycatchers: fat and food affect the time program. Experientia 41, 695–697. ( 10.1007/BF02007727) [DOI] [Google Scholar]
- 12.Gwinner E, Biebach H, Kries I. 1985. Food availability affects migratory restlessness in caged garden warblers (Sylvia borin). Naturwissenschaften 72, 51–52. ( 10.1007/BF00405336) [DOI] [Google Scholar]
- 13.Gwinner E, Schwabl H, Schwabl-Benzinger I. 1988. Effects of food-deprivation on migratory restlessness and diurnal activity in the garden warbler Sylvia borin. Oecologia 77, 321–326. ( 10.1007/BF00378037) [DOI] [PubMed] [Google Scholar]
- 14.Fusani L, Gwinner E. 2004. Simulation of migratory flight and stopover affects night levels of melatonin in a nocturnal migrant. Proc. R. Soc. Lond. B 271, 205–211. ( 10.1098/rspb.2003.2561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauchinger U, Van't Hof T, Biebach H. 2008. Migratory stopover conditions affect the developmental state of male gonads in garden warblers (Sylvia borin). Horm. Behav. 54, 312–318. ( 10.1016/j.yhbeh.2008.03.007) [DOI] [PubMed] [Google Scholar]
- 16.Fusani L, Cardinale M, Schwabl I, Goymann W. 2011. Food availability but not melatonin affects nocturnal restlessness in a wild migrating passerine. Horm. Behav. 9, 187–192. ( 10.1016/j.yhbeh.2010.11.013) [DOI] [PubMed] [Google Scholar]
- 17.Eikenaar C, Bairlein F. 2014. Food availability and fuel loss predict Zugunruhe. J. Ornithol. 155, 65–70. ( 10.1007/s10336-013-0987-7) [DOI] [Google Scholar]
- 18.Aamidor SE, Bauchinger U, Mizrahy O, McWilliams SR, Pinshow B. 2011. During stopover, migrating blackcaps adjust behavior and intake of food depending on the content of protein in their diets. Integr. Comp. Biol. 51, 385–393. ( 10.1093/icb/icr054) [DOI] [PubMed] [Google Scholar]
- 19.Bairlein F. 1985. Body weights and fat deposition of Paleartic passerine migrants in the central Sahara. Oecologia 66, 141–146. ( 10.1007/BF00378566) [DOI] [PubMed] [Google Scholar]
- 20.Yong W, Moore FR. 1993. Relation between migratory activity and energetic condition among thrushes (Turdinae) following passage across the Gulf of Mexico. Condor 95, 934–943. ( 10.2307/1369429) [DOI] [Google Scholar]
- 21.Smith SB, Norment CJ. 2005. Nocturnal activity and energetic condition of spring landbird migrants at Braddock Bay, Lake Ontario. J. Field Ornithol. 76, 303–310. [Google Scholar]
- 22.Fusani L, Cardinale M, Carere C, Goymann W. 2009. Stopover decision during migration: physiological conditions predict nocturnal restlessness in wild passerines. Biol. Lett. 5, 302–305. ( 10.1098/rsbl.2008.0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eikenaar C, Schläfke JL. 2013. Size and accumulation of fuel reserves at stopover predict nocturnal restlessness in a migratory bird. Biol. Lett. 9, 20130712 ( 10.1098/rsbl.2013.0712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindler J, Berthold P, Bairlein F. 1981. On the influence of simulated weather conditions on the endogenous time program for migration in the garden warbler Sylvia borin. Die Vogelwarte 31, 14–32. [Google Scholar]
- 25.Lõhmus M, Sandberg R, Holberton RL, Moore FR. 2003. Corticosterone levels in relation to migratory readiness in red-eyed vireos (Vireo olivaceus). Behav. Ecol. Sociobiol. 54, 233–239. ( 10.1007/s00265-003-0618-z) [DOI] [Google Scholar]
- 26.Maggini I, Bairlein F. 2010. Endogenous rhythms of seasonal migratory body mass changes in different populations of northern wheatears Oenanthe oenanthe. J. Biol. Rhythms 25, 268–276. ( 10.1177/0748730410373442) [DOI] [PubMed] [Google Scholar]
- 27.Maggini I, Bairlein F. 2012. Innate sex differences in the timing of spring migration in a songbird. PLoS ONE 7, e31271 ( 10.1371/journal.pone.0031271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmaljohann H, Korner-Nievergelt F, Naef-Daenzer B, Nagel R, Maggini I, Bulte M, Bairlein F. 2013. Stopover optimization in a long-distance migrant: the role of fuel load and nocturnal take-off time in Alaskan northern wheatears (Oenanthe oenanthe). Front. Zool. 10, 26 ( 10.1186/1742-9994-10-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goymann W, Spina F, Ferri A, Fusani L. 2010. Body fat influences departure from stopover sites in migratory birds: evidence from whole-island telemetry. Biol. Lett. 6, 478–481. ( 10.1098/rsbl.2009.1028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White GC, Burnham KP. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46, 120–139. ( 10.1080/00063659909477239) [DOI] [Google Scholar]
- 31.Nilsson C, Klaassen RHG, Alerstam T. 2013. Differences in speed and duration of bird migration between spring and autumn. Am. Nat. 181, 837–845. ( 10.5061/dryad.82d4q) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the electronic supplementary material associated with this paper.


