Abstract
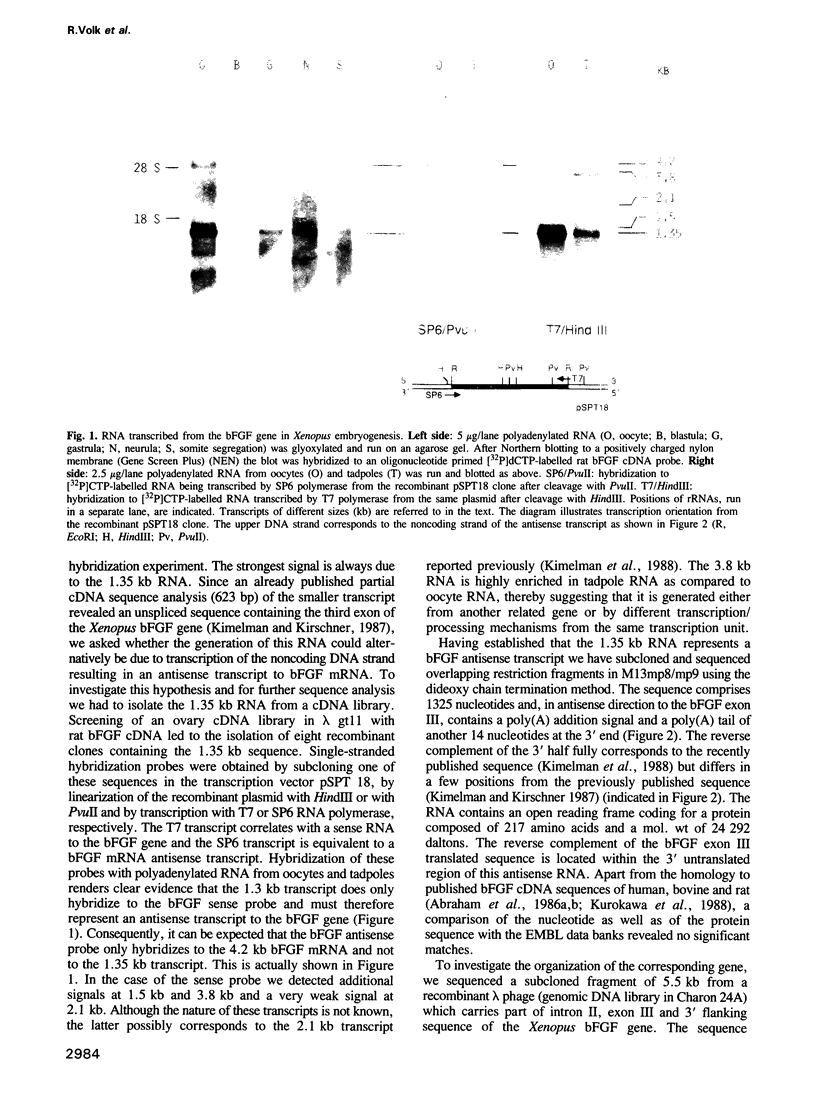
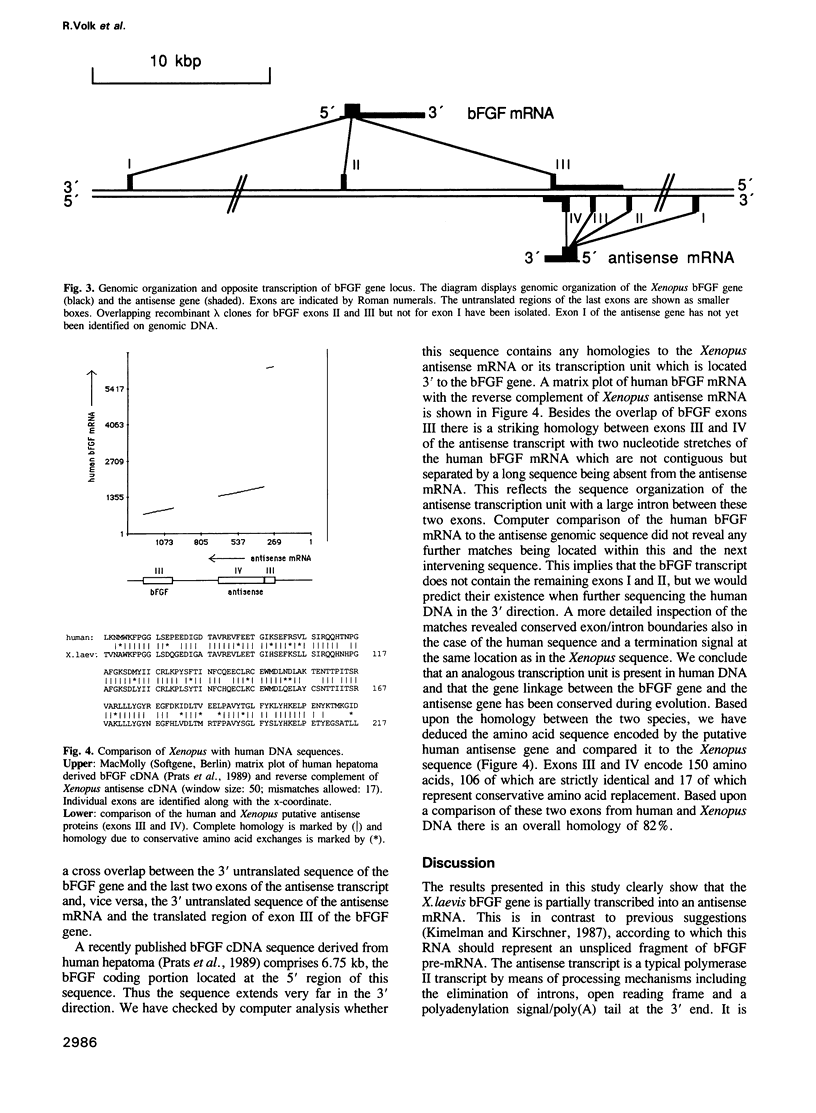
Screening of a Xenopus laevis oocyte cDNA library with a rat basic fibroblast growth factor (bFGF) cDNA led to the isolation of a 1.35 kb sequence containing exon III of the bFGF gene. Reverse complementary listing of this sequence revealed a polyadenylated transcript with an open reading frame coding for an unknown protein of mol. wt 24,292 daltons. The coding part of bFGF exon III is located in this putative mRNA in opposite direction within the 3' untranslated region. By hybridization studies on transcription orientation with single-stranded probes it could be proven that this transcript actually represents an antisense transcript to part of the Xenopus bFGF gene. Sequence organization on corresponding genomic fragments revealed that it is processed from a larger precursor by splicing mechanisms. Sequence comparison with elongated transcripts from the bFGF gene in human hepatoma has shown that the gene coding for the antisense mRNA is evolutionarily conserved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman J. P., Bond C. T., Douglass J., Herbert E. Two mammalian genes transcribed from opposite strands of the same DNA locus. Science. 1987 Mar 20;235(4795):1514–1517. doi: 10.1126/science.3547652. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Born J., Hoppe P., Schwarz W., Tiedemann H., Tiedemann H., Wittmann-Liebold B. An embryonic inducing factor: isolation by high performance liquid chromatography and chemical properties. Biol Chem Hoppe Seyler. 1985 Aug;366(8):729–735. doi: 10.1515/bchm3.1985.366.2.729. [DOI] [PubMed] [Google Scholar]
- Grunz H., McKeehan W. L., Knöchel W., Born J., Tiedemann H., Tiedemann H. Induction of mesodermal tissues by acidic and basic heparin binding growth factors. Cell Differ. 1988 Feb;22(3):183–189. doi: 10.1016/0045-6039(88)90010-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Keene M. A., Fechtel K., Fristrom J. W. Gene within a gene: nested Drosophila genes encode unrelated proteins on opposite DNA strands. Cell. 1986 Jan 17;44(1):33–42. doi: 10.1016/0092-8674(86)90482-4. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Abraham J. A., Haaparanta T., Palisi T. M., Kirschner M. W. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science. 1988 Nov 18;242(4881):1053–1056. doi: 10.1126/science.3194757. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Knöchel W., Born J., Hoppe P., Loppnow-Blinde B., Tiedemann H., Tiedemann H., McKeehan W. L., Grunz H. Mesoderm-inducing factors. Their possible relationship to heparin-binding growth factors and transforming growth factor-beta. Naturwissenschaften. 1987 Dec;74(12):604–606. doi: 10.1007/BF00368525. [DOI] [PubMed] [Google Scholar]
- Knöchel W., Korge E., Basner A., Meyerhof W. Globin evolution in the genus Xenopus: comparative analysis of cDNAs coding for adult globin polypeptides of Xenopus borealis and Xenopus tropicalis. J Mol Evol. 1986;23(3):211–223. doi: 10.1007/BF02115578. [DOI] [PubMed] [Google Scholar]
- Knöchel W., Tiedemann H. Embryonic inducers, growth factors, transcription factors and oncogenes. Cell Differ Dev. 1989 May;26(3):163–171. doi: 10.1016/0922-3371(89)90747-8. [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Seno M., Igarashi K. Nucleotide sequence of rat basic fibroblast growth factor cDNA. Nucleic Acids Res. 1988 Jun 10;16(11):5201–5201. doi: 10.1093/nar/16.11.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster M., Pieler T., Pöting A., Knöchel W. The finger motif defines a multigene family represented in the maternal mRNA of Xenopus laevis oocytes. EMBO J. 1988 Jun;7(6):1735–1741. doi: 10.1002/j.1460-2075.1988.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercola M., Stiles C. D. Growth factor superfamilies and mammalian embryogenesis. Development. 1988 Mar;102(3):451–460. doi: 10.1242/dev.102.3.451. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miyajima N., Horiuchi R., Shibuya Y., Fukushige S., Matsubara K., Toyoshima K., Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989 Apr 7;57(1):31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B. Intragenic pausing and anti-sense transcription within the murine c-myc locus. EMBO J. 1986 Nov;5(11):2859–2865. doi: 10.1002/j.1460-2075.1986.tb04580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C. A mesoderm-inducing factor is produced by Xenopus cell line. Development. 1987 Jan;99(1):3–14. doi: 10.1242/dev.99.1.3. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Yaqoob M., Symes K. Purification, partial characterization and biological effects of the XTC mesoderm-inducing factor. Development. 1988 Jul;103(3):591–600. doi: 10.1242/dev.103.3.591. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Gietz R. D., Hodgetts R. B. Overlapping transcription units in the dopa decarboxylase region of Drosophila. Nature. 1986 Jul 17;322(6076):279–281. doi: 10.1038/322279a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. W., Smith J. E., Cooperman B. S., Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Fried M. A mouse locus at which transcription from both DNA strands produces mRNAs complementary at their 3' ends. Nature. 1986 Jul 17;322(6076):275–279. doi: 10.1038/322275a0. [DOI] [PubMed] [Google Scholar]



