Abstract
Traumatic brain injury (TBI) is the commonest cause of death and disability in those aged under 40 years. Interleukin-1 receptor antagonist (IL1ra) is an endogenous competitive antagonist at the interleukin-1 type-1 receptor (IL-1R). Antagonism at the IL-1R confers neuroprotection in several rodent models of neuronal injury (i.e., trauma, stroke and excitotoxicity). We describe a single center, phase II, open label, randomized-control study of recombinant human IL1ra (rhIL1ra, anakinra) in severe TBI, at a dose of 100 mg subcutaneously once a day for 5 days in 20 patients randomized 1:1. We provide safety data (primary outcome) in this pathology, utilize cerebral microdialysis to directly determine brain extracellular concentrations of IL1ra and 41 cytokines and chemokines, and use principal component analysis (PCA) to explore the resultant cerebral cytokine profile. Interleukin-1 receptor antagonist was safe, penetrated into plasma and the brain extracellular fluid. The PCA showed a separation in cytokine profiles after IL1ra administration. A candidate cytokine from this analysis, macrophage-derived chemoattractant, was significantly lower in the rhIL1ra-treated group. Our results provide promising data for rhIL1ra as a therapeutic candidate by showing safety, brain penetration and a modification of the neuroinflammatory response to TBI by a putative neuroprotective agent in humans for the first time.
Keywords: chemokines, IL1ra, microdialysis, neuroinflammation, randomized control trial, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a common cause of death and disability in those under the age of 40 years, with a global incidence of 200/100,000 per annum.1 Cytokines and chemokines are increasingly recognized as potent molecular mediators of injury after TBI, and provide potentially fruitful therapeutic targets.2 Interleukin-1 receptor antagonist (IL1ra) is a naturally occurring competitive antagonist at the interleukin-1 type-1 receptor (IL-1R). Antagonism at the IL-1R confers neuroprotection in several rodent models of neuronal injury including trauma,3, 4, 5, 6 stroke,7, 8 and excitotoxicity.7, 9 However, several therapeutic agents that have shown remarkable promise in preclinical models have failed to progress to successful human studies. Numerous reasons have been suggested for this failure, including poor blood–brain barrier penetration of the drug, inappropriate timing of drug delivery, a conceptual misunderstanding of the complexity of mechanisms of action, and an inability to determine whether the drug concentration achieved in humans is sufficient to exert a biologic effect.10 We have sought to address these varied limitations by using a translational medicine approach to investigate this promising putative neuroprotective agent.
We describe, for the first time, an open label randomized control trial of human recombinant IL1ra (hrIL1ra) in 20 patients with severe TBI (Supplementary Figure 1). Human recombinant IL1ra (Anakinra, Kineret) is licensed in rheumatoid arthritis at a dose of 100 mg subcutaneously once daily.11 This dose and route of administration was utilized as hrIL1ra, has a well-defined safety profile, and has previously been trialled in stroke,12 subarachnoid hemorrhage,13, 14 and severe sepsis.15 We have used our previously validated cerebral microdialysis methodology16, 17 with a combination of arterial plasma sampling predosing and postdosing (Figure 1) to provide a comprehensive biochemical assessment of the consequences of IL1R antagonism after human TBI and have analyzed the resulting high-dimensional data sets with multivariate projection methods.18
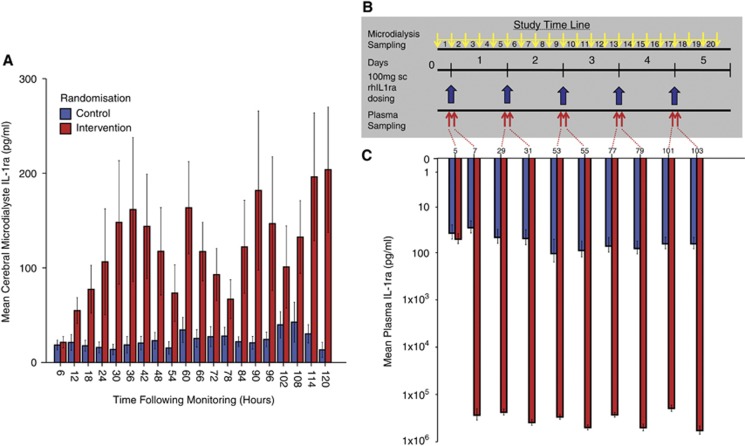
Figure 1.
Cerebral microdialysate and plasma interleukin-1 receptor antagonist (IL1ra) concentration and study time line. (A) Cerebral microdialysate concentration of IL1ra in control (blue) and intervention (red) patients. Error bars denote standard error of the mean. (B) Study time line. After recruitment to the study, patients were monitored for 6 hours (microdialysis time point 1), as a baseline, before administration of 100 mg recombinant human IL1ra (rhIL1ra) by subcutaneous injection daily for 5 days in the intervention group (10 patients) or no drug in the control group (10 patients). Microdialysis monitoring continued throughout with samples pooled into 6 hour time epochs with a total of 20 samples. Plasma sampling was performed 1 hour before and 1 hour after drug administration, giving a total of 10 samples. In control patients, these plasma samples were taken in relation to the hypothetical time at which the drug would have been administered in an identical manner to the intervention patients. (C) Plasma concentrations of IL1ra in control (blue) and intervention (red) patients. Mean IL1ra±standard error of the mean is plotted on a logarithmic scale. Plasma samples were taken 1 hour before and 1 hour after drug administration.
Patients and methods
Patients
Study conduct and reporting are consistent with Good Clinical Practice and CONSORT Guidelines19 (Supplementary Figure 1). The protocol was approved by the ‘Cambridgeshire (2) Local Research Ethics Committee' (06/Q0108/64) and by the appropriate regulatory authorities (Supplementary Table 1). The study recruited patients with severe TBI, defined as patients with a history of cranial trauma, a computed tomography scan consistent with TBI, and a postresuscitation Glasgow Coma Score of ⩽8. Patients were enrolled on the basis of eligibility (Supplementary Table 2) and exclusion (Supplementary Table 3) criteria. To reduce heterogeneity within the patient population, only patients with predominantly diffuse injury were recruited into the study to minimize the effect of variation between patients being related to proximity of microdialysis monitoring to focal lesions. The next of kin was approached for assent in line with the prospective ethical approvals. All patients recruited to the study received sedation (with or without neuromuscular blockade), endotracheal intubation, mechanical ventilation, and multimodality monitoring as a part of a standard clinical management protocol for severe TBI over the period of drug administration.20 Intracerebral monitoring was performed in all patients using a triple lumen cranial access device (Technicam, Newton Abbot, UK) in line with our local clinical protocols. This comprises an intracranial pressure monitor (Codman, Raynham, MA, USA), a microdialysis catheter (CMA 71, 100 kDa molecular weight cutoff) perfused with 3.5% (w/v) Human Albumin Solution (Pharmacy Manufacturing Unit, Ipswich Hospital NHS Trust, Ipswich, UK) composed in central nervous system perfusion fluid and a brain tissue oxygen monitor (Licox Neurosciences, Andover, UK). Patient data on age, sex, mechanism of injury, postresuscitation Glasgow Coma Score, Marshall Grade, Acute Physiology And Chronic Health Evaluation II, and Injury Severity Score were collected (Table 1). All patients had a daily serum hematology and biochemistry screen as a part of standard intensive care management (Supplementary Table 4).
Table 1. Patient admission characteristics and outcomes.
| Trial ID | Age | Sex | Mechanism | GCS | Marshall grade | APACHE II | ISS | GOS |
|---|---|---|---|---|---|---|---|---|
| C01 | 44 | F | Road traffic accident | E1V1M1 | 2 | 17 | 45 | 3 |
| C02 | 25 | M | Road traffic accident | E1V1M1 | 2 | 14 | 38 | 3 |
| C03 | 27 | F | Road traffic accident | E1V1M1 | 2 | 13 | 45 | 5 |
| C04 | 25 | F | Road traffic accident | E1V1M2 | 4b | 10 | 33 | 5 |
| C05 | 58 | F | Road traffic accident | E1V1M1 | 6c | 22 | 38 | 2 |
| C06 | 61 | F | Road traffic accident | E1V1M1 | 6d | 19 | 38 | 3 |
| C07 | 49 | M | Road traffic accident | E1V2M5 | 6d | 10 | 29 | 3 |
| C08 | 60 | M | Road traffic accident | E1V1M5 | 2c | 14 | 36 | 5 |
| C09 | 30 | M | Fall | E1V2M2 | 6d | 21 | 38 | 3 |
| C10 | 39 | F | Road traffic accident | E1V1M1 | 3 | 20 | 50 | 2 |
| I01 | 41 | F | Fall | E1V1M1 | 6 | 15 | 27 | 4 |
| I02 | 37 | M | Road traffic accident | E1V1M1 | 2d | 18 | 43 | 3 |
| I03 | 43 | M | Road traffic accident | E1V1M1 | 2 | 16 | 30 | 4 |
| I04 | 22 | M | Road traffic accident | E1V1M1 | 2 | 15 | 50 | 2 |
| I05 | 18 | F | Road traffic accident | E1V1M5 | 5a | 15 | 30 | 5 |
| I06 | 46 | F | Road traffic accident | E1V1M2 | 3 | 14 | 27 | 3 |
| I07 | 27 | M | Road traffic accident | E1V2M5 | 2b | 13 | 45 | 3 |
| I08 | 47 | M | Road traffic accident | E1V1M3 | 2b | 17 | 66 | 4 |
| I09 | 28 | F | Thrown off horse | E1V2M4 | 2 | 10 | 43 | 3 |
| IN10 | 51 | M | Assault | E1V2M5 | 6c | 15 | 27 | 4 |
C, control group; I, intervention group; GCS, Glasgow Coma Score; E, eye opening; V, verbal response; M, motor response, Modified Marshall Grade; APACHE II, Acute Physiology And Chronic Health Evaluation 2; ISS, injury severity score; GOS, Glasgow Outcome Score.
Randomization and Masking
Patients were randomized using sequential sealed envelopes into intervention or control groups on which basis they received the study drug or no drug (i.e., no placebo was administered). The study was open label and both physician and family were unblinded.
Interventions
All patients were recruited in the first 24 hours after injury, in keeping with the early endogenous production of IL1β after TBI.16 It was a stipulation of the ethics committee that the next of kin should have sufficient time for consideration of study enrolment preventing recruitment in a faster time frame. After an initial monitoring period of 6 hours, to provide a monitoring baseline, patients in the intervention group received 100 mg rhIL1ra (Anakinra; Amgen, Thousand Oaks, CA, USA/Swedish Orphan Biovitrium Ltd, Cambridge, UK) given by subcutaneous injection into the abdomen according to the manufacturer's instructions. Four further doses were given at 24-hour intervals (Figure 1B). Patients in the control group were monitored in an identical manner with sampling time points identical to those in the intervention group, but received no drug. A record of serious adverse events (SAEs; Table 2) was kept for patients during the course of the study. All patients were followed up in a dedicated neurotrauma clinic at 6 months, at which point Glasgow Outcome Score was assessed (Table 1).
Table 2. Serious adverse events.
| Patient | Serious adverse event | Outcome |
|---|---|---|
| Control patients | ||
| C02 | Neutropenia of unknown etiology | Improved spontaneously |
| C04 | Ventilator-associated pneumonia | Improved after antibiotics |
| C10 | Intraabdominal sepsis and acidosis; biliary leak. Prerenal renal failure | Required hemofiltration and antibiotics before resolution |
| Intervention patients | ||
| I02 | Chest infection—mucous plugging, right lower lobe collapse | Improved after bronchoscopy and antibiotics |
| I04 | Right upper lobe lung collapse after bronchoscopy to remove traumatic clots | Improved after change in ventilator settings/physiotherapy |
| I05 | Ventilator-associated pneumonia | Improved after antibiotics |
| I06 | Ventilator-associated pneumonia | Improved after antibiotics |
Sampling
Our microdialysis methodology is published in more detail in previous publications.16, 17 All microdialysis catheters (CMA71, 100 kDa molecular weight cutoff) were perfused at 0.3 μL/min with central nervous system perfusion fluid using CMA 106 microinfusion pumps (M Microdialysis AB, Johanneshov, Sweden). The microdialysis vials were kept at the same height as the microdialysis pump to negate any additional hydrostatic forces. The location of all monitoring devices within the brain was checked on computed tomography scans confirming the placement of all microdialysis catheter into radiologically normal brain parenchyma indicative of diffuse brain injury. Microdialysis vials were changed hourly and analyzed on an ISCUS (M Microdialysis AB) bedside analyser as per standard clinical protocols. The remainder of each sample was then stored at −80°C. Blood samples were collected from in-dwelling arterial catheters in all patients. Samples were collected 1 hour before drug administration and 1 hour after drug administration. Patients in the control group were sampled in an identical manner in relation to the hypothetical time at which drug would have been administered (6, 30, 54, 78, and 102 hours after commencement of monitoring) (Figure 1B). Samples were immediately centrifuged for 15 minutes at 4,000 g. at 4°C. The supernatant (plasma) was decanted and divided into 150 μL aliquots before storage at −80°C.
Cytokine Analysis
All samples were analyzed using the Milliplex Multi-Analyte Profiling Human Cytokine/Chemokine 42 analyte premixed kit (Millipore, St Charles, MI, USA) using the manufacturer's instructions as described previously.16 The 42 cytokines and chemokines assayed are detailed in Supplementary Table 6. Owing to the ultralow flow rates inherent in microdialysis to allow sufficient volume for the assay, microdialysates from a 6-hour time period were pooled immediately before analysis. Plasma samples had sufficient volume for analysis without dilution or pooling. All samples were assayed in duplicate wells (25 μL/well) and the mean of the ensuing results was used. The plates were read using a Luminex 200 analyser (Luminex Corporation, Austin, TX, USA) running STarStation software (Applied Cytometry Systems, Sheffield, UK). Cytokine concentrations were calculated by reference to an eight-point five-parameter logistic standard curve for each cytokine. Microdialysate and plasma samples were run on separate plates as the buffers and background (control) wells for these samples differ within the assay protocol.
Statistical Analysis
Statistical analyses were performed using the SPSS20 (SPSS, Chicago, IL, USA) for Windows and SIMCA 13.0 (Umetrics AB, Umeå, Sweden) for Windows. The concentrations in plasma and microdialysate in the control and intervention groups are plotted in Figure 1 with standard error of the mean. Due to the large number of microdialysis sampling time points, for statistical comparison, the microdialysate IL1ra concentrations were pooled in relation to the time of recombinant human IL1ra (rhIL1ra) administration into four time epochs: 0 to 6 hours before, 6 to 12 hours after, 12 to 18 hours after and 18 to 24 hours after drug administration (Table 3). A mixed (repeated measures and between group) factorial ANOVA was used to explore the effects of both time and drug administration on plasma and cerebral microdialysis IL1ra concentrations. Mauchly's test of sphericity was used for all contrasts. If the assumption of sphericity was violated, then the Greenhouse-Geisser correction was applied.
Table 3. Mean microdialysate IL1ra concentrations (pg/mL)±standard error of mean.
| Time in relation to drug administration | 6 hours predose | 6 hours after dose | 12 hours after dose | 18 hours after dose |
|---|---|---|---|---|
| Control group | 24.3±5.9 | 27.6±7.0 | 23.4±5.2 | 23.1±6.3 |
| Intervention group | 78.8±26.4 | 123.6±34.3 | 138.3±42.7 | 125.0±41.6 |
IL1ra, interleukin-1 receptor antagonist; ANOVA, analysis of variance.
Mixed model ANOVA: Test of between-subjects contrast (control group versus intervention group): F(1,18)=6.45, P=0.02. Test of within-subjects contrast (effect of time on IL1ra concentration): F(1,18)=22.7, P<0.0001). Test of within-subject contrast (interaction between time and intervention/control group): F(1,18)=17.65, P=0.001.
Principal Component Analysis
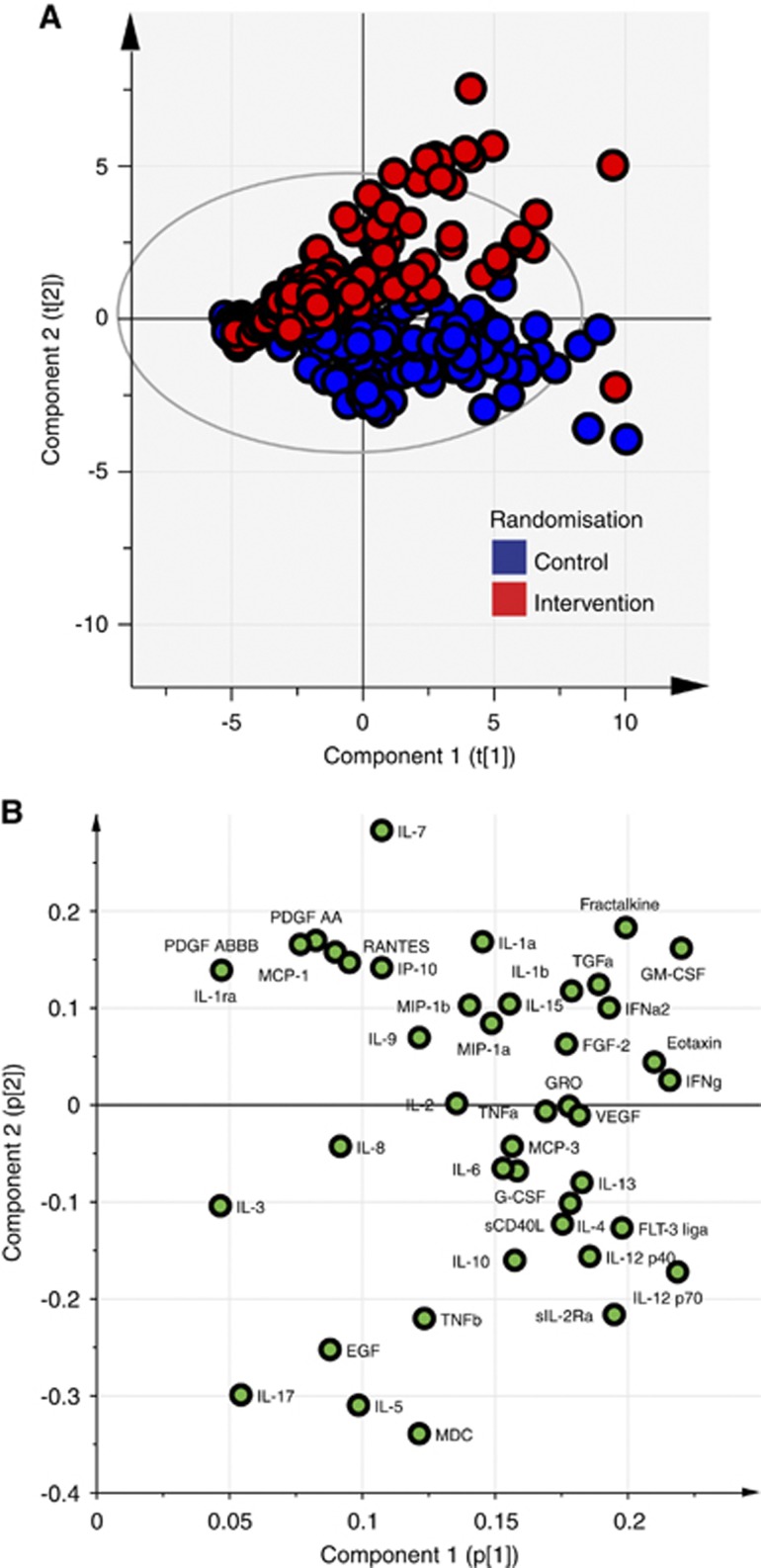
We have previously published a detailed method for the analysis of high-dimensional microdialysis data sets using multivariate projection methods.18 In a principal component analysis (PCA), all microdialysate cytokine data were entered into the model from all 20 patients at every time point. Each time point for each patient was a separate observation consisting of 42 different values (X) for each cytokine assayed. Data for each cytokine were mean centered and then scaled to unit variance to allow direct comparisons between cytokines and chemokines at widely differing native concentrations. The observations were then plotted on the first two principal components (PCs) and color coded by which patient group the original observation came from (Figure 2A). A 95% Hotelling ellipse was projected on the axes. The corresponding loading plot for these two axes is plotted in Figure 2B.
Figure 2.
Principal component analysis (PCA) shows a difference in cytokine profile after rhIL1ra administration. (A) PCA of all microdialysis cytokine observations. Each data point on the plot denotes a single sample (6 hourly) from a single patient assayed for each of the 42 cytokines. Data points are colored by the patient randomization group as control (blue) and intervention (red). (B) Loading plot corresponding to the scores plot in panel A showing the cytokines loading on the principal component (PC) axes. The two PCs of the model explain 43% of the variation in the data set (R2X). EGF, epidermal growth factor; FGF2, basic fibroblast growth factor; Flt3 lig, Fms-related tyrosine kinase 3 ligand; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-monocyte colony stimulating factor; IFNa2, interferon alpha-2; IFNg, interferon gamma; IL, interleukin; IL-1R, interleukin 1 receptor; IL1ra, interleukin-1 receptor antagonist; IL12p40, interleukin 12 subunit beta; IL12p70, interleukin-12; IP10, chemokine (C-X-C motif) ligand 10; MCP, monocyte chemotactic protein; MDC, macrophage-derived chemoattractant; MIP1a, macrophage inflammatory protein-1alpha; MIP1b, macrophage inflammatory protein-1beta; PDGF, platelet-derived growth factor; sCD40L, soluble CD40 ligand; sIL2R, soluble interleuking-2 receptor; TGFa, transforming growth factor alpha; TNFa, tumor necrosis factor alpha; TNFb, tumor necrosis factor beta; VEGF, vascular endothelial growth factor. CSF, cerebrospinal fluid.
Comparison of Candidate Downstream Cytokines Between Control and Intervention Groups
Principal component analysis provides an unbiased representation of the complex multivariate cytokine and chemokine data set that allows selection of candidate cytokines that are impacted by the administration of rhIL1ra. As well as the clear separation on the scores plot (Figure 2A) between the control and intervention group along PC2, we also chose the cytokine/chemokine with the score of the greatest magnitude on this PC to compare between groups using conventional statistical methods. Macrophage-derived chemoattractant (MDC, also termed as CCL22) shows the largest magnitude of loading on the PCA scores plot (−3.4) (Figure 2B). To show whether a difference exists between control and intervention groups, the mean MDC concentration was taken in each group for each study day (Table 4) and a mixed (repeated measures and between group) factorial ANOVA was used to explore the effects of both time and drug administration on mean MDC concentration on each day of the study period.
Table 4. Mean microdialysate MDC concentrations (pg/mL)±standard error of mean.
| Time in relation to drug administration | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Control group | 38.1±11.2 | 39.0±11.8 | 40.3±103.4 | 44.2±16.0 | 45.4±17.5 |
| Intervention group | 8.4±1.7 | 9.4±2.0 | 9.6±2.0 | 9.6±1.8 | 1.04±1.9 |
IL1ra, interleukin-1 receptor antagonist; ANOVA, analysis of variance; MDC, macrophage-derived chemoattractant.
Mixed Model ANOVA: Test of between-subjects contrast (control group versus intervention group): F(1,18)=4.45, P=0.05. Test of within-subjects contrast (effect of time on IL1ra concentration): F(1,18)=0.38, P=0.383). Test of within-subject contrast (interaction between time and intervention/control group): F(1,18)=0.580, P=0.471.
Results
Patient demographic, injury, and clinical outcome data are presented in Table 1. Given the small number of patients within the study, it would not be appropriate to carry out any statistical comparison of clinical outcome between groups, however, all outcome scores are provided for completeness. Serious adverse event in the study patients is listed in Table 2. No SAE was attributable to the study drug. Supplementary Table 4 lists serum hematology and biochemistry results for the two patient groups. Previous studies in subarachnoid hemorrhage and acute stroke have shown a reduction in neutrophil count12, 21 and C-reactive protein12 values after rhIL1ra administration. Although both white-cell count and C-reactive protein are reduced in the rhIL1ra-treated group comparison with paired t-test did not show a statistically significant difference. Supplementary Table 5 provides multimodality data on intracranial pressure control in the two patient groups. As intracranial pressure control is a goal directed target in the management of severe TBI it is necessary to incorporate data on the other physiologic parameters that are manipulated to control intracranial pressure, namely arterial PCO2, serum sodium, and body temperature.
Recombinant Human Interleukin-1 Receptor Antagonist Enters the Brain Extracellular Space after Subcutaneous Administration
We sought to show that the route and dosing of hrIL1ra employed led to consistent plasma and cerebral interstitial levels of IL1ra. Figure 1C shows that rhIL1ra administration leads to a rapid (within 1 hour) and uniform increase in plasma IL1ra, to a mean of 243 ng/mL, consistent with the high reported bioavailability.22 Figure 1A shows that there was also a rapid increase in IL1ra concentration in the cerebral extracellular compartment, detectable within 6 hours of drug administration. Repeated dosing led to an accumulation of IL1ra, but with a wide variation in the absolute concentration achieved. Table 3 lists the mean±standard error IL1ra concentration in cerebral microdialysate when data are averaged across time points in relation to the time at which the study drug was administered. This shows that there is a significant difference between the control and intervention groups (P=0.02), there is a significant variation over time (P<0.0001) and there is a significant interaction between the time point and the group (control versus intervention) from which the data came (P=0.001).
Recombinant Human Interleukin-1 Receptor Antagonist Modulates the Neuro-Inflammatory Response to Severe Traumatic Brain Injury
Having showed that rhIL1ra enters the brain interstitial space after subcutaneous administration, we sought to determine whether this altered the cerebral cytokine and chemokine profile. Principal component analysis was used to model all 6-hourly microdialysis observations without exclusion or modification. Figure 2A shows that there is a separation between control and intervention patients after rhIL1ra administration, and Figure 2B shows the loading plot responsible for this separation.
Mean Macrophage-Derived Chemoattractant Concentrations
The PCA scores plot was used to identify the candidate cytokine most likely to differ between the control and intervention groups based on the loading plot. The mean±standard error for MDC across each study day in each of the study groups is listed in Table 4. There is a significantly lower concentration of MDC in the rhIL1ra-treated patients (P=0.05).
Discussion
In this study, we have shown rhIL1ra to be safe in the human severe TBI population with an equivalent number of SAEs in both study arms, with no SAEs causally attributed to the study drug.
Cerebral microdialysis is unique in its ability to directly sample molecules from the brain extracellular fluid for analysis, and has been used to show penetration of the study drug into the brain parenchyma. Previous rhIL1ra pharmacokinetic studies have measured cerebrospinal fluid concentrations.13 However, cerebrospinal fluid may act as a sump for bulk flow of inflammatory mediators from the cerebral parenchyma23 with some biomarkers at concentrations several orders of magnitude higher than the brain interstitial fluid. This is an important distinction, as ultimately the extracellular fluid is the biologically active compartment to which the cell surface IL1R is exposed.2, 16 However, as a focal monitor, microdialysis provides a sampling volume from a small area of brain that may or may not be representative of the brain as a whole.24 For this reason, manipulation of the inflammatory milieu as evidenced by changes in cytokine composition in the locality of the microdialysis catheter might not necessarily translate into an improved clinical recovery. Nevertheless, penetration of rhIL1ra into the brain extracellular space and a subsequent modification of the inflammatory cascade after TBI are necessary, if not sufficient, for its putative role as a pharmacological neuroprotectant. Such data are rarely provided for promising neurotropic agents before translation into phase III trials and may party explain the universal failure of pharmacological neuroprotectants in human TBI.10
Human TBI is a heterogenous disease making it difficult to compare data from individuals directly.25 We have chosen as homogenous a patient group as possible, using those patients at the most severe end of the spectrum, recruiting those with a predominantly diffuse injury and by placing the microdialysis catheter away from focal injuries such as contusions. There are several overlapping modes of injury in TBI (e.g., contusion, extraaxial hematoma, and diffuse injury with axonal shearing) that may have distinct pathologic mechanisms with different host inflammatory responses.25 Previous rodent models of IL1ra antagonism in TBI have used variations of the controlled cortical impact model.3, 4, 5, 6 In this study, we have tried to minimize interpatient variability rather than recapitulating these rodent models; however, this does not detract from the core conclusions of the study. It is not possible to select out patients with a ‘pure' form of injury for study and therefore human studies are necessarily opportunistic and heterogenous. As a result, microdialysis data can be ‘noisy' and this is further complicated by the limitations in assay technology such that some cytokines/chemokines are near to the lower limit of quantification and may not be reliably quantified.16
This variation is particularly apparent in the concentration of intracerebral IL1ra achieved despite consistent plasma penetration. We do not have a definitive explanation for this variation but we would speculate that there are several potential reasons for this. First, the impact of varying degrees of injury on the function of the blood–brain barrier for the whole brain. Second, blood–brain barrier permeability to macromolecules (rhIL1ra) may respond to injury differently in different parts of the brain such that focal microdialysis monitoring may reflect these specific local variations. Third, there is the interesting possibility that there is a patient-specific host response to administered rhIL1ra such that resident inflammatory cells produce further endogenous IL1ra or release other cytokines that inhibit endogenous IL1ra production. The antibody-based techniques in the luminex assay do not distinguish between exogenous and endogenous forms of IL1ra. Even if this were the case, the differences between patients in the two randomized groups are still likely to be a consequence of the drug administration, whether it is simple blood–brain barrier penetration or a more complex interaction with the host inflammatory response. Fourth, there may be variations between catheters' recoveries due to variations in catheter manufacture. We have already explored this possibility in bench studies of cytokine recovery,17 and we believe it to be a minor contribution. One advantage of using PCA is that data from any given catheter are normalized across the various cytokines and chemokines so providing there is commensurate reduction across all macromolecules in a given catheter the relative balance between recovered molecules will not change and therefore the scores on the PCA plot will be affected minimally. Genuine variations in brain extracellular fluid concentrations are another potential limitation in translation of putative neuroprotective drugs from rigorously controlled animal studies to human TBI. We see this as an additional reason to estimate brain extracellular fluid concentrations directly rather than rely on cerebrospinal fluid concentrations,21 which are much less variable and may not provide an accurate measure of drug penetration to the putative site of action.
Conventional statistical techniques struggle to compare multivariate data from small numbers of patients when there is a wide variation in the underlying concentrations in different patients. We have previously shown that multivariate projection methods can overcome some of these limitations in microdialysis-derived data.18 Principal component analysis is a method that is capable of simplifying multivariate data into a few PCs that contain the majority of the sources of variation within the data set as a whole, thereby assisting with presentation and interpretation of the data. These PCs are made up of a linear combination of the original variables, each of which contributes to a varying degree, termed as the ‘loading'. Variables, in this case cytokines/chemokines, that load to a greater degree will have a larger magnitude of coefficient than those contributing to a lesser degree as illustrated in the loading plot, Figure 2B. Figure 2A, the scores plot, plots each of the individual data points from each individual patient on the PCs. By color-coding the data points by the source of the data (control or intervention group), it can be seen that there is a separation in the observations relating to the administration of rhIL1ra along the PC 2 axis. The visual separation between control and intervention groups in Figure 2B, in itself, provides evidence that the administration of rhIL1ra impacts on the inflammatory milieu after human TBI. As PCA is an ‘unsupervised' technique, the model does not take into account any information about whether the observations come from control or intervention patients, thereby minimizing any potential for bias. Principal component analysis does not generate a measure of statistical significance but it can provide candidate cytokines/chemokines that are likely to differ between the two groups of patients. The visual separation between the two groups in an unsupervised model, in itself, suggests that there is an rhIL1ra-dependent impact on the cytokine milieu after severe TBI.18 A strength of PCA is that it can be used to select candidate biomarkers for further investigation, and MDC was the strongest candidate to emerge from our PCA study; therefore, we explored it further with conventional statistics. While this approach does not preclude investigating other, less strong candidate cytokines by conventional statistics, we limited the present analysis to the strongest candidate (MDC) to avoid the pitfalls of multiple comparisons. We have compared the concentrations of MDC between groups, as it was the chemokine that loaded to the greatest magnitude on PC2. A mixed-model ANOVA shows a statistically significant difference in concentration between the two groups, suggesting that rhIL1ra leads to a reduction in concentration of MDC.
Macrophage-derived chemoattractant has a role in polarizing T cells to Th2 responses,26 however, the specific role in severe TBI is not yet known. Macrophage-derived chemoattractant (also termed as CCL22) exerts its chemokine effect by binding to its receptor CCR4 (also termed as CD194 and K5-5), a type of G protein-coupled receptor found on Th2 cells, monocytes, and various other cell types.27, 28 T-cell infiltration has been implicated in response to central nervous system injury and it has been suggested that a systemic Th2 shift may improve outcome.29 This would imply that MDC might play a part in a healing process. However, MDC can act as a pyrogen, for example injection into the hypothalamus preoptic area of rodents produced a rise in core body temperature and increase in metabolic rate (judged by fluorodeoxyglucose-positron emission tomography) in brown adipose tissue.30 Furthermore, there is growing interest in the development of small-molecule CCR4 antagonists as potential therapies against systemic inflammatory conditions such as asthma.31, 32, 33 It has also been suggested that targeting T-lymphocyte infiltration may improve outcome after TBI, based on an experimental model.34 Whether the overall role of MDC in human TBI is beneficial or detrimental remains unknown. It may be tempting to extrapolate that rhIL1ra is acting as an ‘anti-inflammatory' cytokine and that a reduction in MDC concentration relates to a reduction in macrophage-derived production after TBI. However, inspection of the loading plot shows several β-chemokines: monocyte chemotactic protein-1, macrophage inflammatory protein-1beta, macrophage inflammatory protein-1alpha, and RANTES, responsible for monocyte recruitment,35 load oppositely on PC2 suggesting that they are higher in the rhIL1ra-treated patients. There is conflicting evidence as in some contexts neuroinflammation may be damaging35, 36 while in others it may support repair and recovery.37, 38 Further studies are required to better define the delicate interplay between the humoral cytokine/chemokine response and the variety of cell types that are known to respond to these humoral mediators in TBI.
All previous studies of rhIL1ra in neurologic disease have utilized intravenous administration at much higher doses,12, 14, 21 however, subcutaneous administration, as in this study, may provide a more practical method of drug delivery particularly in the emergency setting. We would expect that the maximal benefit derived from an IL1R antagonist would be in the first few hours after neurologic injury as IL1β, the natural agonist at the IL1R, rises rapidly after injury.16, 39 The putative therapeutic window in TBI may therefore be in the prehospital setting.
In this study, we have shown that subcutaneously administered rhIL1ra results in a large increase in concentration of this cytokine both in the circulation and in the brain extracellular space, in TBI patients. Furthermore, our results of multiplex immunofluorescence analysis of cytokines and chemokines, with data analysis by PCs analysis, suggest that the rhIL1ra treatment has resulted in modulation of the brain extracellular cytokine and chemokine profile. Our ability to show a biologic response to rhIL1ra administration using cerebral microdialysis also provides a biomarker for IL1R antagonism that can be utilized in further dose-ranging studies. While the present study is too small to establish whether the modification of the neuroinflammatory response showed here translates into any beneficial therapeutic effect in terms of clinical outcome, it is only by carrying out detailed studies that illuminate the underlying biology of the IL1 cytokine family, that successful clinical efficacy studies can be designed and implemented. In this regard, microdialysis sampling of cytokines and chemokines from the brain extracellular space, the compartment to which neurons and glia are exposed, can advance our understanding of inflammation in neurologic disease both in TBI and in other pathologies in which rhIL1ra has a putative therapeutic role, such as ischemic stroke.12
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
AH is supported by a joint Medical Research Council/Royal College of Surgeons of England Clinical Research Training Fellowship (G0802251). AH and MRG are supported by Raymond and Beverly Sackler Fellowships. MRG is supported by the Royal College of Surgeons of England Research Fellowship. KLHC is supported by the National Institute for Health Research Biomedical Research Centre, Cambridge (Neuroscience Theme; Brain Injury and Repair Theme). DKM and JDP are supported by National Institute for Health Research Senior Investigator Awards. PJAH is supported a National Institute for Health Research Professorship. Study support was provided by the Medical Research Council (Grant number G0600986 ID 79068) and the National Institute for Health Research Biomedical Research Centre, Cambridge. PJAH and JDP are directors of Technicam. AH and MRG have received specialist training in multivariate projection methods from CAMO Software AS (Oslo, Norway). PJAH and JDP are directors of Technicam.
Supplementary Material
References
- Fearnside M.EpidemiologyIn: Reilly PL ed. Head injury pathophysiology and management2nd edn.Hodder Arnold: London; 2005 [Google Scholar]
- Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95:352–372. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Clausen F, Hanell A, Bjork M, Hillered L, Mir AK, Gram H, et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2009;30:385–396. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- Clausen F, Hanell A, Israelsson C, Hedin J, Ebendal T, Mir AK, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2011;34:110–123. doi: 10.1111/j.1460-9568.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, et al. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- Lazovic J, Basu A, Lin HW, Rothstein RP, Krady JK, Smith MB, et al. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke. 2005;36:2226–2231. doi: 10.1161/01.STR.0000182255.08162.6a. [DOI] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15:547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Allan SM, Rothwell NJ. Interleukin-1beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci Transl Med. 2010;2:27rv1. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- British Medical Association British National Formulary64th edn.BMJ Publishing Group: London, UK; 2012 [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J, Ogungbenro K, Hulme S, Greenhalgh A, Aarons L, Scarth S, et al. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cereb Blood Flow Metab. 2010;31:439–447. doi: 10.1038/jcbfm.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, et al. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28:387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- Opal SM, Fisher CJ, Jr., Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Skepper JN, Kirkpatrick PJ, Pickard JD, Hutchinson PJ. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–561. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
- Helmy A, Antoniades CA, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Principal component analysis of the cytokine and chemokine response to human traumatic brain injury. PLoS ONE. 2012;7:e39677. doi: 10.1371/journal.pone.0039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007;99:32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- Galea J, Ogungbenro K, Hulme S, Greenhalgh A, Aarons L, Scarth S, et al. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cereb Blood Flow Metab. 2011;31:439–447. doi: 10.1038/jcbfm.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BB, Baughman S, Sullivan JT. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin Pharmacol Ther. 2003;74:85–94. doi: 10.1016/S0009-9236(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, et al. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004;21:1113–1122. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- Shannon RJ, Carpenter KL, Guilfoyle MR, Helmy A, Hutchinson PJ. Cerebral microdialysis in clinical studies of drugs: pharmacokinetic applications. J Pharmacokin Pharmacodyn. 2013;40:343–358. doi: 10.1007/s10928-013-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC) J Leukoc Biol. 2000;68:400–404. [PubMed] [Google Scholar]
- Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AE, et al. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- Cadosch D, Al-Mushaiqri MS, Gautschi OP, Chan E, Jung FJ, Skirving AP, et al. Immune response deviation and enhanced expression of chemokine receptor CCR4 in TBI patients due to unknown serum factors. Injury. 2010;41:e4–e9. doi: 10.1016/j.injury.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184:100–112. doi: 10.1016/j.jneuroim.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Osborn O, Sanchez-Alavez M, Dubins JS, Gonzalez AS, Morrison B, Hadcock JR, et al. Ccl22/MDC, is a prostaglandin dependent pyrogen, acting in the anterior hypothalamus to induce hyperthermia via activation of brown adipose tissue. Cytokine. 2011;53:311–319. doi: 10.1016/j.cyto.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdi DF, Chi S, Mattia K, Harrington C, Shi Z, Chen S, et al. Small molecule antagonists of the CC chemokine receptor 4 (CCR4) Bioorg Med Chem Lett. 2007;17:3141–3145. doi: 10.1016/j.bmcl.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Gong H, Qi H, Sun W, Zhang Y, Jiang D, Xiao J, et al. Design and synthesis of a series of pyrido[2,3-d]pyrimidine derivatives as CCR4 antagonists. Molecules. 2012;17:9961–9970. doi: 10.3390/molecules17089961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn A, Hodgson S, Wilson R, Robertson J, Watson J, Beerahee M, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study. BMC Pharmacol Toxicol. 2013;14:14. doi: 10.1186/2050-6511-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F, Lorant T, Lewen A, Hillered L. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J Innate Immun. 2012;4:463–477. doi: 10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, London A, Shechter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158:1133–1142. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Cox AL, Coles AJ, Nortje J, Bradley PG, Chatfield DA, Thompson SJ, et al. An investigation of auto-reactivity after head injury. J Neuroimmunol. 2006;174:180–186. doi: 10.1016/j.jneuroim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Schneider S, Bertsch T, Schlueter D, Fatar M, Ragoschke A, et al. Temporal profile of release of interleukin-1beta in neurotrauma. Neurosci Lett. 2000;284:135–138. doi: 10.1016/s0304-3940(00)00977-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.