Abstract
Cerebral white matter lesions (WMLs) have been consistently related to cognitive dysfunction but the role of white matter (WM) damage in cognitive impairment is not fully determined. Diffusion tensor imaging is a promising tool to explain impaired cognition related to WMLs. We investigated the separate association of high-grade periventricular hyperintensities (PVHs) and deep white matter hyperintensities (DWMHs) with fractional anisotropy (FA) in middle-aged individuals. We also assessed the predictive value to cognition of FA within specific WM tracts associated with high-grade WMLs. One hundred participants from the Barcelona-AsIA Neuropsychology Study were divided into groups based on low- and high-grade WMLs. Voxel-by-voxel FA were compared between groups, with separate analyses for high-grade PVHs and DWMHs. The mean FA within areas showing differences between groups was extracted in each tract for linear regression analyses. Participants with high-grade PVHs and participants with high-grade DWMHs showed lower FA in different areas of specific tracts. Areas showing decreased FA in high-grade DWMHs predicted lower cognition, whereas areas with decreased FA in high-grade PVHs did not. The predictive value to cognition of specific WM tracts supports the involvement of cortico-subcortical circuits in cognitive deficits only in DWMHs.
Keywords: brain imaging, cerebrovascular disease, cognition, diffusion tensor imaging, vascular cognitive impairment, white matter disease
Introduction
Cerebral white matter lesions (WMLs) comprise diffuse areas of hypodensity on computerized tomography and high signal intensities on T2, proton density, and fluid-attenuated inversion recovery (FLAIR) magnetic resonance image (MRI) sequences. White matter lesions are commonly found in normal aging.1 They are considered an expression of cerebrovascular small vessel disease (SVD), so that WMLs are commonly associated with other signs of SVD, such as lacunar infarcts and microbleeds.2
White matter lesions have been consistently related to cognitive dysfunction and decline.3 Cognitive consequences have been attributed to frontal-subcortical circuit involvement,4 with executive function and processing speed impairing the most. The association of WMLs with cognitive function is probably mediated by severity of white matter (WM) damage, with mild lesions unlikely to be related to cognitive dysfunction.3 White matter lesions are usually divided into two groups: those immediately adjacent to the ventricles (periventricular hyperintensities (PVHs)) and those located in the deep white matter (deep white matter hyperintensities (DWMHs)).5 The relative contribution of PVHs or DWMHs to cognitive function is still controversial.3
Despite the abundant evidence for the association between WMLs and cognition, the role of WM damage in cognitive impairment is not fully determined. Previous research has found that correlations between WMLs and cognitive function are modest.3, 6 The evidence of a penumbra of subtle WM injury surrounding lesions supports the notion that WMLs may fail to capture the full extent and degree of WM damage.7 Novel imaging techniques that allow a more direct assessment of the composition and organization of WM are promising tools to explain impaired cognition related to WMLs beyond what can be expected from conventional MRI.8 Diffusion tensor imaging (DTI) enables the measurement of diffusion of water molecules within the brain. In regions with few or no constraints imposed by physical boundaries, such as cerebrospinal fluid in the ventricles, water movement is random and uniform in every direction and is therefore isotropic. In contrast to cerebrospinal fluid, the motion of water molecules in the WM is restricted by the parallel-oriented fibers and diffusion is therefore highly anisotropic.9 Degradation of the WM microstructure organization is accompanied by changes in measurable DTI parameters, such as a decrease in fractional anisotropy (FA).10 Prior studies have related diffusion metrics to cognitive function in community-dwelling samples11 and patients with ischemic leukoaraiosis.12, 13 Fractional anisotropy has been associated with cognitive abilities such as executive function,13 processing speed,11 verbal fluency,12 or motor function.11
White matter lesions detected by conventional MRI and lower FA values measured by DTI may represent two processes affecting the WM that are commonly seen in aging.9 There is scarce evidence of the relation between WMLs and FA on a voxel-by-voxel basis in normal aging. Only one study has reported a widespread pattern of reduced FA in the WM of participants with WMLs compared with healthy controls,13 whereas another study has found an association of WMLs volume and decreased FA in extensive areas of WM.14 Further, most research relating FA to cognitive function in participants with WMLs has employed a region of interest approach12 or has applied segmentation methods to calculate mean FA and other histogram metrics within specific brain tissues (i.e., gray matter, WM, WMLs).11 These approaches may overlook the relative contributions of major WM tracts to cognitive function, which may have some importance given the complex functional anatomy of cognitive processes.15
In this study, our objective is twofold. First, we aim to investigate the separate association of high-grade PVHs and DWMHs with FA on a voxel-by-voxel basis in a community-dwelling sample of middle-aged participants. After previous research,13, 14 we expect that participants with high-grade WMLs will show a spatial pattern of lower FA throughout the whole WM compared with participants with low-grade WMLs. Some data suggest that DWMHs are distributed in a more extensive area than PVHs and that their histopathological correlates may exert more severe damage to WM tracts than PVHs.16 Accordingly, we also expect that this spatial pattern of lower FA will be found in a greater extent in participants with high-grade DWMHs. Second, we aim to assess the predictive value to cognition of lower FA within specific WM tracts related to high-grade PVHs and DWMHs. Cognitive consequences may rely on severity and location of WMLs.3, 17 In a previous study, we found a predominant role of high-grade DWMHs in cognitive dysfunction of middle-aged individuals.18 Consequently, we hypothesize that lower FA in tracts related to high-grade DWMHs will be more associated with cognitive function than lower FA in tracts related to high-grade PVHs.
Materials and methods
Study Design and Sample Selection
The Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) Study is an ongoing population-based study that involves 28 primary healthcare centers and a tertiary stroke center. Complete details for the Barcelona-AsIA protocol have been described elsewhere.19 In brief, a random sample of participants over 50 years old without previous history of stroke or ischemic heart disease underwent clinical examination, blood analysis, complete extra and transcranial Duplex ultrasound study, and neuropsychological assessment. The Barcelona-AsIA Neuropsychology Study is a related prospective study whose objectives are (1) to investigate the associations between cognition and vascular risk factors, asymptomatic cervicocerebral atherosclerosis, and MRI signs of SVD, and (2) to identify clinical and radiologic features and biologic mechanisms underlying these associations.
Our participants were recruited from the PERART Study, a related ongoing population-based study to determine the prevalence of peripheral arterial disease and to evaluate the predictive value of ankle–arm index in relation to cardiovascular mortality and morbidity.20 Details of the recruitment process have been previously described.18 Briefly, a total of 132 participants aged 50 to 65 years were selected to undergo a comprehensive neuropsychological assessment and brain MRI. Exclusion criteria were as follows: history of stroke or transient ischemic attack, coronary heart disease, neurologic disease, or severe psychiatric disorder (n=11); a mini-mental examination score⩽25 or severe disability (n=3); other medical diseases that could affect cognitive assessment and function (n=4); contraindications to undergo MRI (n=10), unexpected findings seen on brain MRI (n=2) or other causes (i.e., less than 75% of neuropsychological assessment available) (n=2). Consequently, the final sample included 100 participants aged 50 to 65 stratified by sex and educational level.
This study has been approved by the ethics committees of the University of Barcelona and the Germans Trias i Pujol University Hospital. It was conducted in accordance with the ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Evaluation of Vascular Risk Factors
Diagnosis of a particular vascular risk factor, such as arterial hypertension, diabetes mellitus, dyslipidemia, or current smoking status, was based on clinical history or use of medication for the particular condition at the time of the clinical examination. Ten-year cardiovascular risk was calculated after the REGICOR function, which is a validated calibration of the Framingham function for Spanish population.19 The REGICOR function includes non-modifiable, such as age and sex, and modifiable vascular risk factors, such as arterial hypertension, diabetes mellitus, dyslipidemia, and smoking status.
Neuropsychological Assessment
All participants completed an extensive neuropsychological battery. Cognitive measures were grouped into eight cognitive domains, which include tests assessing similar cognitive functions:21 executive functioning, working memory, attention, verbal fluency, verbal memory, visual memory, visuospatial skills, and psychomotor speed. Executive functioning (i.e., conceptualization, planning, and inhibition) was assessed with the 64-item computerized version of the Wisconsin Card Sorting Test22 and the interference score of the Color-Word Stroop Test.21 Working memory was examined with Digit Span Backwards from the Wechsler Adult Intelligence Scale 3rd edition (WAIS-III)21 and part B of the Trail Making Test.21 Attentional abilities were assessed with the Continuous Performance Test21 and Digit Span Forward, Symbol Search, and Digit Symbol Coding subtests from the WAIS-III. Verbal fluency was measured with letter fluency (letters P, M, and R) and semantic category fluency (animals) in 60 seconds each.21 Word List and Visual Reproduction from the Wechsler Memory Scale 3rd edition (WMS-III)21 were administrated to measure verbal and visual memory, respectively. Visual Discrimination and the Copy from the Visual Reproduction subtest (WMS-III) were used to evaluate visuospatial skills. Psychomotor speed was assessed with part A of the Trail Making Test and Grooved Pegboard.21 Participants' raw scores were normalized to z-scores using the mean and standard deviation (s.d.) of the sample. Composite z-scores for each participant in each cognitive domain were calculated by averaging the z-scores of all tests within that domain.
Neuropsychological assessment also included the Mini-Mental State Examination21 as a global cognitive function test and the Vocabulary subtest from the WAIS-III as a measure of premorbid intelligence. Depressive symptoms were assessed with the Geriatric Depression Scale 15-item version.23
Magnetic Resonance Imaging Acquisition Protocol
The MRI scanning protocol was performed with a Siemens Magnetom Trio 3T scanner (Siemens Diagnostics Healthcare, Erlangen, Germany) at the Medical Image Core Facility (IDIBAPS, Hospital Clinic, Barcelona, Spain).
The MRI protocol included a set of three-dimensional magnetization-prepared rapid gradient echo T1-weighted images (repetition time: 2,300 milliseconds; echo time: 3 milliseconds; flip angle: 15° field of view: 245 mm; and voxel size: 1 × 1 × 1 mm, no gap), and two sets of DTI images acquired along 30 non-collinear directions (repetition time: 9,300 milliseconds; echo time: 94 milliseconds; flip angle: 15° field of view: 240 mm; voxel size: 2 × 2 × 2 mm, no gap; and b=1,000 seconds/mm2) with an additional acquisition for each set without diffusion weighting (b=0 second/mm2). The two acquisitions of DTI were averaged.
Axial FLAIR images (repetition time: 9,040 milliseconds; echo time: 85 milliseconds; inversion time: 2,500 milliseconds; and voxel size: 1.1 × 0.9 × 5 mm, gap: 1.5 mm) and axial T2-weighted images (repetition time: 5,520 milliseconds; echo time: 92 milliseconds; and voxel size: 0.5 × 0.4 × 5 mm, gap: 1.5 mm) were also collected for rating WMLs and lacunar infarcts (see below).
Magnetic Resonance Imaging Data Processing
Individual processing of DTI data was performed using the FMRIB's Diffusion Toolbox, part of the FMRIB Software Library (FSL) version 4.1.6.24 First, the raw DTI images were visually inspected and corrected for eddy currents and head motion by registering each participant's images to their reference volume (b=0). Brain extraction was performed to remove non-brain structures using the Brain Extraction Tool implemented in FSL. Then, diffusion tensor was reconstructed by fitting a tensor model to each image voxel of the preprocessed DTI data, using the DTIfit program included in FSL.
The resulting FA maps were fed into Tract-Based Spatial Statistics,25 which is also a part of FSL, to carry out the voxel-wise statistical analysis. The FA maps for all participants were first aligned into the MNI standard space using the higher-resolution FA template by the nonlinear registration method FNIRT, which uses a B-spline representation of the registration warp field. The nonlinearly registered images were further averaged to generate a mean FA image of all participants. Next, the mean FA image was thinned to create a mean FA skeleton, which represents the centers of all common tracts. The mean FA skeleton was further thresholded by a FA value of 0.25 to exclude the skeleton voxels that may contain partial volume (i.e., gray matter) or cross-subject image misalignment. After the thresholding of the mean FA skeleton, each subject's aligned FA map was then projected onto this skeleton and the resulting skeletonized, fully nonlinearly aligned FA data were then used for voxel-wise statistical analysis.
Brain tissue volumes (gray matter, WM, cerebrospinal fluid) were calculated with SIENAX software (http://www.fmrib.ox.ac.uk/fsl/siena/index.html) on high resolution T1-weighted images.26 The ratio between brain parenchymal volume (BP=GM+WM) and total brain volume was computed to obtain a normalized measure of brain atrophy.27
Rating of White Matter Lesions and Lacunar Infarcts
Location and severity of WMLs were estimated on T2 and FLAIR images by a trained and masked neuroradiologist (NB) using the Fazekas scale.28 On MRI, WMLs appear hyperintense on T2-weighted images. They also remain bright on FLAIR, a T2-weighted sequence that suppresses the signal from fluid-filled spaces. The Fazekas scale provides two different scores (PVHs and DWMHs), rated on a 0- to 3-point scale of increasing severity within the whole brain. The sum of the PVHs and the DWMHs scores provides a total score. Participants were classified as having no lesions or mild, moderate, or severe lesions (0, 1, 2, or 3 points, respectively) in each location. The intra-rater reliability was determined on 20 randomly selected scans scored twice. Reliability was good for grading both PVHs (weighted kappa=0.66, 95% CI: 0.33–0.96), and DWMHs (weighted kappa=0.7, 95% CI: 0.41–0.99).
Lacunar infarcts were defined as lesions with increased signal intensity on T2-weighted images and decreased signal intensity on T1-weighted and FLAIR images with a diameter of 5 to 15 mm, which were not located in areas with high prevalence of widened perivascular spaces.5
Statistical Analyses
The distribution of the WMLs scores was positively skewed as expected, with most participants having no lesions or mild lesions. We dichotomized our sample into low-grade WMLs (participants with no lesions or mild lesions) and high-grade WMLs (participants with moderate or severe lesions). High-grade PVHs were thus defined as PVH>1 and high-grade DWMHs as DWMH>1. We compared voxel-by-voxel skeletonized FA values between high-grade and low-grade WMLs, with separate analyses for PVHs and DWMHs groups, regressing out age, sex, vascular risk factors (REGICOR function) and the other Fazekas score (PVHs score in the comparison between DWMHs groups, DWMHs score in the comparison between PVHs groups).
For the voxel-wise analysis of group differences, a permutation-based program (randomise) with standard general linear model implemented in FSL was performed with 5,000 random permutations. A developed algorithm, known as Threshold-Free Cluster Enhancement29 was used to obtain the skeleton voxels significantly different between groups at significance level of P<0.05, after accounting for multiple comparisons by controlling for Family-Wise Error rates. Significant clusters were also required to have an extent of at least 50 voxels.
From the results of voxel-wise group comparisons, the skeleton areas showing significant lower FA were located and labeled anatomically by mapping the Family-Wise Error-corrected statistical map of P<0.05 to the Johns Hopkins University DTI WM atlas.30 A binarized mask of significant results was made and superimposed onto probabilistic masks of labeled WM tracts showing lower FA for each group comparison. The mean FA value within areas showing significant differences of FA between groups was then extracted in each WM tract for the linear regression analyses.
Linear regression analyses were carried out using the Statistical Package for Social Sciences (SPSS for Windows, version 18.0, SPSS, Chicago, IL, USA). A standardized z-score of the extracted mean FA value for each tract was calculated and entered into a series of linear regression analyses to assess its specific contribution to cognitive function, with separate analyses for WM tracts showing differences in PVHs and DWMHs groups. The z-scores for all WM tracts were first entered as predictive variables with cognitive domains as the dependent variables. Linear regression models were first adjusted (Model 1) for age, sex, years of education, and treatable vascular risk factors associated with WMLs or cognitive function (P⩽0.1) that we have reported previously.18 Briefly, hypertensive participants had lower scores on verbal fluency, visuospatial skills, and psychomotor speed. Participants with dyslipidemia had lower scores on working memory. Participants with diabetes mellitus had lower scores on executive functioning and psychomotor speed. Higher scores on verbal memory and visuospatial skills were observed in current non-smoker participants. Models were also adjusted (Model 2) for other brain changes usually related to cognitive disturbances (brain atrophy and lacunar infarcts).
Results
Sample Characteristics
Owing to technical difficulties on MRI acquisition, four participants were excluded from the sample. There were no differences in demographic and clinical variables between the remaining 96 participants and those recruited from the PERART Study but excluded from the analyses. Demographic, clinical, and MRI characteristics of the remaining 96 participants (mean age=59.7 years, 59% women, median education=8 years) are summarized in Table 1. Their estimated premorbid intelligence, general cognitive function and depressive symptoms were within the normal range. A significantly higher proportion of participants with high-grade DWMHs (68.8%) had arterial hypertension compared with participants with low-grade DWMHs (42.5%).
Table 1. Demographic, clinical and MRI data.
| Low-grade PVHs (without or mild) (n=80) | High-grade PVHs (moderate) (n=16) | P | Low-grade DWMHs (without or mild) (n=80) | High-grade DWMHs (moderate) (n=16) | P | |
|---|---|---|---|---|---|---|
| Age (years)a | 59.48 (3.48) | 61.00 (2.48) | 0.10 | 59.48 (3.35) | 61.00 (3.25) | 0.10 |
| Sex (n (%) female)b | 48 (60.0) | 9 (56.3) | 0.78 | 48 (60.0) | 9 (56.3) | 0.78 |
| Education (years)c | 8 (6–9) | 8 (6.25–10) | 0.71 | 8 (6–10) | 8 (6.25–8.75) | 0.98 |
| MMSEc | 29 (28–30) | 30 (28.25–30) | 0.20 | 29 (28–30) | 30 (28–30) | 0.71 |
| Vocabulary (WAIS-III)a | 38.91 (8.76) | 37.56 (10.56) | 0.59 | 39.36 (8.85) | 37.50 (9.47) | 0.50 |
| GDS-15c | 2 (1–3) | 1 (0–2) | 0.08 | 2 (0–3) | 1 (1–2.75) | 0.89 |
| REGICORc | 5 (4–7) | 6.5 (4.25–8.5) | 0.26 | 5 (4–7) | 6.5 (4.25–8) | 0.32 |
| Vascular risk factors (n (%)) | ||||||
| Hypertensionb | 38 (47.5) | 7 (43.8) | 0.78 | 34 (42.5) | 11 (68.8) | 0.04* |
| Dyslipidemiab | 48 (60.0) | 9 (56.3) | 0.78 | 47 (58.8) | 10 (62.5) | 0.78 |
| DMd | 15 (18.8) | 2 (12.5) | 0.73 | 15 (18.8) | 2 (12.5) | 0.73 |
| Current smokerd | 12 (15.0) | 3 (18.8) | 0.71 | 13 (16.3) | 2 (12.5) | 1 |
| MRI measuresa | ||||||
| GM (cm3) | 590.82 (35.38) | 580.80 (45.11) | 0.41 | 586.12 (36.22) | 581.74 (45.14) | 0.72 |
| WM (cm3) | 564.90 (60.69) | 542.24 (63.28) | 0.19 | 543.56 (55.53) | 546.50 (64.83) | 0.87 |
| BP (cm3) | 1,155.72 (93.52) | 1,123.04 (104.70) | 0.25 | 1,129.68 (88.42) | 1,128.24 (106.39) | 0.96 |
| TBV (cm3) | 1,450.51 (118.78) | 1,420.00 (128.76) | 0.38 | 1,424.18 (109.25) | 1,425.27 (130.96) | 0.98 |
| Ratio GM/TBV (%) | 40.97 (1.73) | 40.83 (1.35) | 0.73 | 41.22 (1.51) | 40.89 (1.39) | 0.38 |
| Ratio WM/TBV (%) | 38.74 (1.55) | 38.25 (1.50) | 0.27 | 38.11 (1.51) | 38.27 (1.54) | 0.71 |
| Ratio BP/TBV (%) | 79.71 (2.03) | 79.08 (1.44) | 0.25 | 79.33 (1.80) | 79.16 (1.52) | 0.67 |
| LI present (n (%))d | 4 (5.1) | 3 (18.8) | 0.09 | 6 (7.5) | 1 (6.3) | 1 |
BP, brain parenchymal volume=GM+WM; DM, diabetes mellitus; DWMHs, deep white matter hyperintensities; GDS-15, geriatric depression scale, 15-item version; GM, gray matter volume; LI, lacunar infarcts; MMSE, mini-mental state examination; MRI, magnetic resonance imaging; PVHs, periventricular hyperintensities; REGICOR, Registre Gironí del Cor; TBV, total brain volume; WAIS-III, Wechsler Adult Intelligence Scale 3rd edition; WM, white matter volume.
Values are means (s.d.) in Student's t-test or medians (interquartile range) in Mann–Whitney test for continuous variables. Values are n (%) for categorical variables in χ2 test and Fisher's exact test. P shows statistical comparison between participants with high-grade and low-grade white matter lesions.
*P<0.05.
Student's t-test.
χ2 test.
Mann–Whitney test.
Fisher's exact test.
According to the PVHs score, there were 80 participants (83.3%) with low-grade PVHs and 16 participants (16.7%) with high-grade PVHs. Among the participants with low-grade PVHs, 51 participants (53.1% from the total sample) were without lesions and the remaining 29 participants (30.2%) showed mild (caps and pencil-thin lining) PVHs. All the 16 participants with high-grade PVHs had moderate (smooth halo) PVHs. According to the DWMHs score, 80 participants (83.3%) were classified as low-grade DWMHs and 16 participants (16.7%) were classified as high-grade DWMHs. Among the participants with low-grade DWMHs, 19 participants (19.8% from the total sample) were without DWMHs and 61 participants (63.5%) had mild (punctuate) DWMHs. All the 16 participants with high-grade DWMHs presented moderate (beginning to confluent) DWMHs. Seven participants (7.3%) had both high-grade DWMHs and high-grade PVHs (moderate lesions). None of the participants had severe (irregular) PVHs or severe (confluent) DWMHs. Eleven lacunar brain infarcts were present across seven participants (7.3%). Eight of the lacunar infarcts were located in the basal ganglia, and there was one lacunar infarct each in the pons, intern capsule, and corona radiata.
Areas of Lower Fractional Anisotropy in Participants with High-Grade White Matter Lesions
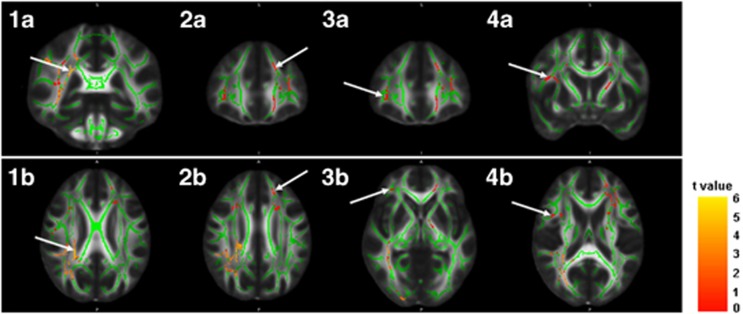
Participants with high-grade DWMHs showed a pattern of lower FA in several areas throughout the whole WM skeleton compared with participants with low-grade DWMHs, after regressing out age, sex, vascular risk factors and the PVHs score (Table 2 and Figure 1). Specifically, peak-value voxels of decreased FA were located in the right anterior thalamic radiation, the right superior longitudinal fasciculus, and the bilateral inferior fronto-occipital fasciculus (IFOF). No significant results were found for the reverse contrast.
Table 2. Areas of lower FA in participants with high-grade DWMHs.
|
MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Location | Lobe | x | y | z | t | P | Vox |
| Right ATR | Sub-lobar | 25 | −37 | 26 | 4.16 | 0.014 | 4,868 |
| Right SLF | Frontal | 42 | 6 | 18 | 3.18 | 0.048 | 459 |
| Right IFOF | Frontal | 35 | 36 | −1 | 3.21 | 0.048 | 299 |
| Left IFOF | Frontal | −17 | 36 | 30 | 3.06 | 0.041 | 2,502 |
ATR, anterior thalamic radiation; DWMHs, deep white matter hyperintensities; FA, fractional anisotropy; IFOF, inferior fronto-occipital fasciculus; MNI, Montreal Neurological Institute; SLF, superior longitudinal fasciculus; Vox, cluster size in number of voxels.
MNI coordinates (mm) represent peak-value voxel location.
Figure 1.
Regional areas of reduction of fractional anisotropy (FA). Clusters showing significant reduction of FA in participants with high-grade deep white matter hyperintensities are displayed on coronal and axial sections of an FA map. The FA skeleton used for statistical analyses is superposed in green. White arrows at images 1a (y=−37) and 1b (z=26) show peak-value voxel location of reduced FA in the right anterior thalamic radiation. White arrows at images 2a (y=36) and 2b (z=30) show peak-value voxel of reduced FA in the left inferior fronto-occipital fasciculus (IFOF). White arrows at images 3a (y=36) and 3b (z=−1) show peak-value voxel of reduced FA in the right IFOF. White arrows at images 4a (y=6) and 4b (z=18) show peak-value voxel of reduced FA in the right superior longitudinal fasciculus. The color scale indicates the magnitude of t-values with lowest appearing in dark red and the highest in bright yellow. Images are displayed in radiologic convention (right side represents left side and left side represents right side of the brain).
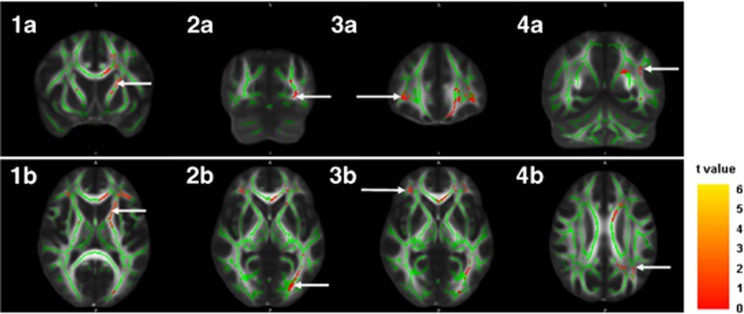
Participants with high-grade PVHs also showed lower FA values in several areas throughout the whole WM skeleton compared with participants with low-grade PVHs, after regressing out age, sex, vascular risk factors, and the DWMHs score (Table 3 and Figure 2). Peak-value voxels of decreased FA were located in the left anterior thalamic radiation, the left superior longitudinal fasciculus and the bilateral IFOF. No significant results were found for the reverse contrast.
Table 3. Areas of lower FA in participants with high-grade PVHs.
|
MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Location | Lobe | x | y | z | t | P | Vox |
| Right IFOF | Frontal | 33 | 37 | 0 | 4.22 | 0.042 | 239 |
| Left ATR | Sub-lobar | −20 | 11 | 11 | 3.46 | 0.035 | 3,199 |
| Left IFOF | Occipital | −24 | −79 | 1 | 2.84 | 0.048 | 571 |
| Left SLF | Temporal | −35 | −55 | 28 | 3.43 | 0.048 | 300 |
ATR, anterior thalamic radiation; FA, fractional anisotropy; PVHs, periventricular hyperintensities; MNI, Montreal Neurological Institute; IFOF, inferior fronto-occipital fasciculus; PVHs, periventricular hyperintensities; SLF, superior longitudinal fasciculus; Vox, cluster size in number of voxels.
MNI coordinates (mm) represent peak-value voxel location.
Figure 2.
Regional areas of reduction of fractional anisotropy (FA). Clusters showing significant reduction of FA in participants with high-grade periventricular hyperintensities are displayed on coronal and axial sections of an FA map. The FA skeleton used for statistical analyses is superposed in green. White arrows at images 1a (y=11) and 1b (z=11) show peak-value voxel location of reduced FA in the left anterior thalamic radiation. White arrows at images 2a (y=−79) and 2b (z=1) show peak-value voxel of reduced FA in the left inferior fronto-occipital fasciculus (IFOF) within the occipital area. Images 3a (y=37) and 3b (z=0) show peak-value voxel of reduced FA in the right IFOF. Images 4a (y=−55) and 4b (z=28) show peak-value voxel of reduced FA in the left superior longitudinal fasciculus. The color scale indicates the magnitude of t-values with lowest appearing in dark red and the highest in bright yellow. Images are displayed in radiologic convention (right side represents left side and left side represents right side of the brain).
Predictive Value to Cognitive Outcome of Areas of Lower Fractional Anisotropy in Specific White Matter Tracts
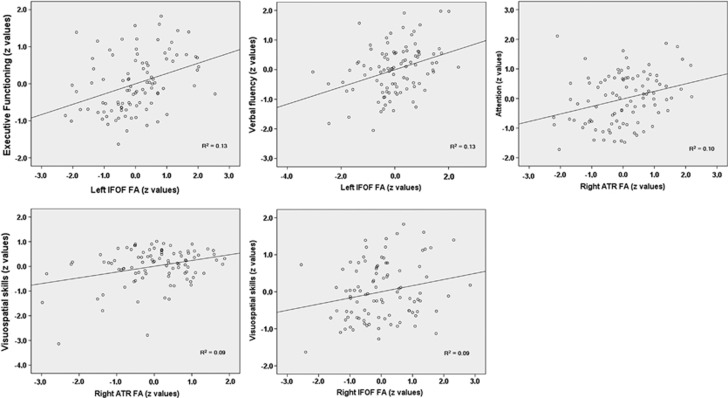
Table 4 shows the predictive value to cognition of areas showing decreased FA between participants with high-grade DWMHs versus participants with low-grade DWMHs. Areas of lower FA in the left IFOF were related to lower scores in executive functioning (R2=0.13; β=0.48) and verbal fluency (R2=0.13; β=0.48). Areas of lower FA in the right anterior thalamic radiation were related to lower scores in attention (R2=0.10; β=0.36) and visuospatial skills (R2=0.09; β=0.35). Areas of lower FA in the right IFOF were also related to visuospatial skills (R2=0.09; β=0.35). Adjustments for age, sex, years of education, and vascular risk factors (Model 1) reduced the associations with executive functioning, attention, and verbal fluency, but they were still significant and increased associations with visuospatial skills. These associations were essentially unaltered by additional adjustment for brain atrophy ratio and lacunar infarcts (Model 2).
Table 4. Predictive value of areas showing FA differences between participants with high-grade DWMHs versus participants with low-grade DWMHs using multivariate linear regression models.
|
Unadjusted model |
Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| Beta | P-value | Beta | P-value | Beta | P-value | |
| EF | ||||||
| r ATR | 0.26 | 0.22 | 0.25 | 0.27 | 0.26 | 0.25 |
| r SLF | 0.28 | 0.16 | 0.27 | 0.20 | 0.30 | 0.16 |
| r IFOF | −0.44 | 0.09 | −0.43 | 0.11 | −0.43 | 0.11 |
| l IFOF | 0.48 | 0.004** | 0.40 | 0.006** | 0.38 | 0.01* |
| W Memory | ||||||
| r ATR | 0.06 | 0.85 | 0.07 | 0.82 | 0.05 | 0.87 |
| r SLF | −0.01 | 0.96 | 0.11 | 0.69 | 0.18 | 0.51 |
| r IFOF | −0.23 | 0.49 | −0.21 | 0.54 | −0.23 | 0.50 |
| l IFOF | 0.17 | 0.27 | 0.05 | 0.77 | 0.03 | 0.86 |
| Attention | ||||||
| r ATR | 0.36 | 0.01* | 0.32 | 0.02* | 0.30 | 0.03* |
| r SLF | −0.15 | 0.50 | 0.04 | 0.85 | 0.07 | 0.75 |
| r IFOF | −0.19 | 0.51 | −0.25 | 0.36 | −0.27 | 0.32 |
| l IFOF | 0.22 | 0.10 | 0.08 | 0.55 | 0.09 | 0.49 |
| Verbal fluency | ||||||
| r ATR | 0.21 | 0.41 | 0.26 | 0.31 | 0.24 | 0.34 |
| r SLF | −0.32 | 0.08 | −0.32 | 0.08 | −0.29 | 0.11 |
| r IFOF | −0.07 | 0.82 | −0.13 | 0.66 | −0.16 | 0.61 |
| l IFOF | 0.48 | 0.001** | 0.41 | 0.005** | 0.43 | 0.004** |
| Verbal memory | ||||||
| r ATR | −0.17 | 0.56 | −0.19 | 0.52 | −0.19 | 0.52 |
| r SLF | 0.28 | 0.28 | 0.23 | 0.39 | 0.21 | 0.45 |
| r IFOF | −0.13 | 0.70 | −0.06 | 0.86 | −0.05 | 0.88 |
| l IFOF | 0.01 | 0.98 | 0.01 | 0.94 | 0.03 | 0.87 |
| Visual memory | ||||||
| r ATR | 0.10 | 0.72 | 0.07 | 0.79 | 0.06 | 0.83 |
| r SLF | 0.06 | 0.80 | 0.09 | 0.73 | 0.12 | 0.65 |
| r IFOF | −0.34 | 0.29 | −0.30 | 0.36 | −0.33 | 0.32 |
| l IFOF | 0.23 | 0.12 | 0.20 | 0.19 | 0.22 | 0.16 |
| VS | ||||||
| r ATR | 0.35 | 0.01* | 0.38 | 0.008** | 0.37 | 0.008** |
| r SLF | 0.18 | 0.42 | 0.25 | 0.28 | 0.26 | 0.26 |
| r IFOF | 0.36 | 0.01* | 0.38 | 0.008** | 0.36 | 0.009** |
| l IFOF | 0.00 | 1.00 | −0.05 | 0.69 | −0.04 | 0.78 |
| PS | ||||||
| r ATR | 0.04 | 0.87 | 0.16 | 0.49 | 0.16 | 0.52 |
| r SLF | 0.21 | 0.35 | 0.24 | 0.28 | 0.25 | 0.28 |
| r IFOF | −0.21 | 0.47 | −0.30 | 0.30 | −0.30 | 0.29 |
| l IFOF | −0.03 | 0.82 | −0.08 | 0.53 | −0.08 | 0.58 |
ATR, anterior thalamic radiation; DWMHs, deep white matter hyperintensities; EF, executive functioning; high-grade, moderate lesions; IFOF, inferior fronto-occipital fasciculus; l, left; low-grade, without or mild lesions; PS, psychomotor speed; r, right; SLF, superior longitudinal fasciculus; VS, visuospatial skills.
Beta values from linear regression models relating areas of decreased FA values in each white matter tract (participants with high-grade versus low-grade DWMHs) to cognitive function. Model 1, adjusted for age, sex, years of education, and treatable cardiovascular risk factors related to cognitive performance (P⩽0.1); Model 2, Model 1 plus adjustment for gray matter ratio (%) and presence of lacunar infarcts.
*P<0.05; **P<0.01.
Areas showing decreased FA between participants with high-grade PVHs versus participants with low-grade PVHs were not related to lower cognitive scores (data not shown).
Figure 3 shows the significant associations between mean FA values extracted within areas showing significant differences of FA in participants with high-grade DWMHs and cognitive domains.
Figure 3.
Predictive value to cognitive outcome of areas of lower fractional anisotropy (FA) in specific white matter tracts. The selected scatter plots illustrate positive associations between areas showing decreased FA in participants with high-grade deep white matter hyperintensities and different cognitive domains. The mean FA value within the areas showing significant differences of FA was extracted in each white matter tract, standardized, and entered into a series of linear regression analyses. The z-values for all WM tracts were first entered as predictive variables with cognitive domains as the dependent variables. Cognitive domains are also represented by z-values. R2=effect size in unadjusted linear regression models.
Discussion
Our study first aimed at investigating the different pattern of lower FA in participants with high-grade PVHs and DWMHs compared with participants with low-grade PVHs and DWMHs on a voxel-by-voxel basis in a community-dwelling sample. We found that participants with high-grade PVHs and participants with high-grade DWMHs showed lower FA in different areas of specific WM tracts. Our second aim was to assess the predictive value to cognitive function of those areas with decreased FA within specific tracts. Interestingly, only areas showing decreased FA in participants with high-grade DWMHs were related to cognitive performance, whereas areas with decreased FA in high-grade PVHs were not. Specifically, lower FA areas in high-grade DWMHs predicted cognitive outcome in executive functioning, attention, verbal fluency, and visuospatial skills. This is the first study showing a specific association of high-grade DWMHs, lower FA areas within specific tracts, and cognitive function.
Participants with high-grade WMLs showed a pattern of reduced FA throughout the WM skeleton. This extensive reduction of FA supports the increased sensitivity of DTI to detect WM damage.8 However, previous research on a voxel-by-voxel basis has reported a more widespread pattern of reduced FA in participants with WMLs than we found.13, 14 Different findings may be due to methodological issues such as sample selection or covariates introduced in the voxel-wise analysis. High-grade DWMHs were associated with areas of decreased FA in the right anterior thalamic radiation, the right superior longitudinal fasciculus and the bilateral frontal IFOF. High-grade PVHs were associated with areas of lower FA in the left anterior thalamic radiation, the left superior longitudinal fasciculus, and the left occipital and right frontal IFOF. These findings confirm our hypothesis that the spatial pattern of lower FA would be found in a greater extent in participants with high-grade DWMHs (∼8,000 voxels) than in participants with high-grade PVHs (∼4,000 voxels).
It has been proposed that WMLs and reduced FA have similar underlying etiologies. Pathologic correlates common to both processes include vascular-related changes, such as myelin reduction and axonal loss, or non-ischemic age-related changes, such as widening of perivascular spaces and gliosis.3 Vascular-related changes are usually associated with high-grade DWMHs, whereas non-ischemic age-related changes are usually related to PVHs.3, 16 Specifically, the diagnosis of arterial hypertension was related to high-grade DWMHs in our sample. Only severe PVHs extending to deep WM are clearly vascular-related changes, but none of our participants with high-grade PVHs were rated with severe lesions. Likewise, it has been suggested that reduced FA may precede the development of WMLs seen on conventional MRI. A decreasing FA may indicate an early stage of WM pathology in the microstructural level and WMLs may represent the extreme end of WM damage in the macrostructural level.7 However, WMLs may also influence reduction of FA in normal-appearing WM via processes such as diaschisis or Wallerian degeneration.14 The exact temporal relation between WMLs and FA changes should be explored further.
We found that only areas showing lower FA within specific WM tracts in participants with high-grade DWMHs were related to cognitive performance. This preeminent association of high-grade DWMHs with areas of decreased FA and cognition reinforces a predominant role for these lesions in cognitive function. Executive functioning, attention, verbal fluency, and visuospatial skills were associated with areas of lower FA in specific tracts. These cognitive domains have been usually related to cortico-subcortical dysfunction and WMLs.3, 17
Areas of decreased FA in the bilateral frontal inferior fronto-occipital fasciculus were positively related to executive functioning, verbal fluency, and visuospatial skills. A decrease of 1 s.d. in FA was related to a 0.48 s.d. decrease in executive functioning and verbal fluency, and a 0.36 s.d. decrease in visuospatial skills. The IFOF is a long association bundle of fibers, which interconnects the frontal lobe with the posterior part of the parietal and temporal lobe, and with the occipital lobe.31 It is suggested that this tract may have a role in processing visuospatial information,32 which is coherent with the association between lower FA and diminished visuospatial skills that we found. Decreased FA in frontal regions of the IFOF has also been associated with diminished executive function, such as a worse task-switching and inhibition performance.33 Lower FA in left frontal WM regions has also been related to poorer outcome in verbal fluency in participants with WMLs.13
Areas of lower FA in the right anterior thalamic radiation were also positively associated with attention and visuospatial skills. A decrease of 1 s.d. in FA was related to a 0.36 s.d. decrease in attention and a 0.35 s.d. decrease in visuospatial skills. The anterior thalamic radiation is a WM projection bundle that connects the anterior and medial dorsal nuclei with the frontal lobe and the anterior cingulate cortex via the anterior limb of the internal capsule.31 It is a critical component of the cortico-subcortical circuits, thereby serving as an important integrative center for networks underlying the ability to modulate behaviors.34 Some studies have shown that the anterior thalamic radiation may have a role in attention-related disorders such as spatial neglect35 or attention deficit hyperactivity disorder.36 Likewise, a better performance in a visual detection task has been associated with higher FA in the anterior limb of the internal capsule in elderly adults.37
Our findings may provide additional support for the notion that cognitive dysfunction related to WMLs could be a disconnection syndrome,38 as areas of lower FA within specific tracts were related to cognitive performance in our sample. However, the interpretation of reduced FA as a marker of WM damage should be considered with caution. Fractional anisotropy can be related to several tissue characteristics, such as degree of myelination, axon density, axon diameter, or axonal membrane integrity, but it is also quite sensitive to the organization and alignment of fibers within a voxel and partial volume effects.9, 10
The application of a voxel-based approach in this study circumvents the potential limitations of region of interest methodology for DTI analysis, such as subjective operator-dependent placement, limited reliability and reproducibility, and partial volume effects.9, 39 It also overcomes the lack of information on a regional level of segmentation methods.11 In particular, the Tract-Based Spatial Statistics procedure that we applied has been developed to retain the strengths of voxel-based analysis while addressing registration and smoothing issues that are potential pitfalls of data preprocessing for such approach.25 Other main strengths of this study are our extensive neuropsychological assessment and the use of 3T MRI to detect WMLs. Higher magnetic fields can provide improved sensitivity and diagnostic capacity.16 Also, the predictive value of FA to cognitive function was investigated with extensive adjustment for possible confounders, such as education, vascular risk factors (including arterial hypertension, diabetes mellitus, dyslipidemia, and current smoking status), brain atrophy, and lacunar infarcts.
One potential weakness of the present study is the use of a visual rating scale28 to rate location and severity of WMLs. Quantitative methods (i.e., volumetric analyses) have been regarded as more reliable and robust. However, visual scales are considered more appropriate for defining WMLs groups.40 Visual scales usually offer separate assessment of PVHs and DWMHs, which semi- and fully-automated quantitative methods often overlook. Other possible limitations need to be considered. The most severe grades of WMLs are under-represented in our community-dwelling sample. The cross-sectional design also precludes us from making causal inferences regarding high-grade WMLs, areas of lower FA, and cognitive function.
White matter lesions are considered a radiologic expression of SVD. The term SVD encompasses all the pathologic processes that affect the small vessels of the brain. Biologic aging of the brain is partly attributable to aging of the cerebrovascular circulation and the effects of these changes on the brain.41 Small vessel disease is thought to be possibly the most frequent cause of vascular cognitive impairment.2 There is also increasing evidence that cerebrovascular dysfunction has a role in neurodegenerative diseases such as Alzheimer's disease.42 As well as cognitive disorders, other clinical characteristics of SVD also include slower gait, impaired balance, depressed mood, urinary disturbances, and overall functional disability.2, 3 Neuroimaging has a central role in the definition of SVD. Its neuroimaging correlates (i.e., WMLs) have been the object of abundant research and new MRI techniques such as DTI are expected to contribute in the understanding of the pathophysiology of WMLs and their clinical correlates. In this line, two important findings emerge from our study. First, the identification of areas of decreased FA in specific WM tracts. Second, and most novel, we found that only areas of decreased FA in high-grade DWMHs are related to cognitive function. Taken together, these novel results could contribute to understand the different mechanisms and clinical consequences of DWMHs and PVHs. Our results also suggest that the concomitant use of conventional MRI and novel techniques such as DTI may be useful to predict cognitive function in SVD. Alternative approaches (i.e., fiber tracking or functional connectivity) could provide additional information about composition, organization, and functional status of WM networks.
In conclusion, only areas of lower FA within specific WM tracts, associated to high-grade DWMHs, are related to lower cognitive performance in a middle-aged community-dwelling sample. The combination of DTI and voxel-based analysis allows to allocate patterns of reduced FA to WM tracts in participants with high-grade WMLs. Diffusion tensor imaging could therefore serve as a additional tool to conventional MRI to investigate extension of WM damage. The predictive value to cognition of specific WM tracts supports the involvement of cortico-subcortical circuits in cognitive deficits associated with WMLs. Our data also corroborates the hypothesis that PVHs and DWMHs are differentially associated with cognitive function and suggests that the ongoing distinction between both types of WMLs is worthy. Further research is needed to more clearly elucidate whether both types arise from dissociable forms of pathogenesis, as the lack of radiologic–pathologic association studies is a key limitation to our understanding of the neuroimaging findings. The advantages of multimodal imaging should be used to advance understanding of pathophysiology. Further research is also needed to determine if lower FA areas associated with DWMHs might have diagnostic value to assess cognitive dysfunction in community and clinical samples.
Acknowledgments
We are indebted to the Medical Image Core Facility of the IDIBAPS for the technical help. The authors thank all the participants who voluntarily accepted to take part in this project.
The authors declare no conflict of interest.
Footnotes
This work was supported by the grants AP2006-00311 from the Spanish Ministry of Education and Science and 2009FI_B00285 from the Generalitat of Catalunya. The Barcelona-AsIA Neuropsychology Study was financially supported by the grant SEJ2006-15399/PSIC from the Spanish Ministry of Education and Science. The Barcelona-AsIA Study was supported by the grant FIS 07/0393 from the Spanish Ministry of Health. The PERART study was supported by the grant FIS 07/0403 and the ETES 07/90415 from the Spanish Ministry of Health.
References
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Grazer A, Enzinger C, Ropele S, Homayoon N, Pluta-Fuerst A, et al. MRI-detected white matter lesions: do they really matter. J Neural Transm. 2011;118:673–681. doi: 10.1007/s00702-011-0594-9. [DOI] [PubMed] [Google Scholar]
- Linortner P, Fazekas F, Schmidt R, Ropele S, Pendl B, Petrovic K, et al. White matter hyperintensities alter functional organization of the motor system. Neurobiol Aging. 2012;33:e1–e9. doi: 10.1016/j.neurobiolaging.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13 (Suppl 2:31–36. doi: 10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Galluzzi S, Pantoni L, Filippi M. The effect of white matter lesions on cognition in the elderly—small but detectable. Nat Clin Pract Neurol. 2007;3:620–627. doi: 10.1038/ncpneuro0638. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol. 2013;34:54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke. 2010;41 (10 Suppl:S154–S158. doi: 10.1161/STROKEAHA.110.595314. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- Quinque EM, Arélin K, Dukart J, Roggenhofer E, Streitbuerger DP, Villringer A, et al. Identifying the neural correlates of executive functions in early cerebral microangiopathy: a combined VBM and DTI study. J Cereb Blood Flow Metab. 2012;32:1869–1878. doi: 10.1038/jcbfm.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Shepel J, Williams VJ, Lipsitz LA, McGlinchey RE, Milberg WP, et al. Associations between T(1) white matter lesion volume and regional white matter microstructure in aging Hum Brain Mappadvance online publication, 30 January 2013 doi: 10.1002/hbm.22236(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann NYAcad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008;64:273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- Soriano-Raya JJ, Miralbell J, López-Cancio E, Bargalló N, Arenillas JF, Barrios M, et al. Deep versus periventricular white matter lesions and cognitive function in a community sample of middle-aged participants. J Int Neuropsychol Soc. 2012;18:874–885. doi: 10.1017/S1355617712000677. [DOI] [PubMed] [Google Scholar]
- López-Cancio E, Dorado L, Millán M, Reverté S, Suñol A, Massuet A, et al. The population-based Barcelona-Asymptomatic Intracranial Atherosclerosis Study (ASIA): rationale and design. BMC Neurol. 2011;11:22. doi: 10.1186/1471-2377-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzamora MT, Baena-Díez JM, Sorribes M, Forés R, Toran P, Vicheto M, et al. Peripheral Arterial Disease Study (PERART): prevalence and predictive values of asymptomatic peripheral arterial occlusive disease related to cardiovascular morbidity and mortality. BMC Public Health. 2007;7:348. doi: 10.1186/1471-2458-7-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O.A Compendium of Neuropsychological Tests3rd edn.Oxford University Press: New York, NY; 2006 [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version. Professional Manual. Psychological Assessment Resources: Lutz, FL; 2000. [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian Stroke Prevention Study. Ann Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PC. MRI Atlas of Human White Matter. Elsevier: Amsterdam, The Netherlands; 2005. [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48:82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Della Nave R, Ginestroni A, Tessa C, Giannelli M, Piacentini S, Filippi M, et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR Am J Neuroradiol. 2010;31:1675–1681. doi: 10.3174/ajnr.A2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Bickerton WL, Hansen PC, Deb S, Humphreys GW. Separating neural correlates of allocentric and egocentric neglect: distinct cortical sites and common white matter disconnections. Cogn Neuropsychol. 2010;27:277–303. doi: 10.1080/02643294.2010.519699. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O'Neill J, et al. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings J Am Acad Child Adolesc Psychiatry 201352431–440.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man: part I. 1965. Neuropsychol Rev. 2010;20:128–157. doi: 10.1007/s11065-010-9131-0. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, et al. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]
- van Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]