Abstract
Intermittent fasting (IF) is neuroprotective across a range of insults, but the question of whether extending the interval between meals alters neurogenesis after ischemia remains unexplored. We therefore measured cell proliferation, cell death, and neurogenesis after transient middle cerebral artery occlusion (MCAO) or sham surgery (SHAM) in mice fed ad libitum (AL) or maintained on IF for 3 months. IF was associated with twofold reductions in circulating levels of the adipocyte cytokine leptin in intact mice, but also prevented further reductions in leptin after MCAO. IF/MCAO mice also exhibit infarct volumes that were less than half those of AL/MCAO mice. We observed a 30% increase in basal cell proliferation in the hippocampus and subventricular zone (SVZ) in IF/SHAM, relative to AL/SHAM mice. However, cell proliferation after MCAO was limited in IF mice, which showed twofold increases in cell proliferation relative to IF/SHAM, whereas AL/MCAO mice exhibit fivefold increases relative to AL/SHAM. Attenuation of stroke-induced neurogenesis was correlated with reductions in cell death, with AL/MCAO mice exhibiting twice the number of dying cells relative to IF/MCAO mice. These observations indicate that IF protects against neurological damage in ischemic stroke, with circulating leptin as one possible mediator.
Keywords: caloric restriction, hippocampus, intermittent fasting, neurogenesis, stroke, subventricular zone
Introduction
Proliferation and differentiation of new neurons are ongoing features of the hippocampus and olfactory bulb. Cell division, migration, differentiation, and functional integration are regulated by environmental demands in both regions.1 Rates of progenitor cell proliferation and neurogenesis also fluctuate in the context of pathological conditions, such as traumatic brain injury, epilepsy, and stroke.2 Transient or permanent interruptions in cerebral blood flow induce neurogenesis, which may occur in response to widespread neuronal loss under ischemic conditions.3 Although this initially spurred substantial interest in stroke-induced neurogenesis as a mechanism for brain repair, more recent data suggest that new neurons formed after middle cerebral artery occlusion (MCAO) fail to integrate properly and may in fact contribute to circuit dysfunction in the hippocampus.4 In this regard, new neurons born into a pathological molecular environment may impede, rather than promote, recovery after stroke due to aberrant functional integration into the dentate gyrus circuitry.
Rates of adult neurogenesis in the intact brain increase after energetic challenges, such as exercise5 and intermittent fasting (IF).6 Although the quantitative impact of neurogenesis on the function of neural circuitry in the intact and injured brain has yet to be entirely resolved, computational studies suggest that both the number and functional integration of newly generated neurons may contribute to plasticity under intact conditions, and recovery after injury.7 The IF regimen, which involves increasing the interval between meals, enhances hippocampal neurogenesis, but the extent to which this enhancement extends to the subventricular zone (SVZ) remains unclear. Because newly generated cells in the ischemic SVZ can migrate into the striatum and differentiate into neurons,8 it is important to understand how SVZ progenitors can potentially be recruited for neuroprotection after stroke. Moreover, while IF reduces infarct size and evokes a molecular profile consistent with neuroprotection in mice after transient MCAO,9 the question of whether this dietary regimen alters the magnitude and extent of stroke-induced neurogenesis has yet to be explored. IF evokes widespread alterations in metabolism and alters numerous neuroendocrine signals known to regulate adult neurogenesis, such as the adipocyte cytokine leptin.10 Given the consistently demonstrated relationship between circulating factors and adult neurogenesis, and the broad nature of physiological responses to IF, we hypothesize that metabolic intervention exerts opposite effects on neurogenesis under intact and ischemic conditions. Specifically, we predict that extending the interval between meals will increase neurogenesis in the intact brain and reduce the extent of cellular damage in the ischemic brain, thereby constraining the proliferative response to stroke.
To address this question, we measured changes in cell death, cell proliferation, and neurogenesis after IF under basal conditions and after stroke. Under intact conditions, extending the interval between meals reduces body weight and circulating leptin levels, and enhances basal neurogenesis in the hippocampus and SVZ. After an ischemic insult, IF limits increases in cell proliferation and neurogenesis, reduces the extent of cell death, and prevents the ischemia-induced drop in circulating leptin. These observations suggest that IF enhances basal neurogenesis and improves the defense of a proliferative set point after an ischemic insult, possibly by maintaining leptin levels, which may emerge as a novel biomarker for the extent of neurological damage after stroke.
Materials and Methods
Animals, Diets, and Bromodeoxyuridine Administration
Male C57Bl6J mice were obtained from the Animal Resources Centre in Canning Vale, Australia, and group housed on arrival in the University of Queensland animal facility. At 10 weeks of age, mice were randomly assigned to either the ad libitum (AL, n=27) or IF (n=27) diet conditions. IF mice were fed for 8 hours out of every 24-hour period, with food available between 0700 and 1500 hours (lights on at 0600 hours, lights out at 1800 hours). This feeding schedule was applied for 3 months before random assignment into either the SHAM (n=8) or MCAO (n=19) condition. Within the MCAO condition, (n=10) mice from each diet group were used for 2,3,5-triphenyltetrazolium chloride (TTC) staining, and the remainder were used for the adult neurogenesis studies. Mortality rates in the MCAO group were comparable across diet conditions (n=3 from AL; n=2 from IF) and food was freely available to mice in both dietary conditions during the 1-week recovery period after MCAO. Body weights were collected on a weekly basis and all procedures followed guidelines set out by the National Institutes of Health (USA) and were approved by the Animal Care and Use Committee of the University of Queensland.
The DNA synthetic marker bromodeoxyuridine (BrdU; Sigma-Aldrich, St Louis, MO) was administered at a dose of 50 mg/kg (intraperitoneally) every 12 hours for 2 days, beginning 5 days after MCAO or sham surgery. This BrdU treatment regimen is widely used for pulse labeling of neuronal progenitor cells11 and does not induce toxicity, even at doses greater than that used in the present study.12 A week after MCAO or sham surgery, mice used for immunohistochemistry were transcardially perfused with 4% paraformaldehyde in phosphate buffer under deep Isoflurane anesthesia. Subsets of MCAO mice from each diet condition were not perfused; instead, these mice were sacrificed by decapitation under Isoflurane anesthesia three days after MCAO or sham surgery, and the brains were immediately processed for TTC staining, as described.9 In brief, 2.0-mm-thick brain sections were stained in 2% TTC in phosphate buffer and photographed. The resultant images were analyzed in a masked fashion using NIH ImageJ software; infarct areas were determined by subtracting the area of intact tissue in the ipsilateral hemisphere from the area of the contralateral hemisphere and summing the infarct area across all slices from each brain.
Ischemia/Reperfusion and Behavioral Assessment of Neurological Damage
MCAO was carried out under Isoflurane anesthesia as described.9 Briefly, mice were anesthetized 15 minutes before occlusion and maintained under anesthesia throughout the 1-hour occlusion period. To induce MCAO, an incision was made along the midline of the neck to expose the left external carotid and pterigopalatine arteries, which were isolated and tied off with 6-0 silk thread. The internal carotid artery (ICA) was then occluded distal to the bifurcation of the ICA and the pterigopalatine artery using small clip, and the common carotid artery was ligated with 6-0 silk thread. The external carotid artery (ECA) was then incised and a blunt-tip 6-0 nylon monofilament was inserted into the ECA. The junction below the ECA and the inserted nylon thread was tightened with 6-0 silk suture to prevent bleeding during manipulation of the nylon thread, which was rotated during its advancement into the ICA. The clip at the ICA was then removed and the nylon thread was advanced to a distance slightly more than 6 mm from the tip of the nylon thread the ICA-pterygophalatine artery bifurcation. This distance relative to the ICA-ECA bifurcation is slightly less than 9 mm. During MCAO, the parietal bone on the occluded side becomes slightly transparent, and laser Doppler flowmetry revealed that blood flow in this area decreases to less than 10% of baseline. After a 1-hour occlusion period, the nylon thread and the common carotid artery ligature are removed and the neck incision is closed with wound clips. In the sham surgery group, the arteries are visualized but not disturbed.
Functional outcomes were assessed daily throughout the 7 days after ischemia and reperfusion injury. Behavioral deficits were evaluated using a 5-point score as described9 (0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; and 4, unable to walk spontaneously), with higher scores reflecting greater sensorimotor impairment. All behavioral analyses were conducted by an experimenter who was blind to the dietary condition of the animals.
Quantification of Serum Leptin
Circulating levels of leptin were determined using a commercial ELISA kit (EZML-82K, Mouse Leptin ELISA; Millipore, Temecula, CA) in serum samples collected from sham and MCAO animals 3 days after surgery. Assay procedures followed the manufacturer's specifications.
Immunohistochemistry and Immunofluorescence
Brains were postfixed for 24 hours in 4% paraformaldehyde in phosphate buffer, then moved into 0.1 M phosphate-buffered saline, before generation of 40-μm transverse sections using a vibrating tissue slicer (Leica). Sections were collected as a 1:6 series and stored in cryoprotectant at −20 °C in preparation for immunohistochemical and immunofluorescence reactions. Peroxidase detection of BrdU and the endogenous proliferation marker, Ki67, followed previously published methodology,13 with the exception that the current study used different primary antibodies for detection of BrdU (1:200; BD Pharmingen, San Jose, CA) and Ki67 (1:500, Millipore). Primary antibodies against BrdU and Ki67 were detected with biotin-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA), amplified via avidin–biotin–horseradish peroxidase, and visualized with diaminobenzadine. Nuclei were counterstained with Cresyl Violet (Sigma-Aldrich).
For TUNEL staining, we used a peroxidase labeling kit from Promega (Madison, WI, USA), according to the manufacturer's instructions. For Fluoro-Jade B histology, slides were pretreated with 0.06% KMnO4 for 15 minutes, rinsed, and incubated in Fluoro-Jade B working solution (0.01% Fluoro-Jade B from Molecular Probes (Carlsbad, CA) in ddH2O with 0.1% acetic acid) overnight. Fluoro-Jade-positive nuclei were quantified throughout the rostrocaudal extent of the dentate gyrus, including the hilar region. For the SVZ, virtually all Fluoro-Jade-positive cells localized to the striatum, and these cells were counted and normalized to the sampled area with the aid of StereoInvestigator software (Microbrightfield, Williston, VT). Immunofluorescent staining of BrdU and phenotypic markers followed previously published protocols,14 with minor modifications. Free-floating tissue sections were denatured in 2 N HCl:Tris-buffered saline (TBS) for 30 minutes, blocked in 5% normal goat serum in TBS containing 0.5% Triton X 100, then reacted overnight in primary antibody mouse anti-BrdU (1:250, BD Pharmingen). The BrdU antibody was detected using a biotinylated secondary and chromated with Streptavidin Alexa 568 (Molecular Probes). Antibodies directed against the immature neuronal marker doublecortin (DCX; 1:250, Abcam, Cambridge, MA), the mature neuronal marker NeuN (1:500, Millipore), and the astroglial marker glial fibrillary acidic protein (GFAP) (1:1,000, Pierce, Rockford, IL) were then applied overnight. Bound primary antibodies for the cell type markers were visualized with Alexa 488-conjugated secondary antibodies (Molecular Probes) directed against the appropriate host, followed by nuclear counterstaining with DAPI. Images were collected on a Zeiss LSM 510 Meta confocal microscope (Jena, Germany).
Stereological Quantification of Labeled Cells
For quantification of BrdU-, Ki67-, and TUNEL-immunoreactive nuclei visualized with peroxidase immunohistochemistry, unbiased stereological sampling schemes were applied using StereoInvestigator software (Microbrightfield) as described.14 All cell counts were conducted blind to the treatment condition of the animals. For analysis of BrdU-, Ki67-, and TUNEL-positive cells in the hippocampus, total cell counts were generated using an XY step size of 150 μm, a disector height of 10 μm, and a 25-μm sampling frame. For quantification of labeled nuclei in the SVZ, the XY step size was reduced to 50 μm, with other sampling parameters consistent with those used in the hippocampus. Numbers of BrdU-, Ki67-, and TUNEL-positive nuclei in the SVZ were normalized to the sampled volume of the SVZ and expressed as cell densities.
For analysis of colabeling, 100 BrdU-labeled cells were sampled from both blades of the dentate gyrus, with sampling anatomically balanced along the dorsal–ventral and septotemporal axes. Similarly, 100 BrdU-labeled cells were scanned throughout the extent of the SVZ. Double-labeled cells were visualized using a × 63 objective (numerical aperture=1.4) on a Zeiss LSM 510 Meta confocal microscope, with images captured under × 2 optical zoom at 512 × 512 pixel density with a 1-μm step size in the z plane. To assess colocalization, cells were analyzed offline in ImageJ software, and fluorescence signals in the two channels used for visualization of BrdU and phenotypic markers were required to exhibit >80% spatial overlap across each captured plane in which the cell nucleus (visualized with DAPI) was visible. For MCAO animals, colabeling was assessed on the contralateral (right) hemisphere; to be consistent, cells scanned in the hippocampus and SVZ of the sham-operated mice were also sampled from the right hemisphere. BrdU-labeled cells in the SVZ were further analyzed based on their proximity to the ventricle, derived from the Euclidean distance (in μm) from the nucleus to the ventricular wall. Nuclei within 20 μm of the ventricle were classified as SVZ progenitors, whereas nuclei >20 μm from the ventricles were characterized as putative differentiated cells. All of the potentially differentiated cells localized exclusively to the striatum.
Statistical Analysis
Body weights and functional outcomes after MCAO were compared across AL and IF mice using repeated measures analysis of variance (ANOVA). Leptin levels and the percentages of cells expressing markers of a given phenotype were compared across diet and surgical conditions using a 2 × 2 ANOVA. Infarct volumes were compared between AL and IF mice using a bidirectional t-test. Comparison of the numbers and densities of BrdU- or Ki67-labeled nuclei in the hippocampus and SVZ used separate 2 × 2 ANOVA designs to evaluate the effects of AL or IF on cell numbers after MCAO or sham surgery in the ipsilateral or contralateral sides. Data from quantitative measures of cell death were analyzed using the same statistical design applied to measures of cell birth. Relationships between markers of cell proliferation and markers of cell death were assessed using Pearson's correlation. All statistical analyses were carried out in SPSS (version 18.0) or GraphPad Prism (version 4.0) with significance at P<0.05. Because these analyses require normally distributed data sets, comparisons between two groups used t-tests to compare variances, and comparisons across more than two groups used ANOVA to compare variances. None of the tests revealed significant differences, and as such were considered normally distributed.
Results
IF Reduces Body Weight and Lowers Circulating Factors Associated with Adipose Tissue
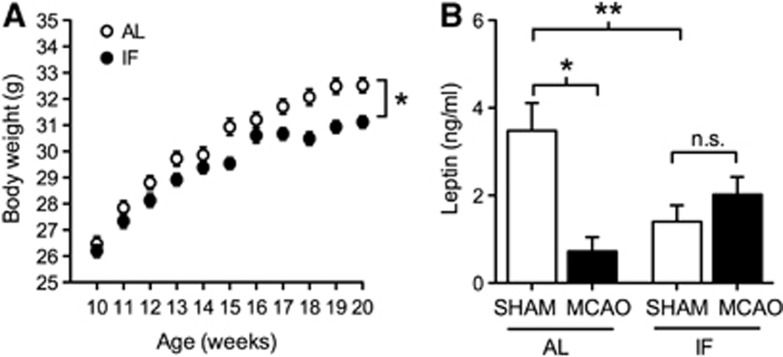
Most previous studies examining the physiological and neurological consequences of IF used alternating 24-hour cycles of fasting and refeeding.15 To determine whether a 16:8 hours schedule of fasting and refeeding might exert comparable physiological effects, we monitored body weight and food intake, and measured serum levels of leptin, a reliable correlate of adiposity. Male C57Bl6J mice maintained on IF under the 16:8 hours schedule gained weight more slowly than mice with ad libitum food access (Figure 1A; F1,11=6.49, P=0.001). Reductions in body weight were accompanied by reduced levels of the adipocyte cytokine leptin in serum from IF mice (Figure 1B; for the effect of IF in sham-operated mice, F1,13=7.73, P=0.001). Unlike the 24-hour fasting and refeeding cycle, in which 48-hour food intake is comparable between IF and AL mice,15 the 16:8 hours schedule was accompanied by reduced food intake (t9=7.12, P=0.009). IF mice on the 16:8 hours fasting and refeeding cycle ate 3.35±0.09 g/24 h, whereas AL mice ate 4.32±0.08 g/24 h (values represent mean±s.e.m.). This pattern of reduced food intake indicates that the 16:8 schedule resembles caloric restriction, with associated declines in body weight and circulating markers of adiposity.
Figure 1.
Intermittent fasting (IF) reduces body weight and prevents stroke-induced reductions in circulating leptin levels. (A) Weight gain is reduced in mice exposed to IF on a 16:8 hours fasting and refeeding schedule. Asterisk (*) indicates statistical significance at P<0.05 after repeated measures analysis of variance (ANOVA). (B) Stroke dramatically reduces circulating leptin levels in mice on the ad libitum diet, but not in mice maintained on IF. Asterisk (*, **) indicates statistical significance at P<0.05 (*) or P<0.01 (**) following 2 × 2 ANOVA. Leptin levels were assessed 3 days after middle cerebral artery occlusion (MCAO) or sham operation and error bars represent the s.e.m. AL, ad libitum.
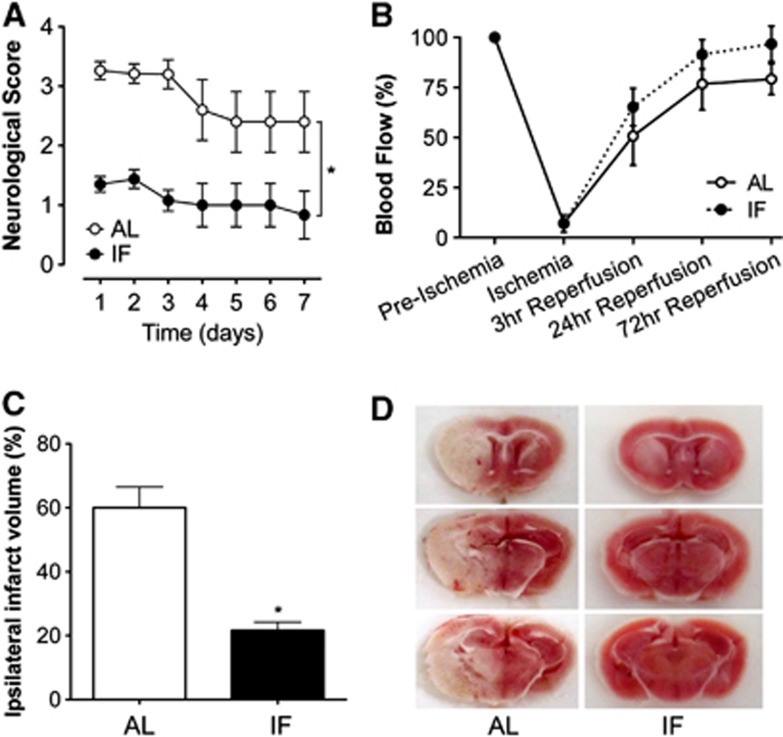
IF Reduces Sensorimotor Impairment and Infarct Size After Ischemia and Reperfusion
The functional consequences of ischemia and reperfusion injury were evaluated over a 1-week period, and throughout that period, mice maintained on the IF diet consistently demonstrated less impairment relative to mice fed ad libitum (Figure 2A; F1,13=2.73, P=0.02). Protection against stroke-induced sensorimotor deficits was accompanied by complete prevention of the drastic reduction in circulating leptin observed after stroke in AL mice (Figure 1B). Leptin levels fell to less than 15%t of sham-operated animals at 3 days post MCAO in AL mice, but were maintained at this same time point in IF mice (for the interaction between diet and stroke, F1,13=4.5, P=0.01). We measured leptin levels 3 days after MCAO because this is the time point when previous studies9 detected differences in molecular markers of neuroprotection between IF/MCAO and AL/MCAO mice. In this regard, changes occurring 72 hours post-stroke may determine the transition between the acute phase, characterized by widespread cellular damage, and the post-stroke recovery phase, involving recruitment of cell growth and plasticity pathways.
Figure 2.
Intermittent fasting (IF) improves functional outcomes and reduces infarct size in male mice. (A) Three months of IF attenuates sensorimotor deficits after middle cerebral artery occlusion (MCAO). (B) Laser Doppler flowmetry shows no differences between groups in the extent to which MCAO compromises blood flow. (C) IF reduces infarct size in male C57Bl6J mice. (D) Staining for triphenyltetrazolium chloride (TTC) in 2-mm-thick brain sections taken after reperfusion reveals smaller infarcts in mice on the IF diet. Asterisk (*) indicates statistical significance at P<0.05 following t-test (C) or repeated measures analysis of variance (A, B). AL, ad libitum.
IF mice exhibit smaller infarcts 3 days after ischemia and reperfusion injury, relative to mice on the ad libitum diet (Figures 2C and 2D; t13=5.18, P=0.002). Improved outcomes could not be explained by differences in the extent to which MCAO compromised blood flow during the stroke, as laser Doppler flowmetry measures conducted in a subset of AL and IF mice during MCAO revealed comparable reductions in blood flow during and immediately after ischemia and reperfusion (Figure 2B; F1,13=2.08, P=0.18). Although we cannot rule out vasogenic contributions to improved functional recovery, it is not likely that the smaller infarcts observed in IF mice were attributable to differences in flow during and after MCAO.
IF Enhances Basal and Suppresses Stroke-Induced Cell Proliferation in the Hippocampus
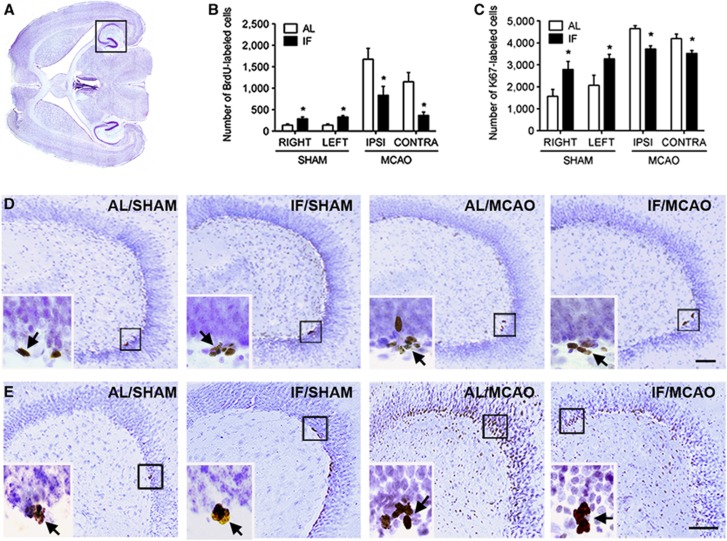
To assess the proliferative response to MCAO, we administered the thymidine analog BrdU (50 mg/kg, intraperitoneally) for 2 days, with injections every 12 hours, starting 5 days after MCAO or sham surgery. This time course was based on early reports identifying the week after ischemia and reperfusion as a temporal window for increases in proliferative activity in the hippocampal dentate gyrus and SVZ.11 Previous work using 24-hour feeding and fasting schedules revealed that this schedule increases BrdU-labeled cell number in the hippocampus.6 We also detected increases in the number of BrdU-labeled cells in the dentate gyrus of sham-operated mice on the 16:8 hours fasting and refeeding schedule (Figure 3A and 3B; F1,18=25.68, P=0.001). However, stroke-induced increases in the number of BrdU-labeled cells were substantially attenuated in both the ipsilateral and contralateral hemispheres of mice on the 16:8 hours fasting and refeeding schedule (F1,19=16.04, P=0.009).
Figure 3.
Intermittent fasting (IF) enhances cell proliferation and survival in the dentate gyrus of intact mice, and attenuates pathological increases after stroke. (A) To facilitate anatomical visualization of both the hippocampus and subventricular zone, transverse sections were used for peroxidase labeling. (B) Graph shows stereological estimates of bromodeoxyuridine (BrdU)-labeled cell numbers in the indicated hemispheres for each treatment group. Asterisk (*) indicates statistical significance following 2 × 2 analysis of variance. (C) Stereological estimates of Ki67-labeled cell number in each treatment condition revealed that IF promotes cell proliferation under intact conditions, and suppresses pathological increases in mice that received middle cerebral artery occlusion (MCAO). (D) Low-power micrographs of hippocampal BrdU labeling on transverse sections of the mouse hippocampus. The boxed areas are shown in the insets, which depict clusters of BrdU-labeled cells in each condition. Arrows indicate labeled cells. Scale bar, 100 μm. (E) Low-power micrographs showing sections of mouse hippocampus stained with antibodies against the endogenous marker of cell proliferation Ki67. Boxed area for each micrograph is shown in the inset, which depicts clusters of Ki67-labeled cells in each treatment group. Arrows indicate labeled cells. Scale bar, 100 μm. AL, ad libitum.
Administration of multiple pulse injections of BrdU labels a mixed population of proliferating cells and their progeny. Moreover, BrdU is an exogenous marker, and its incorporation could be influenced by differences in availability and/or uptake. To measure cell proliferation without the confound of availability of an exogenous marker, we performed immunohistochemical detection of Ki67, an endogenous proliferative marker expressed throughout the cell cycle. Using this marker, we again detected increases in the number of labeled cells in the dentate gyrus of sham-operated mice on the IF diet (Figures 3C and 3D; F1,18=11.53, P=0.002), indicating enhancement of cell proliferation in these mice. Similarly, elevations in Ki67-labeled cell numbers observed after MCAO in AL mice were blunted in mice on the IF feeding schedule (F1,19=22.56, P=0.001). This suggests that the neurogenic response to stroke is commensurate with the extent of neurological damage, as the IF mice, which exhibit smaller infarcts, also show attenuation of the proliferative response to MCAO.
IF Promotes Cell Proliferation in the Intact SVZ and Attenuates Increases after MCAO
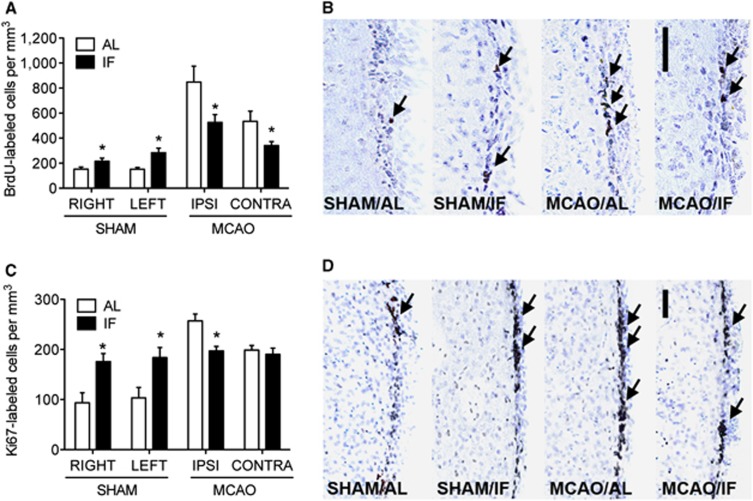
The SVZ also responds to stroke with increases in cell proliferation and neurogenesis, but the dietary regulation of proliferation among SVZ stem and progenitor cells has never been assessed, either in the intact or in the injured brain. We measured the density of BrdU-labeled cells in the SVZ of mice maintained on AL or IF feeding schedules, and observed increased numbers of BrdU-positive cells in sham-operated mice after 3 months of the IF feeding schedule (Figures 4A and 4B; F1,18=15.48, P=0.002). Although IF increased BrdU-labeled cell numbers in shams, the opposite effect was observed after MCAO, with smaller increases in BrdU-labeled cell numbers in mice on the IF schedule (F1,16=9.54, P=0.007). In this regard, the SVZ responds to IF in a manner that is similar to the hippocampus, with enhancement of BrdU-labeled cell numbers in the intact SVZ, and reductions in stroke-induced BrdU incorporation.
Figure 4.
Intermittent fasting (IF) enhances cell proliferation and survival in the intact subventricular zone (SVZ), and protects against pathological increases with stroke. (A) The density of bromodeoxyuridine (BrdU)-labeled cells in the SVZ is significantly increased with IF under basal conditions. After stroke, pathological increases in BrdU-labeled cell numbers are suppressed by IF. (B) Micrographs depict BrdU-labeled cells in the SVZ of mice in each condition. (C) The density of Ki67-labeled cells increases in intact mice on the IF diet, and pathological elevations in cell proliferation after stroke are suppressed. (D) Images of Ki67-labeled cells in the SVZ of mice in each condition. Scale bars, 50 μm (B, D). Arrows indicate labeled cells. For all graphs, asterisk (*) indicates significance at P<0.05 following 2 × 2 analysis of variance. AL, ad libitum.
We also examined the number of nuclei expressing the endogenous marker Ki67 in the SVZ of MCAO or sham-operated mice exposed to IF or ad libitum feeding. Based on the number of Ki67-labeled nuclei in sham-operated mice, the IF feeding schedule elicits increases in the number of immunoreactive nuclei, suggestive of an expanded pool of proliferating progenitors (Figures 4C and 4D; F1,16=17.65, P=0.008). By contrast, the increase in Ki67-labeled cell numbers observed after MCAO was smaller in mice on the IF feeding schedule (F1,15=9.15, P=0.007). Taken together, these observations suggest that the proliferative response to MCAO is dampened by IF feeding, which also promotes cell proliferation and survival among neurogenic regions in the intact brain.
Differentiation of Neurons and Glia is Unaffected by Diet or Ischemia and Reperfusion
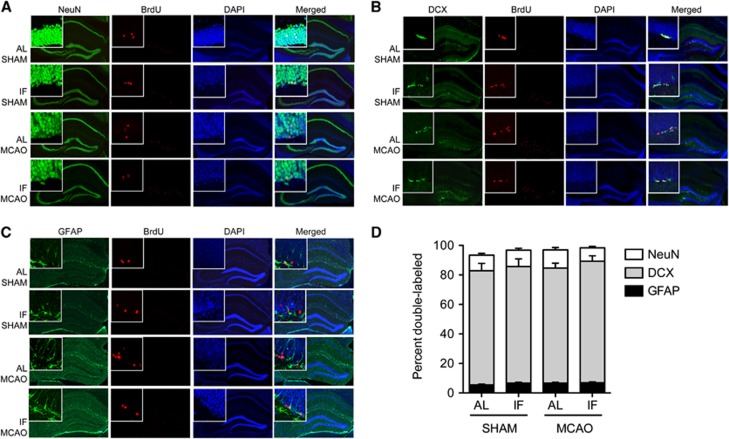
Newly generated cells in the subgranular and SVZs differentiate into functional neurons and astrocytes, but the proportion of cells expressing neuronal or astroglial markers is unchanged by MCAO or dietary manipulation (Figures 5 and 6). After four injections of BrdU (50 mg/kg), administered every 12 hours starting 5 days after MCAO or sham surgery, comparable numbers of BrdU-labeled cells in the subgranular zone expressed the mature neuronal marker NeuN across all treatment groups (Figures 5A and 5D; percent double-labeled; mean±s.e.m., SHAM/AL=77.43±4.93, SHAM/IF=78.98±5.18, MCAO/AL=77.89±3.37, MCAO/IF=82.37±3.64). Coexpression of BrdU and the immature neuronal marker doublecortin in the dentate gyrus was also unaffected by diet or MCAO (Figures 5B and 5D; percent double-labeled; mean±s.e.m., SHAM/AL=10.54±1.24, SHAM/IF=11.07±1.23, MCAO/AL=12.31±1.62, MCAO/IF=9.08±0.86). A smaller proportion of BrdU-labeled cells expressed GFAP, a marker of astrocytes. Again, the proportion of cells that coexpress BrdU and GFAP in the dentate gyrus was unaffected by stroke or dietary intervention (Figures 5C and 5D; percent double-labeled; mean±s.e.m., SHAM/AL =5.43±0.59, SHAM/IF=6.71±0.51, MCAO/AL=6.73±0.50, MCAO/IF=6.93±0.65).
Figure 5.
Bromodeoxyuridine (BrdU)-labeled cells in the hippocampal dentate gyrus express neuronal and astroglial markers. (A) Immunodetection of BrdU and NeuN in the hippocampal dentate gyrus for each condition. (B) Coexpression of BrdU and the immature neuronal marker doublecortin (DCX) in the dentate gyrus of the hippocampus. (C) BrdU-labeled cells also express the astroglial marker glial fibrillary acidic protein (GFAP) in the hippocampal dentate gyrus. (D) Similar proportions of BrdU-labeled cells express neuronal or astroglial markers across groups. For the micrographs from middle cerebral artery occlusion (MCAO) mice in panels A–C, the contralateral hippocampus is shown. AL, ad libitum; IF, intermittent fasting.
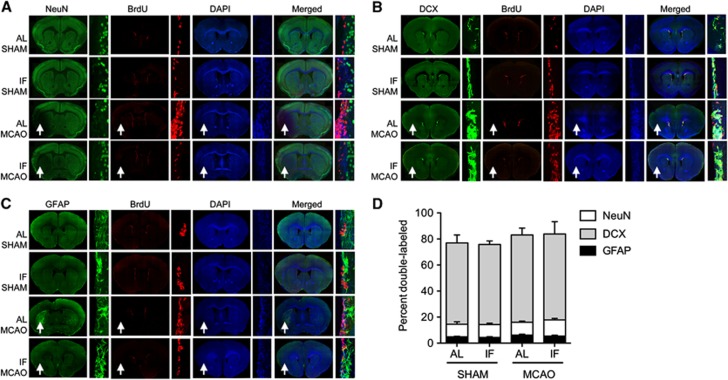
Figure 6.
New cells in the subventricular zone (SVZ) express neuronal and astroglial markers, with no change in cell fate acquisition after intermittent fasting. (A) Bromodeoxyuridine (BrdU)-labeled cells in the SVZ express the mature neuronal marker NeuN. (B) Newly generated cells also coexpress BrdU and the immature neuronal marker doublecortin (DCX). (C) BrdU-labeled cells in the SVZ also express glial fibrillary acidic protein (GFAP), a marker of astrocytes. (D) Comparable proportions of cells express DCX, NeuN, and GFAP across conditions. For the low-magnification micrographs from middle cerebral artery occlusion (MCAO) mice in panels A–C, the ipsilateral hemisphere is indicated by the arrows and the contralateral hemisphere is shown in the insets. AL, ad libitum; IF, intermittent fasting.
Consistent with previous reports,11 far fewer BrdU-labeled cells in the SVZ were colabeled with the mature neuronal marker NeuN, relative to the dentate gyrus, and virtually all of the NeuN/BrdU double-positive cells localized to the ischemic striatum distal to the ventricular wall. The proportion of cells double-labeled with BrdU and NeuN in the striatum was comparable between sham-operated and MCAO mice in the different dietary conditions (Figures 6A and 6D; percent double-labeled; mean±s.e.m., SHAM/AL =12.35±1.56, SHAM/IF=11.67±1.16, MCAO/AL=10.76±1.37, MCAO/IF=14.48±1.56). Although few BrdU-labeled cells expressed NeuN in the SVZ proper, the vast majority of BrdU-labeled cells in this region expressed the immature neuronal marker doublecortin, with no change in the rate of colabeling across groups (Figures 6B and 6D; percent double-labeled; mean±s.e.m., SHAM/AL=62.36±6.18, SHAM/IF=61.46±2.76, MCAO/AL=67.02±5.32, MCAO/IF=66.01±5.32). Coexpression of BrdU and GFAP was also unaffected by diet or MCAO in the SVZ (Figures 6C and 6D; percent double-labeled; mean±s.e.m., SHAM/AL=4.85±0.41, SHAM/IF=4.38±0.56, MCAO/AL=6.17±0.68, MCAO/IF=5.43±0.72).
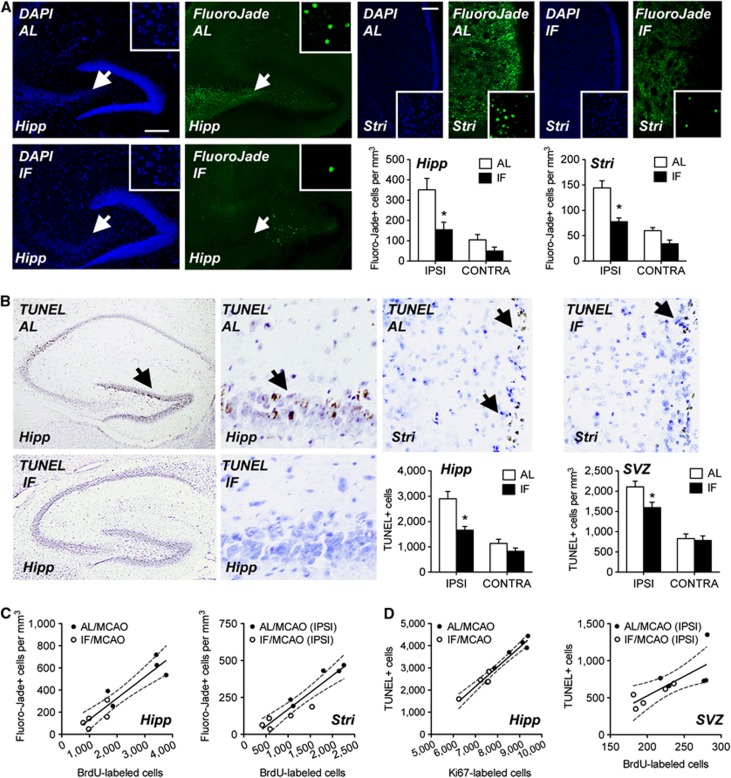
Reduced Cell Loss in Mice on IF and Correlated Rates of Cell Death and Proliferation
To investigate the relationship between neuron loss and neurogenesis in greater detail, we performed Fluoro-Jade B histology and TUNEL staining in the same set of mice used for measurement of BrdU- and Ki67-labeled cell numbers. Consistent with their smaller infarct sizes, mice on the IF diet had fewer cells that stained positive for Fluoro-Jade B (Figure 7A; for the dentate gyrus, F1,16=11.10, P=0.001, for the SVZ, F1,16=4.86, P=0.04) or TUNEL (Figure 7B; for the dentate gyrus, F1,16=5.90, P=0.02, for the SVZ, F1,16=4.93, P=0.04). Negligible labeling for Fluoro-Jade B or TUNEL was detected in mice after sham surgery (data not shown). Importantly, the hypothesis that increases in cell proliferation after MCAO represent a response to cellular damage and loss was upheld in our analysis of correlations between markers of cell death and birth (Figures 7C and 7D; for the correlation between BrdU-labeled cell number and Fluoro-Jade-positive cell numbers in the hippocampus, Pearson's r=0.92, P=0.001; for the correlation between BrdU-labeled cell densities in the ipsilateral SVZ and Fluoro-Jade-positive cells in the ipsilateral striatum, Pearson's r=0.94, P=0.001; for the correlation between hippocampal Ki67-labeled cell number and hippocampal TUNEL-positive cell number, Pearson's r=0.97, P=0.001; for the correlation between ipsilateral BrdU-labeled cell numbers and TUNEL-positive cell densities in the SVZ, Pearson's r=0.75, P=0.02). This pattern suggests that the neuroprotective consequences of IF lead to reduced injury, thereby preventing pathological increases in cell proliferation and neurogenesis after MCAO.
Figure 7.
Intermittent fasting (IF) reduces cell death in neurogenic regions, limiting the proliferative response to stroke. (A) Fluoro-Jade B staining and DAPI counterstaining in the ipsilateral hemisphere of the indicated regions (Hipp, hippocampus; Stri, striatum) after middle cerebral artery occlusion (MCAO). Graphs depict Fluoro-Jade-labeled cell counts from the indicated regions. For all micrographs, the dorsal surface is oriented at absolute north and lateral surface is oriented at west. (B) TUNEL staining in the indicated regions from an ipsilateral section from the brains of mice on the ad libitum (AL) or IF diets. Graphs depict TUNEL-positive cell counts from the indicated regions. (C) Left graph depicts a positive correlation between the number of Fluoro-Jade B-positive cells in the hippocampus, and the number of bromodeoxyuridine (BrdU)-labeled cells in the hippocampus after MCAO. Right graph indicates that increased density of Fluoro-Jade B-positive cells in the ipsilateral striatum is associated with greater numbers of BrdU-labeled cells in the ipsilateral subventricular zone (SVZ). (D) Left graph shows that the number of TUNEL-positive cells in the dentate gyrus is positively correlated with the number of Ki67-labeled cells. Right graph demonstrates that TUNEL-positive cell density is positively correlated with BrdU-labeled cell density in the ipsilateral SVZ. For all graphs, error bars represent the s.e.m. and asterisk (*) indicates statistical significance at P<0.05 following (A, B) 2 × 2 analysis of variance or Pearson's correlation (C, D).
Discussion
We have observed that extending the interval between meals reduces basal leptin levels and protects against stroke-induced reductions in circulating leptin concentrations. This endocrine pattern was accompanied by an enhancement of basal cell proliferation and attenuation of stroke-induced cell proliferation in the hippocampal subgranular zone and in the SVZ, two regions of prominent ongoing neurogenesis in the adult brain. Dampening of the proliferative response to stroke may be attributable to the smaller infarcts and reduced cell death detected in mice on the IF regimen. Although alterations in the number of new cells generated across these regions occurred after stroke and dietary intervention, the proportion of cells expressing neuronal markers was unchanged, in support of changes in neurogenesis because alterations in cell proliferation rather than differentiation. Taken together, these observations suggest that IF enhances plasticity in the intact brain and is neuroprotective after ischemia and reperfusion injury.
We used a 16:8 fasting–feeding regimen in this study to determine whether extending the interval between meals is neuroprotective in a mouse model of focal cerebral ischemia. Because infusion of exogenous leptin is neuroprotective in ischemia and may improve outcome by activating the PI3K/Akt pathway,16, 17 IF-induced alterations in leptin levels or sensitivity could be a contributing factor to the decreased infarct sizes observed in IF animals. IF reduced leptin levels in control animals and prevented decreases in leptin 3 days after ischemia and reperfusion, suggestive of improved defense of a metabolic set point. Given previous work demonstrating that exogenous leptin reduces infarct size,16, 17 and that leptin receptor deficient mice exhibit greater impairment and more widespread cell death after ischemia,18 changes in leptin levels or leptin sensitivity represent a likely mechanism for neuroprotection in IF mice. Because both AL and IF mice had food freely available during the post-stroke recovery period, we cannot rule out the possibility that IF mice simply consumed more food than AL mice during the period immediately after MCAO induction. However, whether due to intake or other metabolic factors, the pattern of neuroprotection observed after MCAO in IF mice is likely to be associated with their maintenance of leptin levels within the range of intact animals. The observation that circulating leptin levels are substantially reduced by stroke in AL mice, but not in IF mice, is consistent with the idea that improved defense of a metabolic set point confers neuroprotection after ischemia.
Stroke increases cell proliferation in both the SVZ and the hippocampus,11, 19 but the extent to which new cells promote or suppress functional recovery is not yet known. The pathological microenvironment encountered by new neurons generated as a result of stroke could impair their synaptic integration, leading to functional deficits across multiple behavioral domains. We observed that IF reduces both cell death and cell birth after stroke, and improves sensorimotor outcomes. Future studies will be necessary to determine whether IF also protects against functional decrements in other domains, such as learning and memory. Labeling for BrdU and Ki67 revealed that IF increased basal cell proliferation in sham-operated mice; based on earlier studies, this effect likely depends on diet-induced increases in levels of brain-derived neurotrophic factor.6, 20 As previously reported,11 ischemia/reperfusion injury enhanced cell proliferation, particularly in the ipsilateral hemisphere, but IF animals in the current experiment exhibit smaller increases in cell proliferation after ischemia when compared with AL mice. It is not fully known if less severe infarcts account for a lower degree of neurogenesis, but the concept of proliferation after injury as a mechanism for population control is an intriguing possibility that is supported by the correlations that we observed between markers of cell death and birth. In this regard, IF appears to precondition neurons, blunting cell death after ischemic insult and limiting pathological increases in cell proliferation as a result of reduced damage. This pattern underscores the importance of energy metabolism and diet as determinants of cellular and functional outcomes after stroke.
Acknowledgments
The authors are grateful to Alexander Widiapradja and Yi-Lin Cheng for technical assistance, and to Drs Adviye Ergul and William Hill for comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
This work was supported by an ARC Future Fellowship (FT100100427) awarded to TVA, and by start-up funds from the Medical College of Georgia (AMS).
References
- Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Niv F, Keiner S, Krishna, Witte OW, Lie DC, Redecker C. Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke. 2012;43:2468–2475. doi: 10.1161/STROKEAHA.112.660977. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Andrade N, Torp M, Wattananit S, Arvidsson A, Kokaia Z, et al. Meteorin is a chemokinetic factor in neuroblast migration and promotes stroke-induced striatal neurogenesis. J Cereb Blood Flow Metab. 2012;32:387–398. doi: 10.1038/jcbfm.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J Comp Neurol. 2012;520:1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhang J, Deng Z, Liao J, Song C, Liang C, Xue H, et al. Leptin attenuates cerebral ischemia injury through the promotion of energy metabolism via the pi3k/akt pathway. J Cereb Blood Flow Metab. 2013;33:567–574. doi: 10.1038/jcbfm.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces bdnf expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]