Abstract
Regional hypometabolism of glucose in the brain is a hallmark of Alzheimer's disease (AD). However, little is known about the specific alterations of neuronal and astrocytic metabolism involved in homeostasis of glutamate and GABA in AD. Here, we investigated the effects of amyloid β (Aβ) pathology on neuronal and astrocytic metabolism and glial-neuronal interactions in amino acid neurotransmitter homeostasis in the transgenic McGill-R-Thy1-APP rat model of AD compared with healthy controls at age 15 months. Rats were injected with [1-13C]glucose and [1,2-13C]acetate, and extracts of the hippocampal formation as well as several cortical regions were analyzed using 1H- and 13C nuclear magnetic resonance spectroscopy and high-performance liquid chromatography. Reduced tricarboxylic acid cycle turnover was evident for glutamatergic and GABAergic neurons in hippocampal formation and frontal cortex, and for astrocytes in frontal cortex. Pyruvate carboxylation, which is necessary for de novo synthesis of amino acids, was decreased and affected the level of glutamine in hippocampal formation and those of glutamate, glutamine, GABA, and aspartate in the retrosplenial/cingulate cortex. Metabolic alterations were also detected in the entorhinal cortex. Overall, perturbations in energy- and neurotransmitter homeostasis, mitochondrial astrocytic and neuronal metabolism, and aspects of the glutamate–glutamine cycle were found in McGill-R-Thy1-APP rats.
Keywords: dementia, GABA, glutamate, neurotransmitters, MR spectroscopy
Introduction
Regional hypometabolism of glucose in the brain is a hallmark of Alzheimer's disease (AD). Compromised mitochondrial function and bioenergetics in AD have also been reported, and among the most robust findings are diminished activity of several enzymes involved in oxidative metabolism of glucose: the pyruvate dehydrogenase (PDH) complex,1, 2 the α-ketoglutarate dehydrogenase complex,1, 2 and cytochrome c oxidase/complex IV of the electron transport chain.3 Since the tricarboxylic acid (TCA) cycle intermediate α-ketoglutarate (α-KG) is the precursor for glutamate and subsequently for GABA in GABAergic neurons and glutamine in astrocytes, the metabolism of glucose and amino-acid neurotransmitters in the brain is closely linked. The homeostasis of glutamate and GABA also requires glial–neuronal interactions, since the transporters and enzymes involved in uptake, synthesis, and degradation of these neurotransmitters are differentially distributed in neurons and astrocytes. Therefore, diseases that encompass changes to glucose metabolism may involve alterations in cellular energy metabolism, amino-acid neurotransmitter homeostasis, and glial–neuronal interactions. Indeed, reduced brain glutamate levels in patients with AD point toward impairment of neurotransmitter homeostasis in the disease.4 Results from 13C nuclear magnetic resonance (NMR) spectroscopy studies in AD patients and in brain extracts from APP-PS1 mice have shown reduced oxidative metabolism of glucose in neurons and reduced neuronal TCA cycle turnover, with possible impairment of the glutamate–glutamine cycle.5, 6 Investigation of astrocytic metabolism in AD patients and in cultured astrocytes exposed to various fragments of amyloid β (Aβ) have, however, provided conflicting results.7, 8, 9 Thus, despite the efforts to understand the metabolic consequences of AD pathology, the contribution of neurons and astrocytes to the deficits in amino-acid neurotransmitter homeostasis in AD remains to be clarified.
Transgenic rodent models expressing familial AD mutations recapitulate key pathologic features of the disease, and enable investigation of the metabolic dysfunction following altered amyloid precursor protein (APP) processing and Aβ pathology. In the present study, the effect of Aβ pathology on neuronal and astrocytic metabolism and glial–neuronal interactions in neurotransmitter homeostasis was assessed in the transgenic McGill-R-Thy1-APP rat model of AD. In these rats, accumulation of Aβ oligomers appears 1 week after birth and cognitive symptoms are apparent by 3 months of age. Extracellular Aβ plaques start accumulating in the subiculum area at age 6 months, appear in most areas of the hippocampal formation and some areas of the cerebral cortex at age 13 months, and are found in most areas of the brain by 20 months of age.10 We have previously reported that changes in metabolite concentrations are readily detected by in vivo 1H NMR spectroscopy at both early and more advanced age in these rats.11 In the present study, neuronal and astrocytic metabolism was studied simultaneously by injecting transgenic McGill-R-Thy1-APP rats and age-matched controls with [1-13C]glucose and [1,2-13C]acetate followed by analysis with ex vivo 1H and 13C NMR spectroscopy and high-performance liquid chromatography (HPLC). We investigated metabolic alterations in the hippocampal formation, frontal-, entorhinal-, and retrosplenial/cingulate cortices since regional hypometabolism of glucose in AD occurs in brain regions such as the posterior cingulate cortex and the medial temporal lobe, as well as in the frontal cortex in later stages of the disease.12, 13
Materials and methods
Materials
[1-13C]glucose and [1,2-13C]acetate were purchased from Cambridge Isotope Laboratories (Andover, MA, USA), deuterium oxide (D2O, 99.9%) from CDN Isotopes (Point-Claire, Quebec, Canada), ethylene glycol from Merck (Darmstadt, Germany) and 2,2-Dimethyl-2-silapentane-5-sulfonate sodium salt (DSS sodium salt) from Sigma-Aldrich (St Louis, MO, USA). All other chemicals of the purest grade were available from local commercial suppliers.
Animals
Ten female McGill-R-Thy1-APP rats and eleven female Wistar controls (HanTac:WH/Wistar Hannover GALAS rats from Taconic, Ejby, Denmark) of age 15 months were included in the experiment. McGill-R-Thy1-APP rats express the 751 isoform of the human APP carrying the Swedish and Indiana mutations under transcriptional control of the murine Thy1.2 promoter.10 All transgenic rats used in this study were homozygous, bred in-house, and genotyped as described previously.11 McGill-R-Thy1-APP and control rats did not differ significantly in weight. All animals were maintained under standard laboratory conditions on a 12/12-hour light/dark cycle, with free access to food and water prior to the experiment. The experiments were approved by the Norwegian Animal Research Authority and performed according to the European Convention (ETS 123 of 1986).
Animal Procedures
The rats were injected intraperitoneally with [1-13C]glucose (543 mg/kg, 0.3 mol/L solution) plus [1,2-13C]acetate (504 mg/kg, 0.6 mol/L solution). Twenty minutes after injection, the animals were subjected to microwave fixation of the head at 4 kW for typically 2 seconds (Model GA5013; Gerling Applied Engineering Inc., Modesto, CA, USA). The hippocampal formation and frontal-, entorhinal-, retrosplenial-, and cingulate cortices were dissected. The retrosplenial and cingulate cortices of each rat were combined to achieve higher tissue weight for analysis with 13C NMR spectroscopy. Blood was collected from the bodies, quickly pipetted into tubes and centrifuged for 10 minutes at 3,000 g at 4°C to obtain blood plasma. All brain and blood plasma samples were stored at −80°C until extraction.
Extraction of Brain Tissue and Blood Plasma
The blood plasma samples were extracted using the perchloric acid method for extraction of blood as described previously.14 Brain tissue samples were extracted using a methanol/chloroform extraction method: samples were homogenized in 300 μL ice-cold methanol using a VibraCell Sonicator (model VCX 750; Sonics & Materials, Newtown, CT, USA), and α-ABA was added as an internal standard for HPLC analysis. In all, 150 μL purified water (Elga Purelab Ultra Analytic, Marlow, UK) and 200 μL chloroform were added to each sample, which was subsequently centrifuged at 9,830 g for 15 minutes at 4°C. The methanol/water phase was collected and transferred to a new tube. The remaining chloroform phase was re-extracted by adding 400 μL methanol, 300 μL purified water, and 100 μL chloroform. After centrifugation, the new methanol/water phase was pooled with the methanol/water phase collected previously. The chloroform phase was once again re-extracted and centrifuged, and the methanol/water phase was pooled with those previously collected for each sample. All samples were kept on ice whenever possible during the extraction procedure and stored at −80°C after extraction. After lyophilization, the samples were resuspended in 200 μL D2O, centrifuged at ∼3,000 g for 10 minutes at 4°C, and 5 μL was removed from the supernatants for HPLC analysis. The supernatants were then lyophilized twice with D2O.
Concentrations of metabolites and incorporation of 13C label into metabolites in brain extracts obtained from transgenic McGill-R-Thy1-APP rats and controls were quantified using HPLC, 1H and 13C NMR spectroscopy. Due to the small size of the entorhinal cortex, 13C NMR spectroscopy spectra with adequate signal-to-noise ratio could not be obtained, and these extracts were analyzed with 1H NMR spectroscopy and HPLC only. Blood plasma samples were analyzed using 1H NMR spectroscopy.
High-Performance Liquid Chromatography
High-performance liquid chromatography with fluorescence detection (1100 series; Agilent Technologies, Santa Clara, CA, USA) was used for quantification of the following amino-acid concentrations in the hippocampal formation, frontal-, entorhinal-, and retrosplenial/cingulate cortices: glutathione, serine, glycine, threonine, arginine, tyrosine, methionine, tryptophan, valine, phenylalanine, isoleucine, and leucine. Amino acids were precolumn derivatized with o-phthaldialdehyde, and components were separated on a Zorbax SB-C18 column (4.6 × 150 mm, 3.5 μm; Agilent Technologies). A gradient of two eluents (one with phosphate buffer (50 mmol/L, pH 5.9) and tetrahydrofurane (2.5%) and the other with methanol (98.75%) and tetrahydrofurane (1.25%)) was used to achieve optimal separation and faster elution of the most nonpolar components. Quantification was performed using the internal standard α-ABA, thus correcting for potential metabolite loss during extraction. All amounts were corrected for tissue weight.
1H and 13C Nuclear Magnetic Resonance Spectroscopy
1H NMR spectroscopy was used to determine the content and 13C enrichment of glucose and acetate in the blood plasma samples, and the content of NAD+, ATP+ADP (and AMP), glucose, myo-Inositol (mIns), phosphocreatine, creatine, taurine, phosphocholine, glycerophosphocholine, choline, aspartate, succinate, glutamine, glutamate, GABA, N-acetylaspartate, lactate, and alanine in all brain regions investigated: the hippocampal formation, frontal cortex, entorhinal cortex, and the combined retrosplenial and cingulate cortices. 13C NMR spectroscopy was used to quantify the concentrations of 13C-labeled metabolites in all brain areas except the entorhinal cortex, which was too small for this analysis. A typical 13C NMR spectroscopy spectrum from the retrosplenial/cingulate cortex of a McGill-R-Thy1-APP rat injected with [1-13C]glucose and [1,2-13C]acetate is shown in Figure 1. Lyophilized extracts of brain and plasma were dissolved in 160 μL D2O containing DSS and ethylene glycol as internal standards for quantification. The supernatants were transferred to SampleJet tubes (3.0 × 103.5 mm) for insertion into the SampleJet autosampler (Bruker BioSpin GmbH, Rheinstetten, Germany). All samples were analyzed using a QCI CryoProbe 600 MHz ultrashielded Plus magnet (Bruker BioSpin GmbH). 1H NMR spectroscopy spectra from brain extracts were acquired with the following parameters: pulse angle of 90°, acquisition time of 2.66 seconds and a relaxation delay of 10 seconds. The number of scans was typically 128. 1H spectra from blood plasma extracts were acquired with the same parameters, but the number of scans was 64. Proton decoupled 13C spectra were acquired with the following parameters: pulse angle of 30°, acquisition time of 1.65 seconds and a relaxation delay of 0.5 seconds, 30 kHz spectral width with 98 K data points. The number of scans was typically 8,192. All spectra were recorded at 20°C.
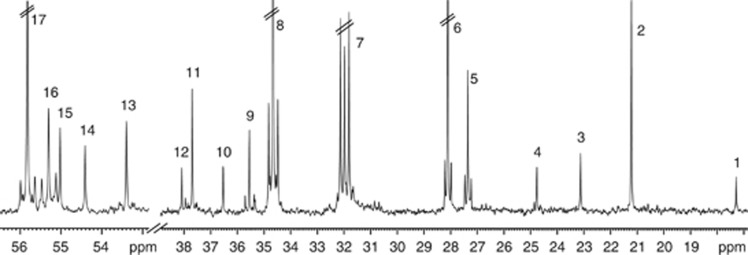
Figure 1.
A typical 13C nuclear magnetic resonance (NMR) spectroscopy spectrum from the retrosplenial/cingulate cortex of a McGill-R-Thy1-APP rat injected with [1-13C]glucose and [1,2-13C]acetate (for details, see Materials and Methods). The singlets are monolabeled metabolites predominantly derived from [1-13C]glucose metabolism, whereas doublets are double-labeled (in consecutive positions) metabolites mainly originating from [1,2-13C]acetate metabolism. Peak assignment: 1—alanine C3, 2—lactate C3, 3—N-acetylaspartate C6, 4—GABA C3, 5—glutamine C3, 6—glutamate C3, 7—glutamine C4, 8—glutamate C4, 9—GABA C2, 10—taurine C2, 11—aspartate C3, 12—creatine C2, 13—aspartate C2, 14—N-acetylaspartate C2, 15—creatine C4, 16—glutamine C2, and 17—glutamate C2. Parallel lines indicate that peaks are truncated.
Relevant peaks in the spectra were identified and integrated using the TopSpin 3.0 software (Bruker BioSpin GmbH). Amounts of metabolites were quantified from the integrals of the peak areas using DSS and ethylene glycol as internal standards for the 1H and 13C spectra, respectively. The amounts obtained from 1H spectra were corrected for the number of protons constituting the peak, for 13C content and for tissue weight. The amounts of 13C-labeled metabolites were corrected for tissue weight, singlets in the 13C spectra were corrected for the 1.1% natural abundance of 13C calculated from 1H spectra, and all peaks were corrected for nuclear Overhauser and relaxation effects in the following way: one 13C NMR spectrum was taken under the experimental conditions with nuclear Overhauser effect, optimized pulse angle and repetition time. Directly thereafter another 13C NMR spectrum was taken of the same sample without nuclear Overhauser effect but with decoupling of the protons briefly before acquisition and a 20 second relaxation delay, well above the 5 × relaxation time for the carbon atoms of interest.15 This was performed with six samples, the averages were taken and applied to all peaks. Percent (%) 13C enrichment was calculated as the 13C amount (corrected for natural 13C abundance) divided by the total concentration of the metabolite (12C+13C) and expressed as percent. The percent 13C enrichment represents the turnover, or the rate of synthesis and degradation, of a metabolite.16
Labeling Patterns from Metabolism of [1-13C]Glucose and [1,2-13C]Acetate
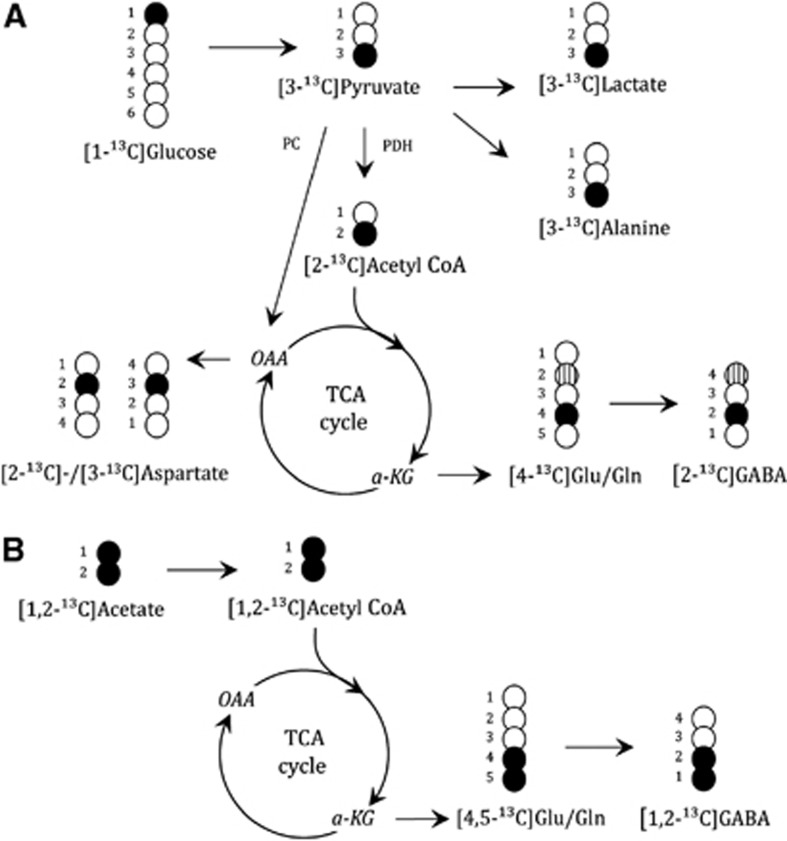
Glucose is taken up by both neurons and astrocytes,17 but the majority of acetyl Coenzyme A (acetyl CoA) derived from glucose is metabolized in neurons.18 Acetate, however, is predominantly taken up and metabolized by astrocytes.19, 20 Therefore, injection of [1-13C]glucose and [1,2-13C]acetate used in conjunction with 13C NMR spectroscopy permits monitoring of the activity of metabolic pathways in neurons and astrocytes as well as interactions between these two compartments. A schematic overview of 13C-labeling patterns is shown in Figure 2.
Figure 2.
13C-labeling patterns from metabolism of (A) [1-13C]glucose in neurons and astrocytes and (B) [1,2-13C]acetate in astrocytes. Black circles are 13C atoms, striped circles show the 13C-label obtained from metabolism via the PC pathway in astrocytes, white circles are 12C atoms. α-KG, α-ketogluratate; glu, glutamate; gln, glutamine (in astrocytes); PC, pyruvate carboxylase (in astrocytes only); PDH, pyruvate dehydrogenase; OAA, oxaloacetate; acetyl CoA, acetyl Coenzyme A; TCA, tricarboxylic acid.
[1-13C]glucose is, via glycolysis, converted to [3-13C]pyruvate that can be further converted to [3-13C]lactate, [3-13C]alanine, or be decarboxylated to [2-13C]acetyl CoA via the PDH pathway. [2-13C]acetyl CoA may enter the TCA cycle through condensation with oxaloacetate (OAA) to form citrate. Subsequently, the TCA cycle intermediate [4-13C]α-KG is formed and can leave the TCA cycle and give rise to [4-13C]glutamate, which can be converted to [2-13C]GABA in GABAergic neurons by the action of glutamic acid decarboxylase. [4-13C]glutamate is released from glutamatergic neurons during neurotransmission, and is predominantly removed from the synaptic cleft by astrocytic uptake. In astrocytes, [4-13C]glutamate is converted to [4-13C]glutamine via the astrocytic enzyme glutamine synthetase and can be sent back to neurons for reconversion to [4-13C]glutamate to replenish their neurotransmitter pool.20 If [4-13C]α-KG remains in the TCA cycle it gives rise to equal amounts of [2-13C]-/[3-13C]OAA, which can be transaminated to aspartate labeled in the same positions, or it can condense with unlabeled acetyl CoA and after several steps give rise to formation of [2-13C]-/[3-13C]glutamate/glutamine or [3-13C]-/[4-13C]GABA (glutamine in astrocytes only). Astrocytes have an additional pathway for metabolism of [3-13C]pyruvate in mitochondria: they can convert it to [3-13C]OAA via the anaplerotic reaction mediated by the astrocytic enzyme pyruvate carboxylase (PC). This gives rise to the formation of [2-13C]glutamate and glutamine after several steps. After being sent to neurons, [2-13C]glutamine is reconverted to [2-13C]glutamate and further to [4-13C]GABA in GABAergic neurons. The neuronal release of glutamate, astrocytic uptake and conversion to glutamine followed by recycling to neurons constitutes the glutamate–glutamine cycle. A similar cycle exists between GABAergic neurons and astrocytes, termed the glutamate–GABA–glutamine cycle. Although the majority of GABA is removed from the synaptic cleft by reuptake into neurons, astrocytes may also take up GABA and degrade it via the GABA shunt and subsequent TCA cycle metabolism to form glutamine which can be transferred to GABAergic neurons for reconversion to GABA via glutamate (reviewed in Bak et al21).
[1,2-13C]acetate is converted to [1,2-13C]acetyl CoA in astrocytes by acetyl CoA synthetase, enters the TCA cycle by condensation with OAA to form citrate, and gives rise to the formation of [4,5-13C]glutamate and [4,5-13C]glutamine. After being sent to neurons, [4,5-13C]glutamine is reconverted to [4,5-13C]glutamate, and also further to [1,2-13C]GABA in GABAergic neurons. If [4,5-13C]α-KG stays in the TCA cycle for a second turn and labeled OAA condenses with unlabeled acetyl CoA, then [3-13C]-/[1,2-13C]glutamate or glutamine can be formed.
Calculation of Metabolite Ratios
Astrocyte–neuron interactions
As previously mentioned, acetate is metabolized predominantly by astrocytes, and [1,2-13C]acetate gives rise to [4,5-13C]glutamate in astrocytes after several steps. [4,5-13C]glutamate is both precursor for [4,5-13C]glutamine in astrocytes and the result of transfer of [4,5-13C]glutamine to neurons followed by reconversion to [4,5-13C]glutamate. However, since the amount of glutamate located in glutamatergic neurons accounts for over 80% of the total glutamate pool,22, 23 [4,5-13C]glutamate quantified by 13C NMR spectroscopy predominantly reflects neuronal conversion of [4,5-13C]glutamine to [4,5-13C]glutamate. This amount will depend on the percent 13C enrichment of glutamine with [4,5-13C]glutamine. Information about transfer of glutamine from astrocytes to neurons can be obtained when comparing the ratio of the amount of [4,5-13C]glutamate divided by the percent enrichment of glutamine with [4,5-13C]glutamine between control and McGill-R-Thy1-APP rats. Similarly, transfer of glutamate from the neuronal to the astrocytic compartment can be obtained from the ratio of the amount of [4-13C]glutamine divided by the percent enrichment of glutamate with [4-13C]glutamate. However, although ∼40% of [4-13C]glutamine is derived from [4-13C]glutamate labeled in the neuronal compartment, ∼60% of [4-13C]glutamine is labeled from [4-13C]glutamate originating from [1-13C]glucose metabolism in astrocytes.20 This ratio should therefore be used with care under circumstances of altered mitochondrial metabolism in astrocytes, which will confound the [4-13C]glutamine level used to reflect glutamate transfer. The transfer of [4,5-13C]glutamine from astrocytes to GABAergic neurons can be estimated by the [1,2-13C]GABA amount divided by the percent enrichment of glutamine with [4,5-13C]glutamine.
Pyruvate carboxylation
The relative contribution from the PC compared with that of the PDH pathway to glutamate and glutamine formation can be evaluated by calculation of the PC/PDH ratio. As [2-13C]glutamate and glutamine may arise both from the anaplerotic PC reaction and from the oxidative PDH reaction, the latter is corrected for by subtraction of [3-13C]glutamate or glutamine, which is formed in equal amounts as [2-13C]glutamate or glutamine from the second turn of the TCA cycle when the 13C label entered via the PDH pathway. However, [3-13C]glutamate or glutamine can also be derived from the second turn of the TCA cycle during [1,2-13C]acetate metabolism, in equal amounts as [1,2-13C]glutamate or glutamine. Thus, [2-13C]glutamate or glutamine in excess of [3-13C]glutamate or glutamine corrected for the contribution labeled from [1,2-13C]acetate is derived from PC activity, and is calculated as [2-13C]−([3-13C]−[1,2-13C]). The PC/PDH ratio for glutamate and glutamine is calculated as follows: ([2-13C]−([3-13C]−[1,2-13C]))/[4-13C].
Acetate/glucose utilization
The acetate/glucose utilization ratio is an estimate of the relative contribution from astrocytes and neurons to the formation of glutamate, glutamine, and GABA. For glutamate and glutamine, it can be expressed as [4,5-13C]/[4-13C] and for GABA as [1,2-13C]/[2-13C].24
Data and Statistical Analysis
One retrosplenial/cingulate cortex sample from a control rat was omitted from all data sets due to incorrect tissue weight. In addition, it was not possible to obtain appropriate 1H NMR spectroscopy signal for one McGill-R-Thy1-APP frontal cortex sample. One control frontal cortex sample was excluded from the 1H and 13C NMR spectroscopy data sets and one McGill-R-Thy1-APP entorhinal cortex sample was excluded from the 1H NMR spectroscopy data set, because these samples were too small to obtain quantifiable spectra. However, these two samples could still be analyzed using HPLC. Also, it was not possible to dissect the entorhinal cortex of one of the McGill-R-Thy1-rats.
All results are presented as the group average±s.e.m. Metabolite concentrations and the amount of 13C-labeled metabolites were compared between control and McGill-R-Thy1-APP rats using the two-tailed unpaired Student's t-test calculated using the Microsoft Excel software, with P<0.05 as the level of significance. It should be noted that the level of significance was not adjusted for multiple comparisons, thus the findings in this study should be interpreted with care.
Results
There were no differences in the concentration and percent 13C enrichment of glucose in the blood plasma between control (7.32±0.28 mmol/L, 36±1% 13C enrichment) and McGill-R-Thy1-APP (7.46±0.64 mmol/L, 34±2% 13C enrichment) rats. The concentration and percent 13C enrichment of acetate in blood plasma of control (0.78±0.08 mmol/L, 66±2% 13C enrichment) and McGill-R-Thy1-APP (0.68±0.13 mmol/L, 65±3% 13C enrichment) were not significantly different either. Furthermore, the concentrations of glucose and of [1-13C]glucose were unchanged compared with controls in all brain regions investigated in McGill-R-Thy1-APP rats, whereas acetate was not detectable in brain extracts in any of the groups. This indicates that there were no differences in substrate transport from blood to brain between the groups. In contrast, the levels of lactate and alanine in the hippocampal formation as well as the lactate level in the frontal cortex were increased in McGill-R-Thy1-APP rats compared with controls (Table 1). In McGill-R-Thy1-APP rats, the level of [3-13C]lactate was significantly elevated in the hippocampal formation and frontal cortex, but the level of [3-13C]alanine did not differ from that of controls in any of the brain areas.
Table 1. Concentrations of glucose, [1-13C]glucose, lactate, [3-13C]lactate, alanine, and [3-13C]alanine.
| nmol/g |
HF |
FCX |
Retrospl/Cing cx |
Entorhinal cx |
||||
|---|---|---|---|---|---|---|---|---|
| Ctrl | AD | Ctrl | AD | Ctrl | AD | Ctrl | AD | |
| Glucose | 2,516±179 | 2,530±200 | 2,507±109 | 2,744±240 | 2,513±127 | 2,551±253 | 2,054±146 | 2,060±197 |
| [1-13C]glucose | 574±40 | 561±66 | 653±50 | 683±96 | 686±51 | 651±68 | — | — |
| Lactate | 1,873±75 | 2,188±87* | 2,323±92 | 2,934±164** | 2,001±114 | 2,416±192 | 2,470±228 | 2,915±240 |
| [3-13C]lactate | 70±11 | 107±13* | 113±11 | 153±14* | 94±9 | 126±18 | — | — |
| Alanine | 468±16 | 525±12* | 524±33 | 593±51 | 432±38 | 428±21 | 527±37 | 554±65 |
| [3-13C]alanine | 23±3 | 30±3 | 29±5 | 25±3 | 23±4 | 19±2 | — | — |
HF, hippocampal formation; FCX, frontal cortex; Retrospl/Cing cx, retrosplenial/cingulate cortex; AD, Alzheimer's disease; NMR, nuclear magnetic resonance.
The concentrations (nmol/g) of glucose, lactate, and alanine were measured using 1H NMR spectroscopy, and those of [1-13C]glucose, [3-13C]lactate, and [3-13C]alanine were measured using 13C NMR spectroscopy. Results are presented as mean±s.e.m. of McGill-R-Thy1-APP (AD, n=9 to 10) and control rats (n=10 to 11). For details, see the Materials and Methods section. The data were analyzed using the unpaired Student's t-test. *P<0.05, **P<0.01, statistically significant difference from control rats.
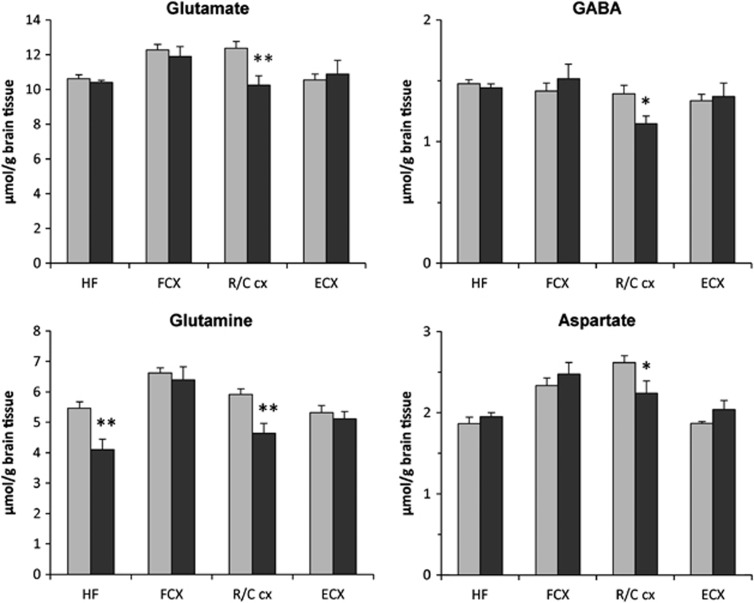
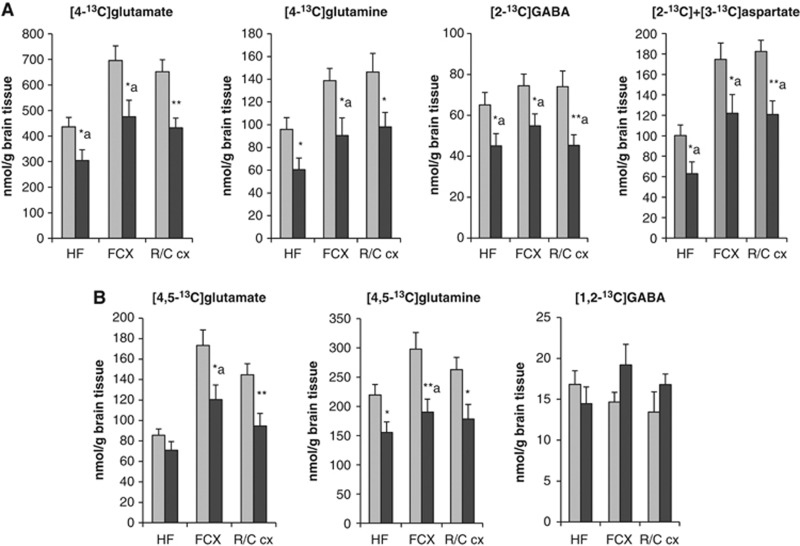
The concentrations of glutamate, glutamine, GABA, and aspartate were significantly decreased in the retrosplenial/cingulate cortex of McGill-R-Thy1-APP rats compared with controls, whereas a reduced level of glutamine was found in the hippocampal formation (Figure 3). In addition, decreased incorporation of 13C label into amino acids labeled from [1-13C]glucose, predominantly reflecting neuronal metabolism, was detected. The concentrations of [4-13C]glutamate, [2-13C]GABA, and [2-13C]+[3-13C]aspartate were significantly decreased in the hippocampal formation, frontal cortex, and retrosplenial/cingulate cortex of McGill-R-Thy1-APP rats (Figure 4). In the frontal cortex and hippocampus, the percent 13C enrichment of glutamate, GABA, and aspartate with [4-13C]glutamate, [2-13C]GABA, and [2-13C]+[3-13C]aspartate was also decreased. There was also a reduction in the level of [4-13C]glutamine, which reflects transfer of glutamate from glutamatergic neurons to astrocytes or/and astrocytic mitochondrial metabolism, in all brain regions analyzed in McGill-R-Thy1-APP rats.
Figure 3.
The concentrations (μmol/g brain tissue) of glutamate, glutamine, GABA, and aspartate in brain extracts from 15-month-old McGill-R-Thy1-APP (AD, black bars) and control rats (gray bars), quantified using 1H nuclear magnetic resonance (NMR) spectroscopy. Results are mean±s.e.m. of McGill-R-Thy1-APP (n=9 to 10) and control rats (n=10 to 11), for details see the Materials and Methods section. The data were analyzed using the unpaired Student's t-test. *P<0.05, **P<0.01, statistically significant difference from control rats. HF, hippocampal formation; FCX, frontal cortex; R/C cx, retrosplenial/cingulate cortex; ECX, entorhinal cortex.
Figure 4.
The concentrations (nmol/g) of 13C-labeled amino acids derived from (A) [1-13C]glucose and (B) [1,2-13C]acetate metabolism in brain extracts of 15-month-old McGill-R-Thy1-APP (black bars) and control rats (gray bars), quantified using 13C nuclear magnetic resonance (NMR) spectroscopy. Results are mean±s.e.m. of McGill-R-Thy1-APP rats (n=10) and control rats (n=10 to 11), for details see the Materials and methods section. The data were analyzed using the unpaired Student's t-test. *P<0.05, **P<0.01, statistically significant difference from control rats, a=percent 13C enrichment is significantly different from control rats (P<0.05). HF, hippocampal formation; FCX, frontal cortex; R/C cx, retrosplenial/cingulate cortex.
The metabolism of [1,2-13C]acetate reflects astrocytic metabolism, and diminished [1,2-13C]acetate metabolism was evident in all brain regions investigated with 13C NMR spectroscopy of McGill-R-Thy1-APP rats compared with controls. Specifically, concentrations of [4,5-13C]glutamine and [4,5-13C]glutamate were significantly decreased in both frontal cortex and retrosplenial/cingulate cortex of McGill-R-Thy1-APP rats compared with controls, whereas in the hippocampal formation, the concentration of [4,5-13C]glutamine, but not [4,5-13C]glutamate, was decreased. The concentration of [1,2-13C]GABA, originating from [4,5-13C]glutamine sent from astrocytes, was unaltered in all brain areas investigated (Figure 4).
The levels of the energy-related metabolites ATP+ADP (and AMP), phosphocreatine, and NAD+ were decreased in the retrosplenial/cingulate cortex, whereas the level of creatine was increased in the frontal cortex of McGill-R-Thy1-APP rats compared with controls (Table 2). The concentration of serine was significantly increased in all brain areas investigated in McGill-R-Thy1-APP rats compared with controls, and the taurine concentration was increased both in the hippocampal formation and in the entorhinal- and frontal- cortices, but not in the retrosplenial/cingulate cortex. Furthermore, there was an increase in the level of arginine in the hippocampal formation, whereas the levels of methionine, isoleucine, and mIns were increased in the frontal cortex of McGill-R-Thy1-APP rats. In the retrosplenial/cingulate cortex, the levels of arginine and fumarate were increased, whereas the levels of threonine, mIns, and phosphocholine were decreased (Table 2). Phenylalanine is a precursor for tyrosine, which is converted to the monoamine neurotransmitters dopamine, norepinephrine, and epinephrine. The phenylalanine contents of the frontal- and the retrosplenial/cingulate cortices of McGill-R-Thy1-APP rats were significantly increased, whereas the levels of tyrosine and the serotonin precursor tryptophan were normal in all brain regions (Table 2).
Table 2. Concentrations of metabolites.
| nmol/g |
HF |
FCX |
Retrospl/Cing cx |
Entorhinal cx |
||||
|---|---|---|---|---|---|---|---|---|
| Ctrl | AD | Ctrl | AD | Ctrl | AD | Ctrl | AD | |
| Energy-related metabolites | ||||||||
| PCr | 2,568±89 | 2,674±47 | 2,001±101 | 2,000±254 | 2,162±100 | 1,343±247** | 1,382±192 | 1,405±215 |
| Cr | 6,236±295 | 6,244±112 | 5,660±200 | 6,612±320* | 6,437±290 | 6,776±351 | 5,955±257 | 6,541±558 |
| NAD+ | 269±17 | 279±4 | 299±12 | 303±10 | 311±12 | 262±18* | 252±15 | 237±14 |
| ATP+ADP | 2,288±197 | 2,582±59 | 2,401±160 | 2,399±178 | 2,362±155 | 1,801±198* | 2,221±189 | 2,030±162 |
| Amino acids | ||||||||
| Taurine | 4,784±152 | 6,140±217** | 5,957±125 | 7,244±337** | 4,726±189 | 5,092±312 | 5,173±219 | 6,226±164** |
| Serine | 965±20 | 1,089±20** | 1,074±30 | 1,242±58** | 952±24 | 1,054±37* | 1,056±19 | 1,143±36* |
| Phenylalanine | 43±2 | 48±1 | 47±2 | 61±4** | 57±3 | 71±4* | 66±4 | 81±7 |
| Tyrosine | 60±5 | 65±2 | 66±6 | 75±5 | 64±6 | 69±6 | 66±11 | 70±7 |
| Tryptophan | 27±1 | 27±1 | 30±1 | 33±3 | 50±3 | 60±5 | 51±3 | 50±2 |
| Threonine | 698±29 | 713±14 | 758±31 | 772±45 | 627±49 | 479±19* | 721±58 | 672±36 |
| Arginine | 144±1 | 170±5** | 181±12 | 201±11 | 207±14 | 256±10* | 234±18 | 259±19 |
| Methionine | 38±2 | 42±1 | 41±2 | 51±3** | 46±3 | 51±3 | 50±3 | 58±4 |
| Isoleucine | 29±02 | 32±1 | 35±2 | 43±3** | 37±3 | 40±3 | 43±3 | 54±4 |
| Various metabolites | ||||||||
| mIns | 6,832±230 | 7,144±149 | 5,279±170 | 5,924±249* | 6,504±355 | 5,532±264* | 7,514±348 | 7,624±453 |
| Fumarate | 46±4 | 52±2 | 65±4 | 134±57 | 64±5 | 82±6* | 48±5 | 76±4** |
| PCh | 521±21 | 510±19 | 460±25 | 521±45 | 414±24 | 312±38* | 474±23 | 457±11 |
Cr, creatine; FCX, frontal cortex; HF, hippocampal formation; PCh, phosphocholine; PCr, phosphocreatine; Retrospl/Cing cx, retrosplenial/cingulate cortex; mIns, myo-Inositol; AD, Alzheimer's disease; NMR, nuclear magnetic resonance; HPLC, high-performance liquid chromatography.
The metabolite concentrations (nmol/g brain tissue) were quantified using 1H NMR spectroscopy and HPLC. Results are presented as mean±s.e.m. of McGill-R-Thy1-APP (AD, n=9 to 10) and control rats (n=10 to 11), for details see the Materials and Methods section. The data were analyzed using the unpaired Student's t-test. *P<0.05, **P<0.01, statistically significant difference from control rats.
Metabolite Ratios
The ratio for transfer of glutamine from astrocytes to glutamatergic neurons (A–N interaction; Table 3) was decreased in the retrosplenial/cingulate cortex of McGill-R-Thy1-APP rats but was unaltered in the hippocampal formation and frontal cortex. The ratio for transfer of glutamine from astrocytes to GABAergic neurons was increased in the frontal cortex of McGill-R-Thy1-APP rats compared with controls, but was unaltered in the hippocampal formation and retrosplenial/cingulate cortex. Unfortunately, the ratio for transfer of glutamate from the neuronal to the astrocytic compartment could not be reliably calculated as it was compromised by the decreased mitochondrial metabolism in astrocytes.
Table 3. Pyruvate carboxylation, acetate/glucose utilization, and glutamine transfer from astrocytes to neurons.
|
HF |
FCX |
Retrospl/Cing cx |
||||
|---|---|---|---|---|---|---|
| Ctrl | AD | Ctrl | AD | Ctrl | AD | |
| PC glutamate, nmol/g | 48.5±4.8 | 27.0±5.4** | 87.5±9.6 | 58.6±11.4 | 71.1±7.1 | 45.2±9.8* |
| PC glutamine, nmol/g | 54.5±5.2 | 36.8±4.5* | 65.6±8.4 | 48.1±7.8 | 63.0±6.5 | 39.3±6.0* |
| Ac/glc utilization GABA | 0.27±0.03 | 0.36±0.08 | 0.19±0.02 | 0.38±0.06** | 0.19±0.03 | 0.32±0.06* |
| Transfer between astrocytes and neurons | ||||||
| Ast–glu neurons | 21.4±1.2 | 18.7±1.6 | 38.7±1.2 | 41.5±3.6 | 33.1±2.0 | 26.1±2.6* |
| Ast–GABA neurons | 4.1±0.5 | 3.5±0.6 | 3.3±0.4 | 9.1±2.5* | 2.7±0.4 | 4.0±0.5 |
HF, hippocampal formation; FCX, frontal cortex; Retrospl/Cing cx, retrosplenial/cingulate cortex; AD, Alzheimer's disease.
The levels (nmol/g) of glutamate and glutamine formed from metabolism via the pyruvate carboxylase (PC) pathway, acetate versus glucose utilization ratio for GABA formation and the transfer of glutamine between astrocytes (ast) and glutamatergic (glu) and GABAergic neurons ratio in McGill-R-Thy1-APP (AD) and control rats were calculated as described in the Materials and Methods section. Results are presented as mean±s.e.m. of McGill-R-Thy1-APP and control rats. The data were analyzed using the unpaired Student's t-test. *P<0.05, **P<0.01, statistically significant difference from control rats.
Neurons rely upon astrocytic TCA cycle anaplerosis to replenish their neurotransmitter pools of glutamate and GABA.21 In both the hippocampal formation and retrosplenial/cingulate cortex of McGill-R-Thy1-APP rats, the levels of glutamate and glutamine resulting from metabolism via the PC pathway (and thus reflecting de novo synthesis) were reduced compared with controls (Table 3). The levels derived from pyruvate carboxylation were equally reduced as those formed via the PDH pathway, leading to unaltered PC/PDH ratios (results not shown). Moreover, significantly more [1,2-13C]acetate relative to [1-13C]glucose was used for GABA synthesis in the retrosplenial/cingulate and frontal cortices of McGill-R-Thy1-APP rats compared with controls, as shown by the increased acetate versus glucose utilization ratio for GABA in these regions (Table 3). For glutamate and glutamine, however, there were no changes in the relative acetate versus glucose utilization (results not shown).
Discussion
In the present study, we investigated the effects of Aβ pathology on regional neuronal and astrocytic metabolism involved in energy- and amino-acid neurotransmitter homeostasis in a transgenic rat model of AD. Although brain metabolism in AD has been extensively studied, few have employed 13C NMR spectroscopy and 13C-labeled precursors, which enables detailed mapping of the activity of metabolic pathways in the brain. The present study assessed neuronal and astrocytic metabolism in several brain regions, thus offering high regional and cellular specificity compared with most previous studies investigating brain metabolism in AD patients or animal models.
Decreased regional cerebral metabolic rate for glucose has been consistently showed in patients with familial or sporadic AD at various disease stages or even before the manifestation of clinical symptoms.25 Our findings of unchanged levels of glucose and [1-13C]glucose in all brain regions under investigation in the McGill-R-Thy1-APP rat model of AD in the present study thus do not replicate previous findings. Similarly, a previous 13C MR spectroscopy study showed an unaltered amount of [1-13C]glucose in the brain of AD patients compared with controls despite several changes in concentrations of 13C-labeled metabolites downstream of glucose.5 The increased level and 13C-labeling of lactate in McGill-R-Thy1-APP rats in the present study reached significance in the hippocampal formation and frontal cortex, which is in agreement with earlier reports of increased brain lactate production in AD patients and transgenic AD mice.5, 26, 27 Together, these findings point toward impaired mitochondrial metabolism in the brain of McGill-R-Thy1-APP rats.
Impaired Neuronal and Astrocytic Mitochondrial Metabolism and Glial–Neuronal Interactions in McGill-R-Thy1-APP Rats
The above-mentioned increase in lactate production in AD patients was accompanied by decreased oxidative glucose metabolism and TCA cycle rate.5 In triple transgenic AD mice, increased lactate production was accompanied by decreased PDH protein level and activity as well as diminished brain mitochondrial respiration.28 Thus, in line with previous studies, our findings suggest impaired glucose oxidation5, 28 and indicate that lactate accumulation might be the result of restricted entry of pyruvate into mitochondria, possibly caused by decreased PDH activity.26, 28
In the present study, impaired neuronal mitochondrial metabolism in the hippocampal formation, frontal- and retrosplenial/cingulate cortices in McGill-R-Thy1-APP rats was showed by the decreased incorporation of 13C label from [1-13C]glucose via the PDH pathway and the TCA cycle into glutamate, GABA, and aspartate. The reduction in the 13C levels and percentage 13C enrichment with [4-13C]glutamate, [2-13C]GABA, and [2-13C]+[3-13C]aspartate concomitant with unaltered overall concentrations in the hippocampal formation and the frontal cortex suggests reduced turnover of these amino acids. Reduced turnover implies that the reduction in synthesis of a 13C-labeled metabolite is accompanied by equal reduction in degradation of unlabeled metabolite, since the overall concentration of the metabolite remains unaltered.16 The reduced turnover of glutamate, GABA, and aspartate suggests reduced TCA cycle flux in both glutamatergic and GABAergic neurons in the frontal cortex and hippocampal formation of McGill-R-Thy1-APP rats. These results are in agreement with previous studies showing reduced concentration of 13C-labeled glutamate, aspartate, and bicarbonate from [1-13C]glucose in AD patients despite unaltered content of amino acids.5 Similarly, decreased turnover of glutamate and GABA was showed in extracts of cortex, hippocampus, and striatum of APP-PS1 mice.6 In the retrosplenial/cingulate cortex of McGill-R-Thy1-APP rats, however, reduced synthesis of amino acids was likely, as there was a reduction in the concentrations of [4-13C]glutamate, [2-13C]GABA, and [2-13C]+[3-13C]aspartate as well as in the overall concentrations of these amino acids. These findings fit well with the reported decrease in glutamate level in the cingulate cortex of AD patients in vivo4 as well as decreased GABA and aspartate levels in postmortem brain tissue from AD patients.29 Changes in glutamate levels are already present before plaques in the McGill-R-Thy1-APP rat model,11 and the findings in the present study show pronounced changes in mitochondrial metabolism at older age with more advanced amyloid pathology. Thus, the promise of indicators of diminished mitochondrial metabolism as supportive biomarkers of AD should be investigated in future clinical studies of AD and mild cognitive impairment.
Compromised astrocytic mitochondrial metabolism in the present study was showed by the reduction in metabolism of [1,2-13C]acetate, and was supported by the decrease in [4-13C]glutamine levels which partly reflects 13C-labeling of the astrocytic α-KG pool. In the hippocampal formation and retrosplenial/cingulate cortex, decreased 13C-labeling of glutamine together with large declines in the glutamine content implicated reduced glutamine synthesis. In addition, reduced amounts of glutamine and glutamate were labeled from metabolism via the PC pathway in astrocytes, indicating compromised de novo synthesis. This is a plausible cause of the reduced synthesis of glutamine in hippocampal formation and of glutamine, glutamate, GABA, and aspartate in retrosplenial/cingulate cortex. A distinct decline in PC activity has previously been detected in postmortem tissue from the frontal and temporal lobes of AD patients,30 but the results in the present study elaborate on this and show the metabolic consequences of a reduction in pyruvate carboxylation. Interestingly, marked reduction in the amounts of [2-13C]glutamate and glutamine was also observed in AD patients after [1-13C]glucose infusion and could partly reflect decreased pyruvate carboxylation, but this was not considered by the authors.5 Altered glutamine levels have previously been shown in the cortex of AD mice.27 The reduction in the amount and percent 13C enrichment with [4,5-13C]glutamine and [4-13C]glutamine together with the unaltered glutamine content in frontal cortex of McGill-R-Thy1-APP rats in the present study suggests decreased glutamine turnover in astrocytes, implicating reduced flux through the astrocytic TCA cycle. This is in line with previous findings of reduced glutamine turnover in AD patients and APP-PS1 mice.5, 6 In contrast, a recent preliminary study in subjects with mild cognitive impairment and AD patients showed an increase in glial metabolic rate in the posterior cingulate gray and white matter.8 More research into astrocyte metabolism in AD is clearly needed to resolve these discrepancies.
The reduced glutamine transfer from astrocytes to glutamatergic neurons in the retrosplenial/cingulate cortex suggests that the metabolic impairment in this region was accompanied by perturbations in aspects of the glutamate–glutamine cycle. The unaltered glutamate content and transfer of glutamine to neurons in the hippocampal formation despite reduced de novo synthesis of glutamate and glutamine via PC suggest that glutamine transfer to neurons for glutamate production is prioritized by hippocampal astrocytes even in the context of reduced mitochondrial metabolism in astrocytes. Even though the reduction in [4-13C]glutamine in all regions may reflect the reduced mitochondrial metabolism in astrocytes, compromised transfer of glutamate from neurons to astrocytes and thus impaired glutamatergic neurotransmission cannot be ruled out. Regarding the contribution of astrocyte-derived glutamine to GABA homeostasis, it can be hypothesized that the unaltered amounts of [1,2-13C]GABA may indicate that [1,2-13C]GABA was derived from an unaffected pool of astrocytic [4,5-13C]glutamine despite decreased glutamine turnover and synthesis. Alternatively, astrocytic supply of glutamine to GABAergic neurons in frontal cortex could be upregulated. The decreased percent enrichment with [4,5-13C]glutamine in this region should be reflected in reduced levels of [1,2-13C]GABA if the amount of glutamine transferred from astrocytes was unchanged. However, this was not the case, and the elevated ratio of glutamine transfer from astrocytes to GABAergic neurons in this region further supports elevated glutamine transfer between astrocytes and GABAergic neurons in the frontal cortex.
Energy Metabolism
Compromised mitochondrial function and energy metabolism was suggested by the reduction in ATP+ADP, phosphocreatine, and NAD+ in the retrosplenial/cingulate cortex in the present study. This region is prone to pronounced early hypometabolism as well as to mitochondrial dysfunction in AD.3, 12, 31 Our findings fit with previous reports of decreased ATP formation in early and advanced AD32 and depleted ATP levels already in young transgenic AD mice33 as well as in cell cultures exposed to Aβ.34 The reduction in energy-related metabolites could also affect the activity of key mitochondrial enzymes that require ATP or NAD+ as cofactors, such as PC, PDH, and the α-ketoglutarate dehydrogenase complex, or that of the cytosolic enzyme glutamine synthetase.
Other Metabolites
Aβ has been shown to directly disrupt mitochondrial function and inhibit key mitochondrial enzymes in cell-culture experiments,35 but there is dissociation between Aβ burden and glucose hypometabolism in vivo.36 Although the present study shows that overexpression of mutated human APP induces cerebral neuronal and astrocytic hypometabolism in McGill-R-Thy1-APP rats, we cannot conclude on whether Aβ directly impaired energy- and neurotransmitter metabolism. The lack of changes in the neuronal marker N-acetylaspartate in the present study indicates that changes in neurotransmitter homeostasis and energy metabolism are not caused by substantial neuronal loss in this rat model of AD. Dystrophic neurites have been detected in peri-plaque areas, indicating neurodegeneration in 20-month-old rats, but neuronal loss has not yet been assessed in detail in the McGill-R-Thy1-APP rat model.10 Neuronal loss as a possible cause of the hypometabolism detected in the present study therefore cannot be fully excluded and should be explored in future studies. Elevated cerebral level of the glial marker mIns is commonly found in AD patients,37 and the increase showed in the frontal cortex of McGill-R-Thy1-APP rats in the present study could suggest astrogliosis. Fibrillar, dense plaques are surrounded by activated microglia in McGill-R-Thy1-APP rats, indicating neuroinflammation,10 which could also mediate the increase in mIns in the present study. Increased concentration of serine has been shown in TgCRND8 mice,27 and although we did not measure whether the widespread increase in brain serine levels represented changes in concentration of the L- or the D-isoform or both, it is interesting to note that D-serine may be involved in NMDA receptor-mediated neurotoxic insults in AD.38 Taurine is thought to exert osmoregulatory and neuromodulatory effects as well as mediating protection against the neurotoxicity of glutamate receptor agonists and Aβ,39, 40 and the increased taurine content observed in all brain regions except the retrosplenial/cingulate cortex could be related to any of these roles. The taurine content is elevated in the brain of some, but not all animal models of AD. We have previously shown elevated taurine content in the dorsal hippocampus at age 9 and 12 months and frontal cortex at the age of 12 months in McGill-R-Thy1-APP rats,11 and the level of taurine was also elevated in APPTg2576 mice.41
Conclusions
The results in the present study show widespread changes in the activity of metabolic pathways in the McGill-R-Thy1-APP rat model of AD, including perturbed energy- and neurotransmitter homeostasis, diminished mitochondrial metabolism in astrocytes and neurons, and impairment of aspects of the glutamate–glutamine cycle. Specifically, reduced turnover of amino acids and thus TCA cycle flux was showed for hippocampal and frontal cortex neurons as well as astrocytes in the frontal cortex. Reduced de novo formation of amino acids via pyruvate carboxylation was showed in hippocampal formation and retrosplenial/cingulate cortex astrocytes, affecting levels of glutamine in hippocampal formation and of glutamate, glutamine, GABA, and aspartate in the retrosplenial/cingulate cortex. Altered amino-acid levels could also be detected in the entorhinal cortex. It is conceivable that the substantial metabolic impairment of glutamatergic and GABAergic neurons as well as astrocytes and the disrupted amino-acid neurotransmitter homeostasis will interfere with glutamatergic and GABAergic neurotransmission, which has implications for neuronal function in the AD brain. Our results thus provide support for therapeutic approaches aimed to improve brain metabolism, and suggest that treatments to enhance mitochondrial metabolism in AD could be beneficial. The potential of diminished mitochondrial metabolism as a biomarker of AD should also be investigated in future clinical studies. Furthermore, the results obtained in the present study show the excellent potential of 13C NMR spectroscopy to detect alterations in cell-specific metabolic pathways in animal models of AD.
Acknowledgments
The authors would like to thank Claudio Cuello who provided the McGill-R-Thy1-APP rat line. The authors would also like to thank Lars G Evje for technical assistance and Kersti Tambet for practical assistance during the experiment and with sample preparations. We are grateful to Ingrid Heggland for genotyping the rats and maintaining and breeding of the colony. We are also grateful to Erling Wold and Knut Sverre Grøn for assistance with injections. We thank the Norwegian Health Association (Dementia) for financial support.
The authors declare no conflict of interest.
References
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Besnard AM. Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer's disease. Metab Brain Dis. 1990;5:179–184. doi: 10.1007/BF00997071. [DOI] [PubMed] [Google Scholar]
- Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K. Brain glutamate levels are decreased in Alzheimer's disease: a magnetic resonance spectroscopy study. Am J Alzheimers Dis Other Demen. 2011;26:450–456. doi: 10.1177/1533317511421780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Shic F, Enriquez C, Ross BD. Reduced glutamate neurotransmission with Alzheimer's disease—an in vivo C-13 magnetic resonance spectroscopy study. Magn Reson Mater Phy. 2003;16:29–42. doi: 10.1007/s10334-003-0004-x. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Patel AB. Impaired glutamatergic and GABAergic function at early age in AbetaPPswe-PS1dE9 mice: implications for Alzheimer's disease. J Alzheimers Dis. 2012;28:765–769. doi: 10.3233/JAD-2011-111502. [DOI] [PubMed] [Google Scholar]
- Schubert D, Soucek T, Blouw B. The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci. 2009;29:1323–1334. doi: 10.1111/j.1460-9568.2009.06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Harris K, Tran T, Ross B. Minimally invasive biomarker confirms glial activation present in Alzheimer's disease: a preliminary study. Neuropsychiatr Dis Treat. 2011;7:495–499. doi: 10.2147/NDT.S23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, et al. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon WC, Canneva F, Partridge V, Allard S, Ferretti MT, DeWilde A, et al. A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alzheimers Dis. 2010;20:113–126. doi: 10.3233/JAD-2010-1349. [DOI] [PubMed] [Google Scholar]
- Nilsen LH, Melo TM, Saether O, Witter MP, Sonnewald U. Altered neurochemical profile in the McGill-R-Thy1-APP rat model of Alzheimer's disease: a longitudinal in vivo (1) H MRS study. J Neurochem. 2012;123:532–541. doi: 10.1111/jnc.12003. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease—FDG-PET studies in MCI and AD. Eur J Nucl Med Mol I. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Nilsen LH, Shi Q, Gibson GE, Sonnewald U. Brain [U-13 C]glucose metabolism in mice with decreased alpha-ketoglutarate dehydrogenase complex activity. J Neurosci Res. 2011;89:1997–2007. doi: 10.1002/jnr.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvestad S, Hammer J, Qu H, Haberg A, Ottersen OP, Sonnewald U. Reduced astrocytic contribution to the turnover of glutamate, glutamine, and GABA characterizes the latent phase in the kainate model of temporal lobe epilepsy. J Cereb Blood Flow Metab. 2011;31:1675–1686. doi: 10.1038/jcbfm.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. J Cereb Blood Flow Metab. 2004;24:1004–1014. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Qu H, Haberg A, Haraldseth O, Unsgard G, Sonnewald U. (13)C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci. 2000;22:429–436. doi: 10.1159/000017472. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46:519–534. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea-pig and Senegalese baboon (Papio papio), with a note on the distribution of gamma-aminobutyrate. Neuroscience. 1985;16:589–606. doi: 10.1016/0306-4522(85)90194-0. [DOI] [PubMed] [Google Scholar]
- Qu H, Eloqayli H, Muller B, Aasly J, Sonnewald U. Glial-neuronal interactions following kainate injection in rats. Neurochem Int. 2003;42:101–106. doi: 10.1016/s0197-0186(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, Nacmias B, De Cristofaro MT, Fayyaz M, Cellini E, et al. Brain metabolic differences between sporadic and familial Alzheimer's disease. Neurology. 2003;61:1138–1140. doi: 10.1212/01.wnl.0000086816.30011.75. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Oesterreich K, Wagner O. Glucose metabolism as the site of the primary abnormality in early-onset dementia of Alzheimer type. J Neurol. 1988;235:143–148. doi: 10.1007/BF00314304. [DOI] [PubMed] [Google Scholar]
- Salek RM, Xia J, Innes A, Sweatman BC, Adalbert R, Randle S, et al. A metabolomic study of the CRND8 transgenic mouse model of Alzheimer's disease. Neurochem Int. 2010;56:937–947. doi: 10.1016/j.neuint.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueli MC, Taibi G. Alzheimer's disease: amino acid levels and brain metabolic status. Neurol Sci. 2013;34:1575–1579. doi: 10.1007/s10072-013-1289-9. [DOI] [PubMed] [Google Scholar]
- Hertz L, Yu AC, Kala G, Schousboe A. Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem Int. 2000;37:83–102. doi: 10.1016/s0197-0186(00)00012-7. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease) Eur J Neurosci. 2003;18:2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Oxidative energy metabolism in Alzheimer brain. Studies in early-onset and late-onset cases. Mol Chem Neuropathol. 1992;16:207–224. doi: 10.1007/BF03159971. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Casley CS, Land JM, Sharpe MA, Clark JB, Duchen MR, Canevari L. Beta-amyloid fragment 25-35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiol Dis. 2002;10:258–267. doi: 10.1006/nbdi.2002.0516. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, et al. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer's disease dementia. J Neurosci. 2012;32:16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res. 2004;1003:26–35. doi: 10.1016/j.brainres.2003.11.074. [DOI] [PubMed] [Google Scholar]
- Inoue R, Hashimoto K, Harai T, Mori H. NMDA- and beta-amyloid1-42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci. 2008;28:14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology. 2005;49:1140–1148. doi: 10.1016/j.neuropharm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Santa-Maria I, Hernandez F, Moreno FJ, Avila J. Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-beta-peptide aggregation. Neurosci Lett. 2007;429:91–94. doi: 10.1016/j.neulet.2007.09.068. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Choi JK, Cormier K, Kowall NW, Jenkins BG. Magnetic resonance spectroscopic analysis of Alzheimer's disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 2004;1012:60–65. doi: 10.1016/j.brainres.2004.02.079. [DOI] [PubMed] [Google Scholar]