Abstract
Objective
Determine if daily bathing with chlorhexidine-based soap decreased methicillin-resistant Staphylococcus aureus (MRSA) transmission and ICU-acquired S. aureus infection among ICU patients.
Design
Prospective pre-post-intervention study with control unit
Setting
1,250 bed tertiary-care teaching hospital
Patients
Medical and surgical intensive care unit (ICU) patients
Methods
Active surveillance for MRSA colonization was performed in both ICUs. In June 2005, a chlorhexidine bathing protocol was implemented in the surgical ICU. Changes in S. aureus transmission and infection rate before and after implementation were analyzed using time-series methodology.
Results
The intervention unit had a 20.68% decrease in MRSA acquisition after institution of the bathing protocol [pre-intervention 12.64 vs. post-intervention 10.03 cases/1000 patient-days-at-risk (95% CI: −5.19 – −0.04, p = 0.046)]. There was no significant change in MRSA acquisition in the control ICU during the study period [10.97 pre-June 2005 vs. 11.33/1000 patient-days at risk post-June 2005 (95% CI −37.40 – 15.19, p = 0.40)]. There was a 20.77% decrease in all S. aureus (including MRSA) acquisition in the intervention ICU from 2002-2007 [19.73 pre-intervention to 15.63 cases per 1000 patient-days at risk post-intervention (95% CI −7.25 – −0.95, p=0.012)]. The incidence of ICU-acquired MRSA infections decreased by 41.37% in the intervention ICU (1.96 pre-intervention vs. 1.15 infections per 1000 patient-days at risk post-intervention; p=0.001).
Conclusions
Institution of daily chlorhexidine bathing in an ICU resulted in a decrease in the transmission of S. aureus, including MRSA. These data support the use of routine daily chlorhexidine baths to decrease rates of S. aureus transmission and infections.
Keywords: Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, chlorhexidine, infection control, intensive-care unit
Introduction
Staphylococcus aureus is a major cause of healthcare-associated infections, particularly in critically ill patients. Methicillin-resistant S. aureus (MRSA) has caused an increasing proportion of intensive care unit (ICU)-acquired S. aureus infections in the United States over the last twenty years. Compared with methicillin-sensitive S. aureus (MSSA), MRSA infections are associated with increased costs1-3 and mortality.4;5
Transmission of S. aureus between hospitalized patients has long been felt to primarily occur via the hands of healthcare workers.6 Multiple interventions have been undertaken to interrupt MRSA transmission in healthcare settings, including improving healthcare worker hand hygiene compliance, instituting contact precautions for patients colonized or infected with MRSA, performing active surveillance to identify asymptomatic colonization and prompt earlier contact precautions, and decolonizing MRSA-colonized patients.7 Chlorhexidine gluconate, a topical antiseptic, has been used in pre-procedural skin antisepsis and to eliminate MRSA carriage. Recent data have emerged to support the use of chlorhexidine skin antisepsis to prevent the transmission of drug resistant organisms such as vancomycin-resistant enterococci (VRE) and MRSA in intensive care units.8-10 Several studies have evaluated the use of chlorhexidine-based skin antisepsis, with or without intranasal therapy to decolonize MRSA-colonized ICU patients,11-14 and reported decreases in MRSA acquisition,11 colonization,12;13 and infection.13;14 Additionally, bathing all ICU patients daily with a chlorhexidine-based soap has been shown to decrease acquisition of MRSA,8;15 colonization with MRSA16 and MRSA infection.17] However, few studies8;17 examining the effect of routine daily chlorhexidine bathing on S. aureus transmission and infection have accounted for secular trends in colonization pressure due to changes in MRSA prevalence or changes in patient mix among patients admitted to the ICU. Additionally, few studies have concomitantly compared the use of chlorhexidine in intervention units with non-intervention units.10;18
The objective of this study was determine if a daily bathing protocol with a chlorhexidine-based soap decreased intra-unit MRSA transmission among ICU patients. A secondary goal was to determine if chlorhexidine-bathing reduced intra-unit overall S. aureus (i.e., regardless of susceptibility to methicillin) transmission and ICU-acquired S. aureus infection. These outcomes were compared to an ICU in which bathing with non-medicated soap was performed. The use of time series methodology allowed for us to address potential confounders such as temporal trends in patient mix and the prevalence of S. aureus colonization at ICU admission over time.
Methods
This study was performed at Barnes-Jewish Hospital (BJH), a 1250-bed, urban, tertiary-care teaching hospital in St. Louis, Missouri. During the period of study, the trauma and surgical ICU (SICU) had 24 beds and approximately 1,400 admissions per year. The 19-bed medical ICU (MICU) had approximately 1,500 annual admissions. Patients with a prior or current history of MRSA, vancomycin-resistant enterococci (VRE), C. difficile-associated diarrhea, and certain multidrug-resistant Gram-negative bacilli were routinely placed in contact precautions, per hospital policy.
S. aureus (both methicillin-sensitive S aureus and MRSA) active surveillance data were available for the intervention unit (SICU) from January 2002 and for the control unit (MICU) from January 2005. The study intervention was performed in June 2005, and study activities continued through December 2007. For the purposes of our analyses, all available data from each ICU were included for primary and secondary outcomes.
Nasal cultures for S. aureus were performed on all patients admitted to these ICUs for more than 12 hours. Nasal cultures were also performed weekly and at ICU discharge for all patients staying in the ICU more than 48 hours. Decolonization therapy for MRSA with topical mupirocin was not routinely performed at BJH during the study period. Microbiological analysis of nasal specimens has been previously described.19 Methicillin resistance among S. aureus clinical isolates was determined using standard microbiological methods. All positive routine clinical cultures for S. aureus (i.e., MRSA and MSSA) were recorded.
Bathing protocol
Patient bathing in both units in the before June 2005 consisted of basin baths with non-medicated soap (Aloe Vesta 2-n-1 Body Wash & Shampoo, ConvaTec, Skillman, NJ) at least daily, and additionally throughout the day if needed after bowel movements or other episodes of blood or body fluid soiling. In June 2005, the surgical ICU switched to using 4% chlorhexidine-based soap (Exidine 4%, Cardinal Health, Dublin, OH) for daily patient bathing. One, four-ounce bottle of chlorhexidine-based soap was added to four quarts of water, to give an approximate final concentration of 0.125% chlorhexidine gluconate in the bath water. Bathing was done with wash cloths using a standard method.20 Chlorhexidine bathing was not performed above the neck, perineum, or on open wounds. Bathing compliance in the surgical ICU during the post-intervention period was assessed weekly by determining the total number of chlorhexidine bottles used versus the total number of patient-days.
Data collection and definitions
Prospective collection of patient-level data occurred in the surgical ICU from January 2002 through December 2007 and in the medical ICU from January 2005 through December 2007 as part of an ongoing study of S. aureus transmission which has been previously described.21 Data collected for all surgical and medical ICU patients included demographic characteristics, hospital and ICU admission and discharge dates, prior admission to BJH in the past 12 months, the patient’s location prior to hospital admission, and use of contact precautions. Additional data were collected for patients who remained in the ICU for more than 48 hours, including their past medical history, ICU processes of care, and use of mechanical ventilation and/or central venous catheters. Enteral tube feeding was defined as feeding via a nasogastric, Dobhoff, gastrostomy, jejunostomy, or gastrojejunostomy tube.
S. aureus colonization at admission was defined as a patient having an admission nasal surveillance culture positive for S. aureus or any clinical culture positive for S. aureus within 48 hours after ICU admission. To determine the number of patients coming into the unit already carrying S. aureus, in-coming colonization pressure was defined as the number of patients colonized or infected with S. aureus at admission per total number of admissions per month. S. aureus acquisition was defined as an admission nasal surveillance culture negative for S. aureus and subsequent isolation of S. aureus from a surveillance or clinical culture performed more than 48 hours after admission. The S. aureus acquisition rate was defined as the number of acquired S. aureus cases per 1000 patient-days at risk, where an ‘day-at-risk’ was defined as a day in the ICU (not in the first 48 hours after admission) without evidence of any S. aureus colonization or infection.18 ICU-related S. aureus infections and device utilization ratio were defined using the Centers for Disease Control and Prevention criteria.19
Analysis
The primary outcome of the analysis was the effect of the bathing intervention on MRSA acquisition in the intervention ICU (SICU) versus the control ICU (MICU). Times series analysis of the primary outcome was performed using data from the intervention ICU (SICU) from January 2002 through December 2007 and from the control ICU (MICU) from January 2005 through December 2007. The secondary outcomes were S. aureus acquisition (i.e., both methicillin-susceptible and –resistant bacteria) as well as ICU-acquired infection due to MRSA and all S. aureus within the intervention ICU. The unit of analysis for each ICU was one month. The monthly proportion of patients admitted to each unit with that characteristic, or who developed it during their ICU admission, was calculated. A time series model was developed for each ICU, using ordinary least squares regression. First-order serial autocorrelation and higher order autocorrelation were assessed for each model using Durbin-Watson statistic and Box-Ljung Q(k)-test, respectively. Since autocorrelation was present in the control ICU, an autoregressive moving average (ARMA) model was created for the primary outcome; an autoregressive (AR) part and a moving average (MA) part were included in this model. Since the hypothesis of the study is that overall colonization pressure should decrease as a result of the intervention, an instrumental variable “adjusted colonization pressure” was created to account for variation in colonization pressure due to changes over time in the proportion of patients already colonized at the time of ICU admission. This is expressed as:
where Dt is the binary variable for the intervention. The Andrews-Ploberger test for a break in mean at an unknown date22 was performed to determine the month with the maximal change in mean MRSA transmission rate in both units. All analysis was conducted in EViews™ 6 (IHS Global, Irvine, CA). The Wilcoxon rank-sum test was used to examine differences between medians. This study was approved by the Washington University Human Research Protection Office.
Results
During the study periods mentioned above, data were collected for a total of 53,526 patient-days, 35,124 from the intervention ICU and 18,402 from the control ICU [Table 1]. There was higher utilization of central lines and ventilators in the intervention ICU than the control ICU, as well as a higher monthly proportion of patients receiving enteral feeds and having tracheostomies. MRSA colonization on admission was higher in the control ICU (median proportion 0.24 vs. 0.17 per month by admission, p <0.001). No other interventions were implemented during the periods of study that substantively affected S. aureus acquisition and/or infection rates.
Table 1. Characteristics of the Intervention and Control Intensive Care Units.
| Variable | Intervention (Surgical) ICU (Dec. 2002-Dec. 2007) |
Control (Medical) ICU (Jan. 2005-Dec. 2007) |
p c |
|---|---|---|---|
| Number of beds | 24 | 19 | NA |
| Patient days, totala | 35124 | 18402 | NA |
| Ventilator utilization ratio, monthly, median (range)a |
0.69 (0.45-0.80) | 0.59 (0.43-0.68) | <.001 |
| Central venous catheter utilization ratio, monthly, median (range)a |
0.66 (0.44-0.81) | 0.59 (0.46-0.76) | 0.008 |
| Patient characteristics, monthly proportion, median (range)b |
|||
| Decubitus ulcer (Stage II or greater) |
0.23 (0.14-0.38) | 0.22 (0.16-0.34) | 0.08 |
| Tracheostomy | 0.20 (0.11-0.54) | 0.18 (0.11-0.25) | 0.003 |
| Enteral tube feeding | 0.28 (0.17-0.69) | 0.22 (0.17-0.40) | 0.004 |
| MRSA colonization on admission |
0.17 (0.06-0.38) | 0.24 (0.13-0.38) | <.001 |
Note: MRSA - methicillin-resistant Staphylococcus aureus; ICU – intensive care unit; NA – not applicable.
Patients with >2 day stay in ICU only.
Proportion of all ICU admissions with variable of interest during ICU stay, per month.
Determined by Mann-Whitney U test
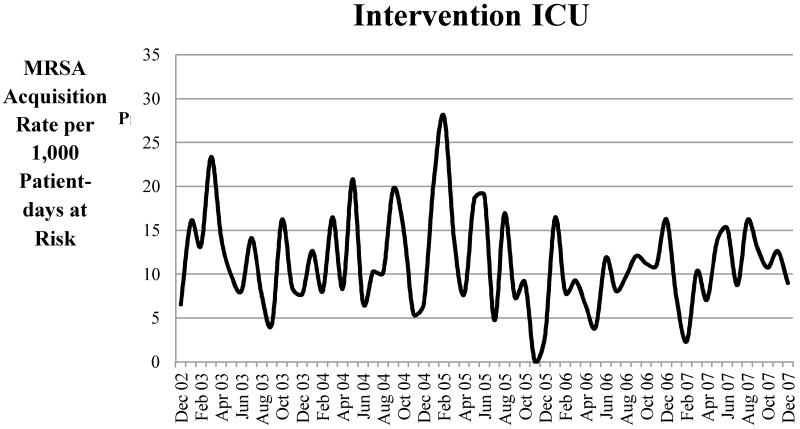
The intervention unit had a 20.68% decrease in MRSA acquisition after institution of the chlorhexidine bathing protocol in June 2005 [pre-intervention 12.64 vs. 10.03 cases per 1000 patient-days at risk post-intervention (beta −2.62, 95% CI:−5.19 – −0.04, p = 0.046)] (Table 2 and Figure 1). The reduction in MRSA acquisition was similar when intervention compliance (measured by antiseptic soap bottles used per patient day) was used in the model [data not shown]. There was no significant change in MRSA acquisition in the control ICU after June 2005 [10.97 per 1000 patient-days at risk before June 2005 vs. 11.33 after June 2005 (beta −11.10, 95% CI−37.40 – 15.19, p = 0.40)]. In order to account for changes in MRSA prevalence at the time of ICU admission, adjusted MRSA colonization pressure was included in the final model (Table 2). Other factors, such as the proportion of ICU patients with decubitus ulcers or the monthly central venous catheter device utilization ratio, were not significant in the multivariable model. By the Andrews-Ploberger test, July 2005 was the month in the intervention unit with the maximal change in MRSA acquisition (one month after the intervention started).
Table 2. Comparison of Methicillin-resistant Staphylococcus aureus (MRSA) Acquisition Rates in the Intervention Intensive Care Unit (ICU) (2002-2007) and Control ICU (2005-2007).
| Intervention (Surgical) ICUa | Control (Medical) ICU | |||
|---|---|---|---|---|
| Crude Rates | Rate (per 1,000 pt-days at riskb) | Rate (per 1,000 pt-days at riskb) | ||
| Pre-intervention | 12.64 | 10.97 | ||
| Post-intervention | 10.03 | 11.33 | ||
| Percent change | −20.68% | +3.28% | ||
|
| ||||
| Time Series Modelc | Beta [95% CI] | p | Beta [95% CI] | p |
| Intervention | −2.62 [−5.19, −0.04] | 0.046 | −11.10 [−37.40, 15.19] | 0.395 |
| Adjusted MRSA colonization pressured |
30.79 [8.74, 52.85] | 0.007 | 22.90 [−13.36, 59.16] | 0.207 |
| AR(1) | -- | -- | 0.33 [−0.02, 0.67] | 0.061 |
| MA(4) | -- | -- | −0.89 [−1.00, −0.79] | 0.000 |
| Constant | 6.75 [2.14, 11.35] | 0.005 | 17.72 [−11.61, 47.04] | 0.227 |
|
| ||||
| Model parameters | ||||
| Adjusted R2 | 0.14 | 0.32 | ||
| Durbin-Watson statistic | 2.08 | 1.84 | ||
| Q(4) p-value | 0.71 | 0.65 | ||
| Q(8) p-value | 0.55 | 0.55 | ||
| Q(12) p-value | 0.62 | 0.73 | ||
Note: AR(1)= auto-regressive variable, 1; MA(4)= moving-average variable, 4. The inclusion of AR and MA terms in the model addressed serial correlation seen, as evidenced by the Durbin-Watson statistic and the Box-Ljung Q(k) tests of the unadjusted model.
Models are based on all available data for the surgical ICU (61months, 2002-2007) and medical ICU (24 months, 2005-2007).
See study methods for definition of patient-days at risk.
In the multivariate time series model, ‘beta’ indicates the magnitude and the direction of the variable in the model, while the p value and 95% CI indicate the precision and significance of the variable within the model.
Other factors considered for inclusion in the final model included: decubitus ulcers, tracheostomy, enteral feedings, central venous catheter utilization ratio, mechanical ventilator utilization ratio.
Figure 1. Unadjusted Rates of Methicillin-resistant Staphylococcus aureus (MRSA) Acquisition per 1000 Patient-Days at Risk for the Intervention Intensive Care Unit (ICU).
Using June 2005 as the breakpoint for analysis in both units, there was a 41.37% decrease in MRSA infection in the intervention ICU (1.96 pre-intervention vs. 1.15 infections per 1000 ICU-days post-intervention). There was no significant change in MRSA infection in the control ICU (2.19 before June 2005 vs. 1.05 infections per 1000 ICU-days after June 2005, beta−1.51, 95% CI −4.01 – 1.00, p=0.228) (Table 3). There was a 20.78% decrease in all S. aureus acquisition in the intervention ICU from 2002-2007 (19.73 pre-intervention vs. 15.63 cases per 1000 patient-days at risk post-intervention, beta −4.1, 95% CI−7.25 – −0.95, p=0.012) (Table 4). There was no change in S. aureus acquisition from 2005-2007 in the control ICU (19.21 before June 2005 vs. 15.33 cases per 1000 patient-days at risk after June 2005, beta −6.75, 95% CI −20.75 – 7.26, p=0.334). ICU-acquired S. aureus infection rates also decreased by 34.31% in the intervention ICU (2.71 pre-intervention vs. 1.78 S. aureus infections per 1000 ICU-days post-intervention) (Table 5), but not in the control ICU (2.19 before June 2005 vs. 1.98 S. aureus infections per 1000 ICU-days after June 2005, beta 1.58, 95% CI −1.31-4.47, p=0.273).
Table 3. Comparison of Methicillin-resistant Staphylococcus aureus (MRSA) Infection Rates in the Intervention Intensive Care Unit (ICU) (2002-2007) and Control ICU (2005-2007).
| Intervention (Surgical) ICUa | Control (Medical) ICU | |||
|---|---|---|---|---|
| Crude Rates | Rate (per 1,000 ICU-days) | Rate (per 1,000 ICU-days) | ||
| Pre-intervention | 1.96 | 2.19 | ||
| Post-intervention | 1.15 | 1.05 | ||
| Percent change | −41.37% | −51.95% | ||
|
| ||||
| Time Series Modelb | Beta [95% CI] | p | Beta [95% CI] | p |
| Intervention | −0.90 [−1.40, −0.40] | 0.001 | −1.51 [−4.01, 1.00] | 0.228 |
| Adjusted MRSA colonization pressure |
10.78 [5.41, 16.15] | 0.000 | 1.37 [−1.76,4.51] | 0.377 |
| AR(1) | -- | -- | −0.57 [−0.91, −0.24] | 0.002 |
| AR(2) | -- | -- | −0.38 [−0.69, −0.07] | 0.018 |
| MA(5) | −0.36 [−0.62, −0.11] | 0.005 | -- | -- |
| MA(9) | −0.30 [−0.53, −0.08] | 0.009 | -- | -- |
| MA(10) | −0.31 [−0.57, −0.06] | 0.017 | -- | -- |
| MA(12) | -- | -- | −0.93 [−1.00, −0.86] | 0.000 |
| Constant | −0.13 [−1.33, 1.06] | 0.822 | 2.32 [−0.18,4.82] | 0.068 |
|
| ||||
| Model parameters | ||||
| Adjusted R2 | 0.42 | 0.70 | ||
| Durbin-Watson statistic | 2.12 | 2.37 | ||
| Q(4) p-value | 0.24 | 0.28 | ||
| Q(8) p-value | 0.66 | 0.54 | ||
| Q(12) p-value | 0.90 | 0.40 | ||
Note: AR(1)= auto-regressive variable, 1; AR(2)= auto-regressive variable, 2; MA(5) = moving average variable, 5; MA(9) = moving-average variable, 9; MA(10) = moving-average variable, 10; MA(12) = moving-average variable, 12.
The inclusion of AR and MA terms in the model addressed serial correlation seen, as evidenced by the Durbin-Watson statistic and the Box-Ljung Q(k) tests of the unadjusted model.
Models are based on all available data for the surgical ICU (61months, 2002-2007) and medical ICU (24 months, 2005-2007).
In the multivariate time series model, ‘beta’ indicates the magnitude and the direction of the variable in the model, while the p value and 95% CI indicate the precision and significance of the variable within the model.
Table 4. Time Series Models for Staphylococcus aureus Acquisition Rates in the Intervention Intensive Care Unit (ICU) (2002-2007) and Control ICU (2005-2007).
| Intervention (Surgical) ICUa | Control (Medical) ICU | |||
|---|---|---|---|---|
| Crude rates | Rate (per 1,000 pt-days at riskb) | Rate (per 1,000 pt-days at riskb) | ||
| Pre-intervention | 19.73 | 19.21 | ||
| Post-intervention | 15.63 | 15.33 | ||
| Percent change | −20.78% | −20.18% | ||
|
| ||||
| Time Series Modelb | Beta [95% CI] | P | Beta [95% CI] | p |
| Intervention | −4.10[−7.25, −0.95] | 0.012 | −6.75[−20.75, 7.26] | 0.334 |
| Adjusted S. aureus colonization pressure |
49.13 [26.21, 72.04] | 0.000 | 25.96 [−15.38, 67.31] | 0.210 |
| AR(1) | 0.24 [−0.13, 0.61] | 0.191 | ||
| MA(4) | −0.38 [−0.74, −0.02] | 0.039 | ||
| Constant | 2.40 [−5.99, 10.79] | 0.569 | 12.50 [−9.77, 34.76] | 0.261 |
|
| ||||
| Model parameters | ||||
| Adjusted R2 | 0.28 | 0.10 | ||
| Durbin-Watson statistic | 1.90 | 1.99 | ||
| Q(4) p-value | 0.83 | 0.81 | ||
| Q(8) p-value | 0.87 | 0.87 | ||
| Q(12) p-value | 0.87 | 0.88 | ||
Note: AR(1)= auto-regressive variable, 1; MA(4)= moving-average variable, 4.
The inclusion of AR and MA terms in the model addressed serial correlation seen, as evidenced by the Durbin-Watson statistic and the Box-Ljung Q(k) tests of the unadjusted model.
Models are based on all available data for the surgical ICU (61months, 2002-2007) and medical ICU (24 months, 2005-2007).
See study methods for definition of patient-days at risk.
In the multivariate time series model, ‘beta’ indicates the magnitude and the direction of the variable in the model, while the p value and 95% CI indicate the precision and significance of the variable within the model.
Table 5. Time Series Models for Staphylococcus aureus Infection Rates in the Intervention Intensive Care Unit (ICU) (2002-2007) and Control ICU (2005-2007).
| Intervention (Surgical) ICUa | Control (Medical) ICU | |||
|---|---|---|---|---|
| Crude rates | Rate (per 1,000 ICU-days ) | Rate (per 1,000 ICU-days ) | ||
| Pre-intervention | 2.71 | 2.19 | ||
| Post-intervention | 1.78 | 1.98 | ||
| Percent change | −34.31% | −9.37% | ||
|
| ||||
| Time Series Modelb | Beta [95% CI] | p | Beta [95% CI] | P |
| Intervention | −0.87[−1.25, −0.49] | 0.000 | 1.58 [−1.31, 4.47] | 0.273 |
| Adjusted S areus colonization pressure |
11.35 [6.86, 15.84] | 0.000 | 11.17 [6.39, 15.95] | 0.000 |
| AR(1) | −0.40 [−0.77, −0.04] | 0.032 | ||
| AR(2) | −0.36 [−0.73, 0.01] | 0.059 | ||
| MA(1) | −0.21 [−0.46, 0.03] | 0.083 | ||
| MA(5) | −0.33 [−0.57, −0.09] | 0.008 | ||
| MA(6) | −0.44 [−0.70, −0.18] | 0.001 | ||
| MA(7) | −0.91 [−0.99, −0.83] | 0.000 | ||
| Constant | −1.37 [−3.04, 0.31] | 0.107 | −3.63 [−7.40, 0.14] | 0.059 |
|
| ||||
| Model parameters | ||||
| Adjusted R2 | 0.35 | 0.46 | ||
| Durbin-Watson statistic | 2.09 | 1.87 | ||
| Q(4) p-value | 0.14 | 0.22 | ||
| Q(8) p-value | 0.66 | 0.18 | ||
| Q(12) p-value | 0.71 | 0.28 | ||
Note: AR(1)= auto-regressive variable, 1; AR(2)= auto-regressive variable, 2; MA(1)= moving-average variable, 1; MA(5)= moving-average variable, 5; MA(6)= moving-average variable, 6; MA(7)= moving-average variable, 7. The inclusion of AR and MA terms in the model addressed serial correlation seen, as evidenced by the Durbin-Watson statistic and the Box-Ljung Q(k) tests of the unadjusted model.
Models are based on all available data for the surgical ICU (61months, 2002-2007) and medical ICU (24 months, 2005-2007).
In the multivariate time series model, ‘beta’ indicates the magnitude and the direction of the variable in the model, while the p value and 95% CI indicate the precision and significance of the variable within the model.
Discussion
Institution of daily chlorhexidine bathing in a surgical ICU resulted in a decrease in the acquisition of and infections with S. aureus, including MRSA. This effect persisted even when accounting for temporal changes in S. aureus and MRSA colonization pressure on admission to the ICU and other patient risk factors. There was no significant decrease in S. aureus and MRSA acquisition or infections during an overlapping time frame in the control ICU which did not implement chlorhexidine bathing.
The use of time-series analysis allows for evaluation of the impact of an intervention while controlling for issues related to changes in S. aureus and MRSA colonization pressure among newly admitted patients, differences in patient comorbidities and severity of illness, and seasonality. This is important since external trends in MRSA prevalence on admission to the ICU can affect subsequent changes in observed rates of S. aureus transmission and infection, independent of a particular intervention. The use of an instrumental variable, adjusted colonization pressure, to account for the changes in the S. aureus and MRSA colonization pressure present at ICU admission is novel.
Further evidence supporting causality includes our finding that the maximal change in the MRSA monthly acquisition rate (as measured by the Andrews-Ploberger test) coincided with the implementation of chlorhexidine bathing in the intervention unit, while no change was seen during the same time frame in a control unit within the same hospital but not using chlorhexidine bathing. This supports our findings and reduces the risk that observed declines in S. aureus and MRSA acquisition and infection rates in the ICU using chlorhexidine were due to seasonal variation or other unmeasured confounders. Even when examined in the time period from 2005-2007 for both units, the reduction in MRSA acquisition was still significant in the intervention unit (data not shown).
These results expand on previous studies12;13 which demonstrated that decolonization of S. aureus carriers identified by active microbiologic surveillance using brief (seven day) periods of daily chlorhexidine baths significantly decreased rates of MRSA in an ICU. Ridenour et al reported a 52% decreased rate of MRSA acquisition in the ICU,13 and Fraser et al demonstrated a 47% decrease in S. aureus colonization incidence and 63% decreased incidence in total S. aureus-related hospital-acquired infections.12 However, these studies did not take into account the impact of routine chlorhexidine bathing in all patients over a longer period of time, which could have an independent and potentially additive effect on S. aureus carriage and acquisition. Several studies have examined the effect of daily chlorhexidine bathing on ICU patients, reporting decreases in the rates of healthcare-associated infections10;16;18;23 and the transmission of VRE.9;10 Milstone et al24 found decreased incidence in bacteremia in pediatric ICUs in per-protocol analysis, but similar rates of S. aureus bacteremia, very possibly due to very small numbers of positive cultures. Huang et al15 found comparable significant decreases in MRSA-positive clinical cultures (37% compared to 41% seen in our study) after the institution of universal decolonization with daily chlorhexidine baths and nasal mupirocin. None of the studies were able to demonstrate decreased MRSA transmission.
Few studies8;11;17 have utilized time-series methodology to evaluate the impact of daily chlorhexidine bathing on MRSA in ICUs. The use of time-series methodology allowed us to address secular trends in colonization pressure, which might otherwise bias our findings. Climo et al8 reported a multi-center study using time-series analysis to evaluate daily bathing with chlorhexidine and reported a 32% decrease in MRSA acquisition but no difference in MRSA bacteremia. The decrease in reported MRSA acquisition was comparable to that seen in our study. However, we also noted a 41.4% decrease in all MRSA infections in the ICU using chlorhexidine bathing. The difference might be explained by accounting for in-coming colonization pressure in our model. In a smaller study, Gould et al17 evaluated daily chlorhexidine bathing as one of multiple simultaneous interventions, and found an 11.4% decrease in MRSA in their ICU and non-significant decreases in MRSA bacteremia. However, since chlorhexidine bathing was only one of multiple interventions adopted (which included active surveillance culturing and contact isolation of colonized and infected patients), the effect of chlorhexidine bathing alone could not be determined.
Our study had some limitations. While the use of time-series methodologies and a concurrent control ICU addresses many potential confounders, this was not a randomized controlled trial and we cannot completely exclude the impact of other unmeasured confounders or temporal trends on the outcome. We did not evaluate length-of-stay (LOS) or mortality using these data; there would be additional confounding factors and/or interventions impacting LOS and mortality that would need to be included in the model. A future dedicated study could be performed to look at the impact of chlorhexidine bathing on those outcomes. We did not evaluate the MRSA strains in this study for the chlorhexidine resistance loci (i.e., qacA/B). Future studies will be needed to determine if widespread use of chlorhexidine for patient bathing will select for chlorhexidine tolerance in S. aureus and MRSA within health care settings and the community.
Our findings support the routine use of daily chlorhexidine baths to decrease rates of S. aureus transmission and infection in ICU settings. Chlorhexidine bathing is an inexpensive and relatively simple measure to adopt. Further studies are needed to evaluate the effect of chlorhexidine bathing in non-tertiary care centers as well as in non-critical care settings in order to define the role of routine chlorhexidine bathing in healthcare and to evaluate for the development of resistance to chlorhexidine and/or potential adverse events that might occur with more widespread use of this topical antiseptic.
Acknowledgements
Financial support. This work was supported through the Barnes-Jewish Hospital Foundation and the CDC Prevention Epicenters Program (CDC U54CK000162). This work was also supported by Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1 TR000448 through The Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences at the National Institutes of Health. The contents are solely the responsibility of the authors and do not necessarily represent the official view of, CDC, NCATS or NIH.
The authors would like to acknowledge the contributions of Michael Climo, MD, (Holmes McGuire Veterans Affairs Medical Center, Richmond, Virginia); Cherie Hill and Dorothy Sinclair (Washington University School of Medicine, Saint Louis, Missouri).
Footnotes
Potential conflicts of interest.
V.F. reports that she has been a consultant to Battelle, has received grant funding from CDC, NIH, AHRQ and the BJH Foundation, and that her spouse is employed by and holds shares in Express Scripts. All other authors report no conflicts of interest relevant to this article.
Reference List
- (1).Anderson DJ, Kaye KS, Chen LF, et al. Clinical and financial outcomes due to methicillin resistant Staphylococcus aureus surgical site infection: a multi-center matched outcomes study. PLoS One. 2009;4:e8305. doi: 10.1371/journal.pone.0008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31:365–373. doi: 10.1086/651094. [DOI] [PubMed] [Google Scholar]
- (3).Rubio-Terres C, Garau J, Grau S, Martinez-Martinez L. Cost of bacteraemia caused by methicillin-resistant vs. methicillin-susceptible Staphylococcus aureus in Spain: a retrospective cohort study. Clin Microbiol Infect. 2010;16:722–728. doi: 10.1111/j.1469-0691.2009.02902.x. [DOI] [PubMed] [Google Scholar]
- (4).de Kraker ME, Wolkewitz M, Davey PG, et al. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2011;55:1598–1605. doi: 10.1128/AAC.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hanberger H, Walther S, Leone M, et al. Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int J Antimicrob Agents. 2011;38:331–335. doi: 10.1016/j.ijantimicag.2011.05.013. [DOI] [PubMed] [Google Scholar]
- (6).MORTIMER EA, Jr., LIPSITZ PJ, WOLINSKY E, GONZAGA AJ, RAMMELKAMP CH., Jr. Transmission of staphylococci between newborns. Importance of the hands to personnel. Am J Dis Child. 1962;104:289–295. doi: 10.1001/archpedi.1962.02080030291012. [DOI] [PubMed] [Google Scholar]
- (7).Humphreys H. Can we do better in controlling and preventing methicillin-resistant Staphylococcus aureus (MRSA) in the intensive care unit (ICU)? Eur J Clin Microbiol Infect Dis. 2008;27:409–413. doi: 10.1007/s10096-008-0469-7. [DOI] [PubMed] [Google Scholar]
- (8).Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- (9).Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006;166:306–312. doi: 10.1001/archinte.166.3.306. [DOI] [PubMed] [Google Scholar]
- (10).Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Batra R, Cooper BS, Whiteley C, Patel AK, Wyncoll D, Edgeworth JD. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis. 2010;50:210–217. doi: 10.1086/648717. [DOI] [PubMed] [Google Scholar]
- (12).Fraser TG, Fatica C, Scarpelli M, et al. Decrease in Staphylococcus aureus colonization and hospital-acquired infection in a medical intensive care unit after institution of an active surveillance and decolonization program. Infect Control Hosp Epidemiol. 2010;31:779–783. doi: 10.1086/654001. [DOI] [PubMed] [Google Scholar]
- (13).Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol. 2007;28:1155–1161. doi: 10.1086/520102. [DOI] [PubMed] [Google Scholar]
- (14).Sandri AM, Dalarosa MG, Ruschel de AL, da Silva EL, Zavascki AP. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol. 2006;27:185–187. doi: 10.1086/500625. [DOI] [PubMed] [Google Scholar]
- (15).Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Evans HL, Dellit TH, Chan J, Nathens AB, Maier RV, Cuschieri J. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg. 2010;145:240–246. doi: 10.1001/archsurg.2010.5. [DOI] [PubMed] [Google Scholar]
- (17).Gould IM, MacKenzie FM, MacLennan G, Pacitti D, Watson EJ, Noble DW. Topical antimicrobials in combination with admission screening and barrier precautions to control endemic methicillin-resistant Staphylococcus aureus in an Intensive Care Unit. Int J Antimicrob Agents. 2007;29:536–543. doi: 10.1016/j.ijantimicag.2006.12.019. [DOI] [PubMed] [Google Scholar]
- (18).Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007;167:2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- (19).Warren DK, Guth RM, Coopersmith CM, Merz LR, Zack JE, Fraser VJ. Epidemiology of methicillin-resistant Staphylococcus aureus colonization in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2006;27:1032–1040. doi: 10.1086/507919. [DOI] [PubMed] [Google Scholar]
- (20).Perry AG, Potter PA. Clinical Nursing Skills and Techniques. Fifth Edition Mosby; 2001. [Google Scholar]
- (21).Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol. 2010;31:584–591. doi: 10.1086/652530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Andrews DWK, Ploberger W. Optimal tests when a nuisance parameter is present only under the alternative. Econometrica. 1994;62:1383–1414. [Google Scholar]
- (23).Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30:959–963. doi: 10.1086/605925. [DOI] [PubMed] [Google Scholar]
- (24).Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381:1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]