Abstract
Rationale
Delaying presentation of a drug can decrease its effectiveness as a reinforcer, but the effect of delaying punishment of drug self-administration is unknown.
Objective
This study examined whether a histamine injection could punish cocaine self-administration in a drug-drug choice, whether delaying histamine would decrease its effectiveness, and whether the effects of delay could be described within a delay discounting framework.
Methods
Monkeys were implanted with double-lumen catheters to allow separate injection of cocaine and histamine. In discrete trials, subjects first chose between cocaine (50 or 100 μg/kg/inj) alone and an injection of the same dose of cocaine followed immediately by an injection of histamine (0.37–50 μg/kg). Next, they chose between cocaine followed immediately by histamine and cocaine followed by an equal but delayed dose of histamine.
Results
When choosing between cocaine alone and cocaine followed immediately by histamine, preference increased with histamine dose from indifference to > 80% choice of cocaine alone. When choosing between cocaine followed by immediate histamine and cocaine followed by delayed histamine, monkeys showed strong position preferences. When delayed histamine was associated with the non-preferred position, preference for that option increased with delay from ≤ 30% to > 85%. The corresponding decrease in choice of the preferred position was well described by a hyperboloid discounting function.
Conclusions
Histamine can function as a punisher in the choice between injections of cocaine and delay can decrease its effectiveness as a punisher. The effects of delaying punishment of drug self-administration can be conceptualized within the delay discounting framework.
Keywords: cocaine, self-administration, punishment, histamine, delay, delay discounting
The effectiveness of a reinforcer is a decreasing function of the delay until its delivery (see Lattal 2010 for a review). When assessed using choice procedures, this phenomenon is termed delay discounting (Ainslie 2001; Mazur 1987; Myerson and Green 1994; Rachlin 2006). There is increasing evidence that a hyperboloid discounting model provides an excellent description of how preference for a reward decreases as a function of the delay until its receipt (see Green and Myerson 2004, for a review):
| (Equation 1) |
where V is the value of an outcome when it is delivered after a delay (D), expressed as a proportion of its value when it is delivered immediately; the parameter k reflects how steeply the outcome is discounted, and the parameter s reflects the nonlinear scaling of time and amount.
The phenomenon of delay discounting has been observed in both human and nonhuman subjects and has assumed an increasingly prominent theoretical role in the study of drug abuse. This may be in large part because substance abusers have been shown to discount delayed reinforcers more steeply than non-abusing control subjects (e.g., Coffey et al. 2003; Madden et al. 1997; see Reynolds, 2006, for a review). It has been argued that steep discounting in drug abusers contributes to the choice to self-administer a drug with its immediate effects rather than choosing competing, but more delayed non-drug reinforcers (Bickel and Marsch 2001).
Recent research with nonhuman subjects also supports the role of delay discounting in drug self-administration (see Perry and Carroll 2008, for a review). The hyperboloid discounting model has been shown to describe the choices of rats and pigeons responding for food and liquid reinforcers (Green et al. 2004; Mazur 2000; Richards et al. 1997), and we have extended this finding to monkeys choosing between immediate and delayed injections of cocaine (Woolverton et al. 2007). Notably, Perry et al. (2005) found that rats that discounted delayed food more steeply were subsequently more likely to self-administer cocaine than rats that discounted food less steeply, suggesting that high rates of delay discounting are predictive of drug self-administration (Carroll et al. 2010).
The effectiveness of a punisher, like that of a positive reinforcer, is a decreasing function of delay until its delivery (see Azrin and Holz, 1966). Early studies with non-human subjects focused on a single operant situation (e.g., Baron 1965; Cohen 1968), but similar effects of delay have been observed in a choice situation (e.g., Deluty, 1978). In human subjects, there has been growing interest in the nature of the relationship between delay and value when choices are between losses (punishers), and how this relationship compares to that between delay and value for gains (reinforcers; e.g., Estle et al. 2006; Holt et al. 2008; Murphy et al. 2001). Discounting functions for both gains and losses have consistently been shown to be better described by the hyperboloid model than by the exponential discounting function derived from the expected utility model favored by economists (Green and Myerson 2004).
The discounting of delayed punishers would seem particularly relevant to the choice to take a drug of abuse. Drug taking has consequences beyond the immediate effect of the drug itself (e.g., hangovers, incarceration, accidents, adverse health and social consequences) that could function as punishers to suppress drug taking. These consequences are often delayed, however, and their effectiveness may be diminished by that delay. Although the number of empirical studies that directly address this point is limited, Odum et al. (2002) reported that smokers discounted hypothetical health losses in a manner that was predicted by the hyperboloid discounting model and that they did so more steeply than never-smokers did. The clear implication is that delayed health losses are not particularly effective punishers of smoking, at least among current smokers. With regard to non-humans, it is known that electric shock can suppress drug taking under single-operant (Bergman and Johanson 1981; Grove and Schuster 1974) and choice (Johanson 1977) situations. However, we are not aware of any research with non-humans on the effect of delay on punishment of drug self-administration.
The present study examined the effect of delay of punishment on the self-administration of cocaine using a method for studying the punishing effects of histamine that we developed previously (Woolverton 2003; see also Negus 2005). In Phase 1, monkeys chose between an injection of cocaine alone and an injection of the same dose of cocaine followed immediately by an injection of histamine. We hypothesized that histamine injections would punish cocaine self-administration and that the effectiveness of histamine punishment would increase with the dose of histamine. In Phase 2, monkeys chose between an injection of cocaine that was followed immediately by a histamine injection and an injection of cocaine that was followed by a delayed histamine injection. For each monkey, the dose of histamine was one that had been determined to be an effective punisher in the previous phase. We hypothesized that delayed histamine would be a less effective punisher than immediate histamine and that the hyperboloid discounting model (Green and Myerson, 2004) would describe the decrease in punishment effectiveness as a function of delay.
Materials and Methods
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (1996).
Subjects
Five male rhesus monkeys, weighing between 7.6 and 12.8 kg, served as subjects. Monkeys 4296, SBR115 and 4300 were experimentally naïve at the start of the study. The other two monkeys had significant histories of lever pressing maintained by drug and/or non-drug reinforcers, most recently as reported in Freeman et al. (2009; monkey BDH) and Freeman et al. (2010; monkey M341). Monkey M341 lost his last catheter before completing the second phase of the study and therefore data only from the first phase are reported. Each monkey had continuous access to water, was fed monkey chow and fresh fruit at 07:00 each day in amounts sufficient to maintain stable body weights, and was given a multi-vitamin tablet 3 days/week. Animal rooms were maintained on a 16:8 hour light:dark cycle with lights on at 06:00.
Apparatus
Each monkey was housed in a ventilated cubicle (Plas-Labs, 1.0 m3) that also served as the experimental chamber. The door of the cubicle was clear plastic and had attached two metal boxes, 33 cm apart. Each box contained a primate response lever (PRL-001, BRS/LVE, Beltsville, MD) and four jeweled stimulus lights, two white and two red. Partway through the experiment, one monkey (4296) developed a strong preference for one lever. For that monkey, the two-lever arrangement was replaced with a custom-designed single “joy stick” type lever that could be pushed either to the left or the right and which had the white and red stimulus lights arranged vertically on each side of the manipulandum. Monkey M4296 completed the delay portion of the study with the joystick.
Each monkey was fitted with a mesh jacket and tether (Lomir Biomedical, Malone, NY) that attached to the rear of the cubicle. This system allowed the monkey essentially unrestricted movement and protected the catheter, which was attached to a double lumen swivel at the distal end of the tether. Outside the cubicle, the catheter was connected to pumps (7540X, Cole-Parmer Instrument Co, Chicago, IL) that injected drug solutions at a rate of approximately 1.5 ml/10 sec. Macintosh computers with custom software controlled experimental events and recorded data.
Procedure
For catheter implantation, each monkey was injected with atropine sulfate (0.04 mg/kg, i.m.) and ketamine hydrochloride (10 mg/kg, i.m.) followed 20–30 minutes later by inhaled isoflurane. When anesthesia was adequate, a double-lumen silicone catheter was implanted into a major vein (internal jugular, external jugular, or femoral) with the tip terminating near the right atrium. The distal end of the catheter was passed subcutaneously to the midscapular region, where it exited the subject’s back. After surgery, the monkey was returned to the experimental cubicle and the catheter was connected to the infusion pumps, one for cocaine injections and one for histamine injections. An antibiotic (usually Kefzol, Eli Lilly & Co., Indianapolis, IN) was administered twice daily for seven days post-operatively to prevent infection. If a catheter became nonfunctional during the experiment it was removed and a new catheter was implanted after a 1–2 week period to allow any infection to clear. Catheters were filled between sessions with a solution of 40 units/ml heparin to help prevent clotting at the catheter tip.
Experimental sessions began at 12:00 each day and were conducted seven days a week. Sessions began with four forced-choice (“sampling”) trials, which were followed by 20 free-choice trials. On forced-choice trials, the white lights were illuminated for one option only and a single response on the corresponding lever (FR 1) extinguished the white lights. The cocaine injection associated with that option began immediately and was delivered over 10 sec. When a histamine injection was programmed to follow the cocaine injection after a delay, the histamine injection was delivered over 8 sec after the delay elapsed. No signal was presented during the delay. During both the cocaine and histamine injections, the red lights were continuously illuminated over the operative lever only. An inter-trial interval (ITI) was programmed to begin with the lever press (as opposed to the end of the injection). That is, the ITI and the delay ran simultaneously to ensure that trials were evenly spaced and that reinforcement density did not covary with delay and to minimize drug accumulation over the session. Free-choice trials were identical to forced-choice trials except that the white lights for both options were illuminated until one was chosen, and different injections and/or different delays were delivered as a consequence of responding on one or the other lever.
In Phase 1 of the experiment, we examined the punishing effect of histamine on cocaine self-administration. One choice option consisted of cocaine only (in different conditions, either 50 or 100 μg/kg/inj), whereas the other option consisted of the same dose of cocaine followed immediately (1-sec between injections) by either saline or different doses of histamine (0.37–50 μg/kg/inj). Histamine doses were tested in an irregular order across monkeys. The ITI was 10 min for this phase of the study.
In Phase 2 of the experiment, the ITI was increased to 20 min and we examined the effect on cocaine choice of delaying a histamine injection. For both choice options, a histamine injection (6, 12 or 25 μg/kg) followed the cocaine injection. For one option, the histamine injection followed cocaine immediately, whereas for the other option there was a delay between the cocaine and histamine injections. A relatively long delay (360 or 720 sec) was tested first in all monkeys to verify that a long delay would be effective. Monkey 4296 did not initially prefer the delayed-histamine option and, for this monkey, choice of the cocaine-only option (as in Phase 1) was re-established. Next, cocaine followed by a low dose of delayed histamine was substituted for the cocaine-only option. Then the dose of the delayed histamine injection was gradually increased to the same level as the immediate dose until choice was stable and maintained after a position reversal. Shorter delays were then tested in an irregular order across monkeys.
Each experimental condition was in effect for at least four sessions and until choice was stable for three consecutive sessions. Stability was defined as three consecutive sessions in which the number of choices of the preferred option was within ± 3 of the three-session mean and in which there was no upward or downward trend. After preference for one option was stable, the positions of the two options were reversed.
Data Analysis
For each monkey, mean percent choices of the cocaine-only option in Phase 1 and of the delayed-histamine option in Phase 2 were calculated for the last three sessions of a condition and its reversal. Both histamine dose-response functions and delay-response functions were analyzed individually using least squares linear regression. Because no preference (i.e, 50% choice) is equivalent to no effect, ED75 values (i.e., 75% choice) were estimated based on the regression of percent choice on log histamine dose in Phase 1 and provided a measure of sensitivity to the punishing effect of histamine.
To examine the applicability of the delay discounting model to punishment, the percentage of trials on which monkeys chose the immediate histamine option when it was associated with the preferred position served as a measure of the effectiveness of histamine as a punisher. Changes in punisher effectiveness as a function of delay were analyzed by fitting a hyperboloid discounting function to the data (Equation 1).
Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse. Histamine dihydrochloride was purchased from Sigma Aldrich, Inc. Both were dissolved in 0.9% saline in concentrations appropriate to doses. Drug dose was varied by varying the concentration of the drug solution.
Results
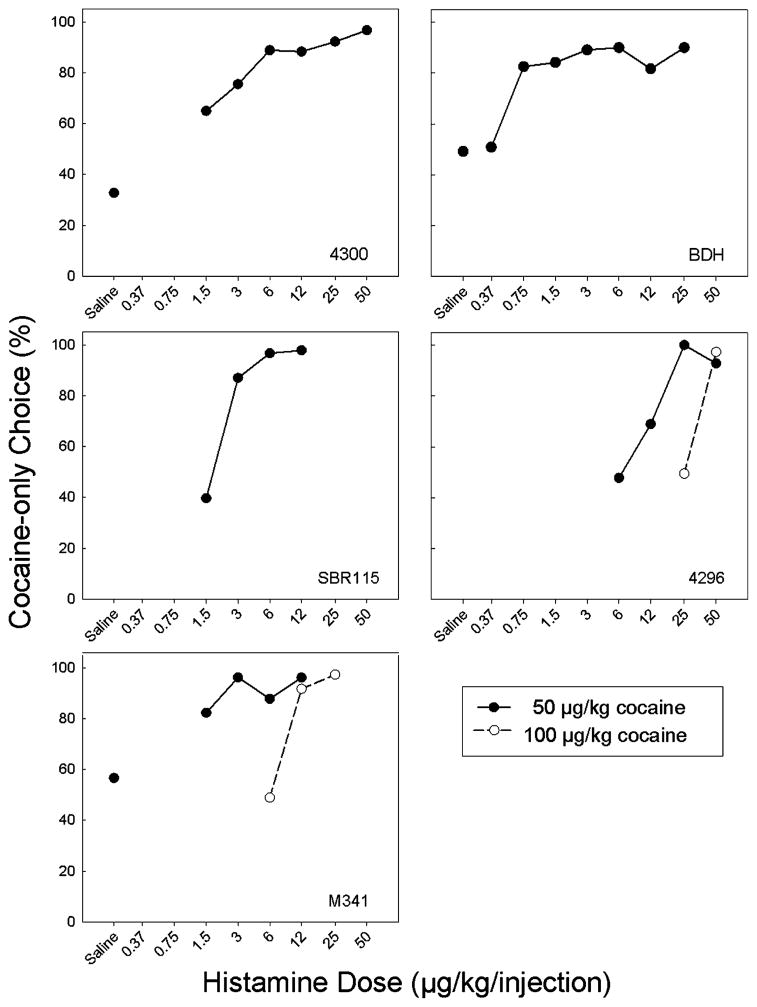
The monkeys completed all trials in all sessions of both Phase 1 and Phase 2. Sessions to stability ranged between four and 22 over the entire experiment. In Phase 1, three of the monkeys (4300, BDH and M341) showed no clear preference between an injection of 50 μg/kg of cocaine alone and the same cocaine dose followed immediately by an injection of saline. The other two monkeys (4296 and SBR115) were not tested with a saline option but showed no clear preference between the cocaine injection alone and the same dose of cocaine followed immediately by an injection of a low dose of histamine (1.5 and 6 μg/kg; see Figure 1). All five monkeys showed an increase in choice of the cocaine-only option as histamine dose was increased with preference for the cocaine-only option increasing to near 100% in all cases. ED75 values for histamine were 0.6 μg/kg (BDH), 3.0 μg/kg (4300, SBR 115) and 12 μg/kg (4296). The ED75 value was not calculated for M341 since choice levels below 75% were not seen with any tested dose of histamine. Monkey BDH was unusually sensitive to histamine, with a clear preference for the cocaine-only option when the histamine dose was 0.75 μg/kg. Two of the monkeys (M341 and 4296) were tested with a cocaine dose of 100 μg/kg/inj in addition to the dose of 50 μg/kg/inj, With the higher dose of cocaine, the histamine dose-response function was shifted to the right relative to that seen when the dose of cocaine was 50 μg/kg, indicating that higher doses of histamine were required to punish responding maintained by a higher dose of cocaine. There were no post-session effects of histamine on food intake.
Figure 1.
Dose-response functions for rhesus monkeys choosing between cocaine (50 or 100 μg/kg/inj) followed immediately by an injection of saline or histamine at the indicated doses, and the same dose of cocaine alone. Symbols represent the mean percent choice of the cocaine-only option averaged across the last 3 sessions of a dose condition and its reversal (i.e., 6 total sessions).
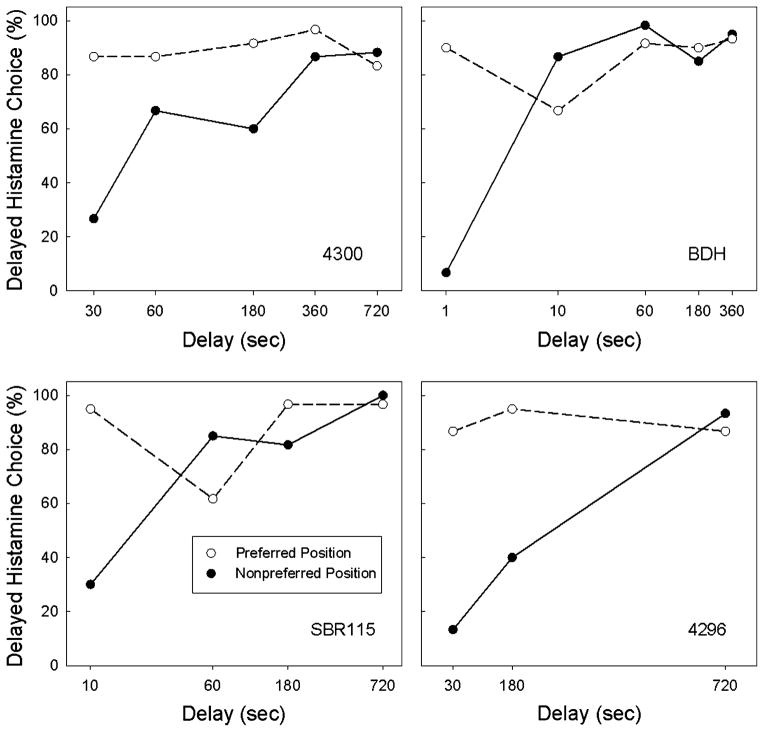
In Phase 2, the options were either cocaine followed immediately by histamine or cocaine followed by the same dose of histamine but delivered after a delay. Under these conditions, every monkey developed a position bias such that one of the two positions was preferred even when it was associated with histamine with a brief delay; choice of the preferred position was greater than 85%, averaged across delays, for each monkey. Choice of that position was not systematically affected by the delay to the histamine injection (Figure 2, open symbols). Across the four monkeys, the change in percent choice from the shortest to the longest delay ranged from −3.4% to 3.0%. Because of these position preferences, analysis focused on the effect of delay until the histamine injection on choice of the other, non-preferred position when it was associated with delayed histamine. Choice of the non-preferred position increased systematically as a function of the delay (Figure 2, solid symbols) in all monkeys. Monkey BDH was particularly sensitive to delay, choosing its non-preferred position 6.7% of the time when either choice resulted in cocaine followed by histamine 1 second later, but choosing this option on 86.7% of the trials when the delay was 10 seconds.
Figure 2.
Effects of delay on choice between cocaine (50 μg/kg/inj) followed by an immediate injection of histamine and the same dose of cocaine followed by a delayed injection of histamine. For monkeys 4300, BDH, SBR115, and 4296 the histamine dose was 6, 12, 12, and 25 μg/kg, respectively. Solid symbols and open represent the percent choice (based on the last 3 sessions of a condition) of the non-preferred and preferred positions, respectively, when the delayed histamine was associated with those positions. Note that delay is plotted on a logarithmic scale.
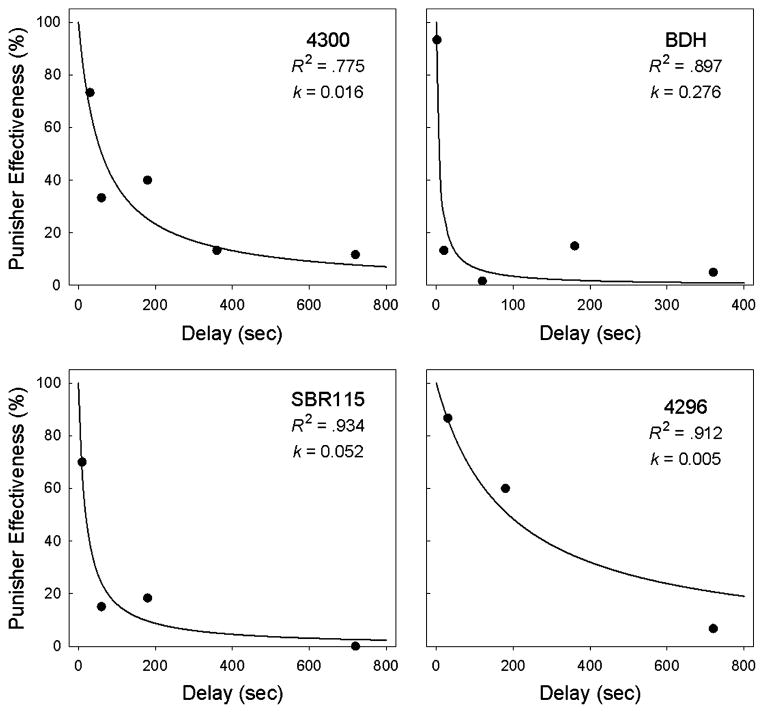
To examine the effects of delay to histamine on punisher effectiveness within the delay discounting framework, we analyzed the decrease in the percentage of trials on which monkeys chose the immediate histamine option when it was associated with the preferred position as a function of the delay to histamine associated with the non-preferred position (i.e., the complement of the data represented by the solid symbols in Figure 2). As predicted by the hyperboloid model, discounting was negatively accelerated (see Figure 3) and Equation 1 accounted for a significant proportion of the variance in the data with R2s ranging from 0.77 to 0.93.
Figure 3.
Discounting functions for rhesus monkeys choosing between cocaine (50 μg/kg/inj) followed by an immediate injection of histamine and the same dose of cocaine followed by a delayed injection of histamine. Punisher effectiveness is measured as the percentage choice of the preferred position, as a function of delay when the delayed histamine option is associated with the non-preferred position. Note that, in contrast to Figure 2, delay is plotted on a linear scale.
Discussion
The present study examined the effect of punishment on cocaine self-administration and whether the role of delay in modulating such punishment may be usefully examined within the context of the delay discounting framework. Given a choice between a cocaine injection that was followed immediately by a histamine injection and the same injection of cocaine alone in Phase 1, monkeys preferred the injection of cocaine alone, suggesting that delivering a histamine injection after a cocaine injection punished cocaine self-administration. Moreover, the frequency with which monkeys chose the cocaine-only option increased systematically as the dose of histamine increased. When given a choice between cocaine only and cocaine followed by a saline injection (or in some cases, a low dose of histamine), the monkeys were relatively indifferent, indicating that simply delivering a second injection after a cocaine injection was not punishing in and of itself.
Histamine has previously been shown to punish food-maintained responding in both single operant and choice situations (Goldberg, 1980; Katz and Goldberg 1986; Negus 2005; Woolverton 2003). In addition, histamine has been shown to punish cocaine self-administration in monkeys given a choice between cocaine and food (Negus 2005). The results of the present experiment demonstrate that histamine can also function as a punisher of cocaine self-administration when monkeys choose between injections of cocaine. There were individual differences in sensitivity to histamine, with ED75s ranging between 0.6 and 12 μg/kg. Overall, effective doses of histamine in the present study were comparable to the doses of histamine that previously have been shown to punish cocaine self-administration when monkeys choose between cocaine and food (Negus, 2005).
In Phase 2, monkeys were given a choice between a cocaine injection followed immediately by histamine and cocaine followed by the same dose of histamine injected after a delay. We hypothesized that adding a delay between a cocaine injection and its punisher would diminish the effectiveness of that punisher. In fact, histamine injected with relatively long delays after cocaine (360 or 720 seconds) proved to be ineffective as a punisher of cocaine self-administration in the present study. When the delay to the histamine injection was brief, and thus the difference between the alternatives was relatively small, all of the monkeys showed strong position preferences. Such position preferences have been amply documented in monkeys responding under conditions similar to those used here (e.g., Iglauer and Woods, 1974; Johanson and Schuster 1975). Although the mechanisms that determine position preferences are unclear, they are most often observed when the differences between the options are relatively small.
When the delayed histamine was associated with the preferred position, increasing the delay had little effect because choice of that option was already near ceiling. In contrast, when the delayed histamine was associated with the non-preferred position, choice of that option increased systematically as a function of the delay to the histamine injection, indicating that the effectiveness of histamine as a punisher of that option decreased. This result was observed in all monkeys, consistent with the hypothesis that a delay between drug self-administration and a punisher weakens the effectiveness of the punisher. Interestingly, the delays that affected choice of cocaine self-administration in the present experiment were comparable to those that were effective in decreasing choice of a delayed cocaine injection in previous experiments (Woolverton and Anderson 2006; Woolverton et al. 2007). That is to say, delays of approximately the same duration reduced the effectiveness of both punishment and positive reinforcement of cocaine self-administration.
We had hypothesized not only that the effectiveness of histamine punishment would decrease as a function of delay, but also that the decrease would be described by the hyperboloid discounting model (Green and Myerson, 2004). Consistent with this hypothesis, increases in choice of the non-preferred option as the delay to histamine injection showed a negatively accelerated increase, reflecting a nonlinear decrease in punishment effectiveness that was well described by the hyperboloid model (Equation 1; see Figure 3). In addition to extending the generality of the discounting framework to situations involving delayed punishment of drug self-administration, these results are important because of the potential implications of the form of the discounting function for our understanding of the effects of punishment on drug taking.
More specifically, the hyperboloid discounting function predicts the occurrence of preference reversals that are not predicted by other forms of discounting (e.g., exponential). For example, a substance abuser might agree to quit next week at which point he (or she) would experience the negative (but time-limited) consequences of abstinence rather than use drugs and experience the larger, but later (and perhaps longer lasting) negative consequences for social and family relationships. Nevertheless, come next week he may choose to continue to use drugs, despite his previously stated preference. Such preference reversals have been typically discussed with respect to choices between smaller, sooner and larger, later rewards, but they have been shown to occur with smaller, sooner and larger, later losses as well (Holt et al. 2008). Thus, the hyperboloid model explains the preference reversals often associated with substance abuse as a consequence of the discounting of delayed punishment.
Discounting research with substance abusers has also focused on delayed rewards, and the steep discounting of such rewards by substance abusers is often assumed to underlie their substance abuse (e.g., Bickel and Marsch, 2001). The present results may serve as a reminder that the negative consequences of substance abuse are governed by similar principles, and these principles must be taken into account in our effort to understand and change such problem behavior. Although the use of punishment (e.g., arrest and incarceration) to influence the choice to take a drug is common, there has been little systematic investigation of its theoretical underpinnings. The discounting framework would appear to provide such underpinnings. Individuals’ tendency to discount delayed punishment may also play a crucial role in their escalating drug use and may contribute to their vulnerability to drug abuse. For purposes of investigating the dynamics of the control of drug taking by punishment, an animal model will be particularly useful, and a behavioral preparation in which histamine drug injections are used as a punisher of monkeys’ drug self-administration would appear to provide such a model.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant R21-DA-0026832 to W.L.W. Preparation of the manuscript also was supported in part by National Institute of Mental Health Grant MH055308. We gratefully acknowledge Lee Hutson, Emily Partridge and Jennifer Naylor for their expert technical assistance.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Ainslie G. Breakdown of will. Cambridge, England: Cambridge University Press; 2001. [Google Scholar]
- Azrin NH, Holz WC. Punishment. In: Honig WK, editor. Operant Behavior: Areas of Research and Application. Prentice-Hall, Inc; Englewood Cliffs, NJ: 1966. pp. 380–447. [Google Scholar]
- Baron A. Delayed punishment of a runway response. J Comp Physiol Psychol. 1965;60:131–134. doi: 10.1037/h0022326. [DOI] [PubMed] [Google Scholar]
- Bergman J, Johanson CE. The effects of electric shock on responding maintained by cocaine in rhesus monkeys. Pharmacol Biochem Behav. 1981;14:423–426. doi: 10.1016/0091-3057(81)90413-5. [DOI] [PubMed] [Google Scholar]
- Bickel W, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL. Delay discounting as a predictor of drug abuse. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. American Psychological Assoc; Washington, DC: 2010. pp. 243–271. [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharm. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cohen PS. Punishment: The interactive effects of delay and intensity of shock. J Exp Anal Behav. 1968;11:789–799. doi: 10.1901/jeab.1968.11-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluty MZ. Self-control and impulsiveness involving aversive events. J Exp Psychol: Anim Behav Proc. 1978;4:250–266. [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Differential effects of amount on temporal and probability discounting of gains and losses. Mem Cogn. 2006;34:914–928. doi: 10.3758/bf03193437. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Green L, Myerson J, Woolverton WL. Delay discounting of saccharin in rhesus monkeys. Behav Proc. 2009;82:214–218. doi: 10.1016/j.beproc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Wang Z, Woolverton WL. Self-administration of (+)-methamphetamine and (+)-pseudoephedrine, alone and combined, by rhesus monkeys. Pharmacol Biochem Behav. 2010;95:198–202. doi: 10.1016/j.pbb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR. Histamine as a punisher in squirrel monkeys: effects of pentobarbital, chlordiazepoxide, and H1- and H2- receptor antagonsist on behavior and cardiovascular responses. J Pharmacol Exp Ther. 1980;214:726–736. [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? J Exp Anal Behav. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2:199–208. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J, Estle SJ. Preference reversals with losses. Psychon Bull Rev. 2008;15:89–95. doi: 10.3758/pbr.15.1.89. [DOI] [PubMed] [Google Scholar]
- Iglauer C, Woods JH. Concurrent performances: Reinforcement by different doses of intravenous cocaine in rhesus monkeys. J Exp Anal Behav. 1974;22:179–196. doi: 10.1901/jeab.1974.22-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE. The effects of electric shock on responding maintained by cocaine in a choice procedure in the rhesus monkey. Psychopharmacology. 1977;53:277–282. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Katz JL, Goldberg SR. Effects of H1-receptor antagonists on responding punished by histamine injection of electric shock presentation in squirrel monkeys. Psychopharmacology. 1986;90:461–467. doi: 10.1007/BF00174061. [DOI] [PubMed] [Google Scholar]
- Lattal KA. Delayed reinforcement of operant behavior. J Exp Anal Behav. 2010;93:129–139. doi: 10.1901/jeab.2010.93-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Exp Clin Psychopharm. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mazur JE. The effect of delay and intervening events on reinforcement value. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior. Vol. 5. Lawrence Erlbaum and Associates; NJ: 1987. pp. 55–73. [Google Scholar]
- Mazur JE. Tradeoffs among delay, rate, and amount of reinforcement. Behav Proc. 2000;49:1–10. doi: 10.1016/s0376-6357(00)00070-x. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Vuchnich RE, Simpson CA. Delayed reward and cost discounting. Psych Rec. 2001;51:571–588. [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: Models of individual choice. J Exp Anal Behav. 1994;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Bickel WK. Discounting of delayed health gains and losses by current, never- and ex-smokers of cigarettes. Nic Tobacco Res. 2002;4:295–303. doi: 10.1080/14622200210141257. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Rachlin H. Notes on discounting. J Exp Anal Behav. 2006;85:425–435. doi: 10.1901/jeab.2006.85-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL. A novel choice method for studying drugs as punishers. Pharmacol Biochem Behav. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Anderson KG. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2006;186:99–106. doi: 10.1007/s00213-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharm. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]