Abstract
Background
This study examined relationships between excess body weight (EBW) loss and current gait and functional status in women 5 years after Roux-en-Y gastric bypass surgery.
Methods
Gait data were analyzed in nine female bariatric patients for relationships with longitudinal changes in weight, body composition, and physical function assessed by the Short Musculoskeletal Functional Assessment (SMFA) questionnaire and the timed “get-up-and-go” (TGUG) test. Gait characteristics in the bariatric sample were also compared to an age- and BMI-matched nonsurgical reference sample from the Fels Longitudinal Study.
Results
Bariatric patients lost an average of 36.4 kg (61.1 %) of EBW between preoperative and 5-year follow-up visits (P <0.01); SMFA function index scores and TGUG times also decreased (both P <0.01). Degree of EBW loss was correlated with less time spent in initial double support and more time in single support (both P =0.02), and for all gait variables, the bariatric sample fell within the 95 % confidence intervals of gait/EBW relationships in the reference sample.
Conclusions
Gait and function 5 years after bariatric surgery were characteristic of current weight, not preoperative obesity, suggesting that substantial, sustained recovery of physical function is possible with rapid surgical weight loss.
Keywords: Bariatric surgery, Quantitative gait analysis, Function, Excess body weight, Obesity
Introduction
Significant functional complications of obesity include altered walking gait [1-4], which limits activities of daily living and health-related quality of life [5]. Functional impairment is especially severe with greater body mass index (BMI) and higher degrees of obesity (Class III obesity: BMI≥40.00) [1, 6, 7]. To reduce the impact of functional impairment on health-care costs and quality of life, lowering the rate of obesity rate is a major public health priority [8].
Diet and exercise are preferred weight control methods, but when these fail to produce sustained weight loss, bariatric surgery is an effective and increasingly common treatment for class III obesity [9]. Roux-en-Y gastric bypass (RYGB), historically the most common procedure, creates a small gastric pouch, a gastrojejunostomy, and a jejunojejunostomy [10, 11], leading to weight loss via accelerated satiety, altered gut hormonal responses, and significant restriction of caloric intake [12]. Typically, RYGB results in loss of 55-75 % of excess weight [13-15], which is associated with functional improvement shortly after surgery [4, 10, 16-18]. It is not well-known whether postsurgical functional gains are sustained over longer time periods.
To our knowledge, this is the first study to present data on long-term functional outcomes following RYGB bariatric surgery. This study uses quantitative gait analysis along with the Short Musculoskeletal Functional Assessment (SMFA) questionnaire and the timed “get-up-and-go” (TGUG) test to examine functional status in women 4- to -5 years after RYGB weight loss surgery. The sample includes women from South-west Ohio who previously participated in a 1-year follow-up-to-surgery study [10] and a comparative age- and BMI-matched nonsurgical reference sample from the Fels Longitudinal Study. The study tests the hypotheses that current gait in the postsurgery bariatric patients is normal for their current BMI and that current gait patterns are related to longitudinal changes in weight, body composition, and functional status.
Patients and Methods
Bariatric Sample
Recruitment targeted previous participants in a 1-year follow-up study of weight loss and function following RYGB surgery [10], and inclusion criteria for which were scheduled RYGB surgery, female sex, age of ≥20 years, and no previous bariatric surgery. Of 47 women in that study, 11 agreed to participate in the present study, and 2 dropped out before data collection, leaving N =9 women in the current sample. This follow-up rate is similar to other medium- to long-term studies of RYGB surgical outcomes [19]. Procedures and risks were described to participants, who gave informed consent prior to data collection. All procedures were approved by the local institutional review board.
Reference Sample
The reference sample (Fels, N =132) consisted of healthy female participants in the community-based Fels Longitudinal Study [20]. Since age and BMI are associated with variation in gait patterns [2, 21], the Fels sample was matched to the bariatric sample by including only participants within the current bariatric sample ranges (age, 25.5–73.5 years; BMI, 25.0–40.0 kg/m2); sample distributions did not differ (Kolmogorov-Smirnov, age, P =0.99; BMI, P =0.52). Participants were excluded for weight loss surgery; lower limb joint replacement; chronic gait-related neuromuscular or musculoskeletal disorders; toe walking; prescription shoe inserts; lower limb, pelvic, or vertebral skeletal injury ≤5 years before testing; or lower limb, pelvic, or back soft tissue injury ≤1 year before testing. Osteoarthritis (OA) was not exclusionary since obesity is a factor in the etiology of lower limb joint OA [22, 23]. Lower limb joint OA incidence was 33 % in the bariatric sample and 9 % in the Fels sample.
Body Composition and Anthropometric Measurements
Both samples were measured for height (in centimeter), sitting height (in centimeter), weight (in kilogram), and abdominal circumference (in centimeter) using standard methods [24]. BMI was calculated from weight and height as in kilogram per square meter. Ideal body weight (IBW) was calculated as IBW (kg) = height – 100 – [(height – 150)/2] [25]. Excess body weight (EBW) was calculated as EBW (kg) = weight – IBW. Lower limb length (in centimeter) was calculated as height – sitting height. Dual-energy X-ray absorptiometry (DXA) measured fat mass (in kilogram) and fat-free mass (in kilogram).
Short Musculoskeletal Functional Assessment Questionnaire
The SMFA test [26] of functional status was administered to the bariatric sample only. This questionnaire results in two indices: the bother index evaluates the degree to which individuals are bothered by musculoskeletal conditions in daily life, and the function index gauges functional limitations in activities of daily living. Higher scores indicate greater bother or poorer function.
Timed Get-Up-and-Go Test
The TGUG is a validated test of balance and function [27, 28] and was administered to the bariatric sample only. Participants are timed as they rise from a seated starting position, stand and walk at 3 m, turn without assistance, and walk back and return to the seated position. Deviations from confident, normal performance are noted. Participants are not formally trained before testing.
Quantitative Gait Analysis
Spatiotemporal gait variables were measured in the Motion Analysis Laboratory at the Wright State University Boonshoft School of Medicine. The lab is equipped with a six-camera three-dimensional quantitative gait analysis system (Motion Analysis Corp., Santa Rosa, CA), which records external passive reflective markers placed according to the Helen Hayes Marker System [29]. Gait data were processed using Cortex and OrthoTrak software (Motion Analysis Corp., Santa Rosa, CA) and were normalized for lower limb length [30]. Additional equipment and methodological details are described elsewhere [31].
Timing of Measurements
In the bariatric sample, data on weight, height, abdominal circumference, TGUG, and the SMFA were collected at both preoperative and follow-up visits. Longitudinal changes in these variables were calculated as value at follow-up – preoperative value. Fat mass and fat-free mass from DXA, as well as sitting height and gait variables, were measured only at the bariatric sample’s follow-up visit. In the Fels sample, all measurements were taken at a single visit.
Statistical Analysis
Analyses were two-sided with significance set to α =0.05, performed using SAS version 9.3 (SAS, Inc., Cary, NC). Due to sample size and nonnormal distributions of variables in the bariatric sample, nonparametric methods were used for most tests. In the bariatric sample, changes in body composition, anthropometric variables, and functional status from the preoperative visit to the follow-up visit were analyzed using Wilcoxon signed rank tests. Differences in means between Fels and bariatric participants at follow-up were assessed using Wilcoxon two-sample tests. In the bariatric sample, Spearman’s partial rank correlation determined associations between variation in gait parameters and changes from preoperative to follow-up visits in functional status, weight, BMI, EBW, and abdominal circumference. In the Fels sample, linear regression analysis determined relationships between gait variables and BMI. Bariatric sample gait data were plotted against the Fels regression lines and their 95 % prediction intervals to assess whether bariatric participants fell within the Fels sample’s range of variation.
Results
In the bariatric sample, average time between the preoperative visit and the follow-up visit was 4.8±0.3 years; average time between surgery and follow-up was 4.7±0.2 years. Prior to surgery, one bariatric participant was classified as class II obese (BMI=35.00-39.99), and eight were class III obese (BMI≥40.00) by World Health Organization standards [6]. From the preoperative visit to the follow-up visit, bariatric participants exhibited significant reductions in weight, EBW, BMI, and abdominal circumference (for each P ≤0.01; see Table 1). On average, bariatric participants lost 36.4 kg or 61.1 % of EBW (range, 11.6-55.7 kg; 19-87 %). At the follow-up visit, six participants were classified as pre-obese (BMI=25.00-29.99), one was class I obese (BMI=30.00-34.99), one was class II obese, and one was class III obese. Change in the SMFA bother index over the same time period did not reach significance (P =0.06), but there were significant improvements in function, with an average reduction in SMFA function index scores of 32.5 points and a decrease in average TGUG time of 3.8 s (both P ≤0.01).
Table 1.
Body composition and function in the bariatric sample before and 5 years after surgery [mean ± SD (range)]
| Preoperative | Five years | |
|---|---|---|
| Weight (kg) | 115.5±8.5 (98.0–129.8) | 78.8±14.4 (64.4–103.2)* |
| EBW (kg) | 59.6±8.3 (43.0–73.5) | 23.2±14.9 (8.1–48.2)* |
| BMI (kg/m2) | 44.2±3.2 (38.3–49.1) | 30.4±6.0 (25.0–40.8)* |
| Abdominal circumference (cm) | 119.2±8.8 (105.0–132.0) | 98.6±13.5 (82.2–122.9)* |
| TGUG (s) | 13.0±3.5 (9.0–20.7) | 9.2±2.4 (6.4–13.4)* |
| SMFA bother index | 41.4±21.2 (17.0–75.0) | 29.6±28 (0.0–65.0)** |
| SMFA function index | 56.0±17.0 (34.0–81.0) | 23.5±21 (0.0–57.0)* |
P ≤0.01, a significant change from preop to 5 years postop;
P=0.06, a change not significantly different
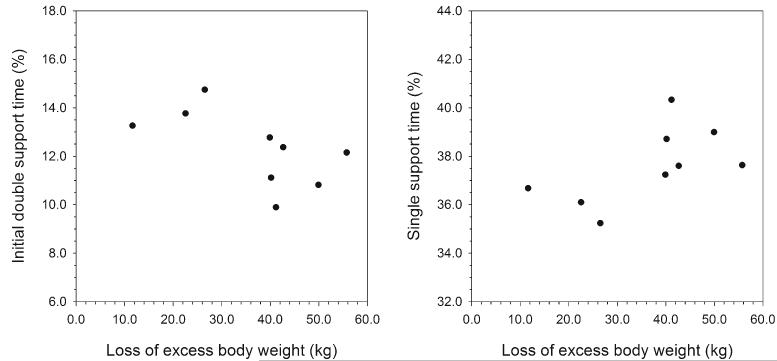
Table 2 presents results from the bariatric sample for Spearman’s partial correlation analysis, controlling for age, between gait variables and changes from the preoperative visit to the follow-up visit in body composition, anthropometric variables, TGUG, and SMFA indices. Percentages of the gait cycle spent in initial double support and in single support were significantly correlated with absolute changes in EBW (see Fig. 1), weight, BMI (P =0.02 for all), and abdominal circumference (P =0.01). Neither initial double support time nor single support time was significantly correlated with changes in SMFA bother or function indices (bother, P =0.34; function, P =0.09), change in TGUG time (P =0.25), or percent EBW loss (P =0.08). None of the other gait variables were significantly correlated with changes in body composition, anthropometric variables, or SMFA indices, and the pattern of correlations remained the same even when not controlling for age.
Table 2.
Correlations between gait and body composition and anthropometric and functional changes in the bariatric sample
| Gait variables | Preoperative to 5-year follow-up changes |
||||
|---|---|---|---|---|---|
| EBW (kg)a | Abdominal circumference (cm) | TGUG (sec) | SMFA bother index | SMFA function index | |
| Normalized forward velocity | 0.23 | 0.40 | −0.13 | 0.37 | 0.25 |
| Normalized cadence | 0.19 | 0.35 | −0.06 | 0.06 | 0.13 |
| Normalized step length | 0.21 | 0.35 | −0.36 | 0.67 | 0.46 |
| Normalized step width | 0.11 | 0.13 | −0.32 | 0.22 | 0.30 |
| Initial double support time (%) | −0.83* | −0.87** | −0.50 | −0.42 | −0.69 |
| Single support time (%) | 0.83* | 0.87** | 0.50 | 0.42 | 0.69 |
Spearman’s correlation coefficients (rs) are presented. Gait variables were measured at 5-year follow-up visit. Preoperative to 5-year follow-up changes are calculated as value at preoperative visit – value at 5-year follow-up visit
P ≤0.05;
P ≤0.01.
Statistical results for weight and BMI change were identical to results for EBW, so only EBWis presented
Fig. 1.
Percent of the gait cycle spent in initial double support and single support plotted against excess body weight loss between the preoperative and 5-year follow-up visits in the bariatric sample. EBW was significantly correlated (Spearman’s partial rank correlation) with both support times (P=0.02 for each; initial double support time, rs=−0.83; single support time, rs=0.83)
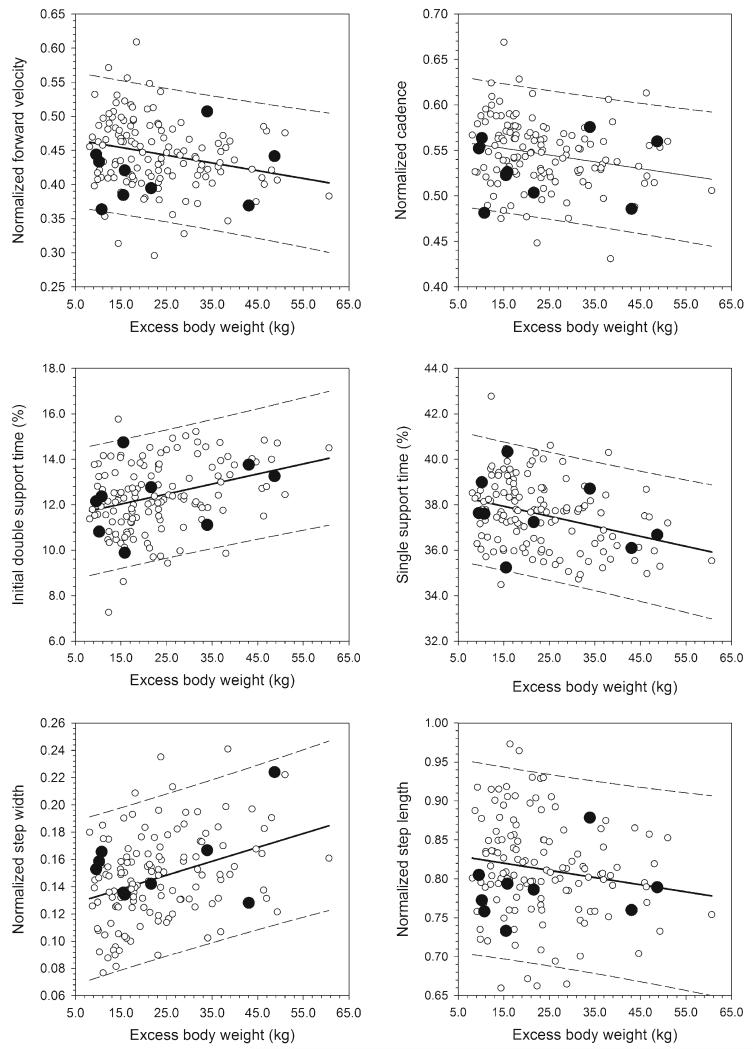
Mean values for the study variables in the bariatric sample at 5-year follow-up and in the Fels sample are presented in Table 3. The sole variable for which the two samples differed significantly was lower limb length (P =0.05). Within the Fels sample, significant linear regression relationships were found between EBW and normalized forward velocity (b =−0.0011; P <0.01), normalized cadence (b =−0.0007; P =0.01), normalized step width (b =0.0010; P <0.01), and percent of the gait cycle spent in initial double support (b =0.044; P <0.01) and single support (b =−0.044; P <0.01). The relationship between normalized step length and EBW was not significant (b =−0.0009; P =0.07). For all regression relationships, the bariatric participants fell on or within the Fels sample’s 95 % prediction interval lines (Fig. 2).
Table 3.
Descriptive statistics for the bariatric sample (current visit) and Fels reference sample [mean ± SD (range)]
| Bariatric (N =9) | Fels (N=132) | |
|---|---|---|
| Age (years) | 48.7±14.7 (25.5–73.4) | 49.5±13.5 (25.9–73.2) |
| Height (cm) | 161.1±2.0 (159.0–165.5) | 165.3±6.7 (153.0–181.3) |
| Weight (kg) | 78.8±14.4 (64.4–103.2) | 80.7±12.2 (60.0–124.6) |
| EBW (kg) | 23.2±14.9 (8.1–48.2) | 23.1±10.8 (8.1–60.7) |
| BMI (kg/m2) | 30.4±6.0 (25.0–40.8) | 29.5±3.8 (25.0–40.0) |
| Fat mass (kg) | 31.2±13.0 (15.3–54.4) | 31.0±6.8 (18.1–51.0) |
| Fat free mass (kg) | 48.5±3.7 (41.8–52.8) | 49.7±6.3 (33.9–70.3) |
| Lower limb length (cm) | 74.3±2.5 (70.9–79.0) | 77.2±4.4 (66.9–89.0)* |
| Abdominal circumference (cm) | 98.6±13.5 (82.2–122.9) | 100.0±10.6 (80.6–132.9) |
| Normalized forward velocity | 0.42±0.05 (0.36–0.51) | 0.45±0.05 (0.30–0.61) |
| Normalized cadence | 0.53±0.03 (0.48–0.58) | 0.55±0.04 (0.43–0.67) |
| Normalized step length | 0.79±0.04 (0.73–0.88) | 0.81±0.06 (0.66–0.97) |
| Normalized step width | 0.16±0.03 (0.13–0.22) | 0.15±0.03 (0.08–0.24) |
| Initial double support time (%) | 12.3±1.5 (9.9–14.7) | 12.4±1.5 (7.3–15.8) |
| Single support time (%) | 37.6±1.6 (35.2–40.3) | 37.6±1.5 (34.5–42.8) |
P ≤0.05, sample means differ significantly
Fig. 2.
Significant relationships between gait variables and excess body weight. Regressions (solid lines) and their 95 % prediction intervals (dashed lines) are derived from the Fels reference sample only (open circles). Bariatric sample data (filled circles) are plotted for comparison, falling largely on or within the prediction intervals for the age- and BMI-matched Fels sample. Step length was the only gait variable not significantly related to EBW in the Fels sample
Discussion
Functional recovery is an important correlate of the significant weight loss that results from bariatric surgery and can occur rapidly in the short term [4, 10, 16-18]. This study provides new evidence that improvements in walking gait are maintained even 4 to 5 years after surgery. Gait signatures of women in the bariatric sample were consistent with their current EBW, BMI, and obesity status, rather than what would be expected for their preoperative status. Compared to published data for class III obese women [32, 33], women in the bariatric sample including the two women who remained obese (classes I and II) took longer and narrower steps and walked at higher cadence and velocity. Greater postsurgical weight loss also correlated with less time spent in double support and more time spent in single support during the gait cycle. The direction and magnitude of these results are consistent with changes in gait observed 3 to 12 months after surgery in other studies [4, 17, 18], indicative of a shift toward more normal gait. Thus, these findings show that simply moving into a lower obesity class via surgical weight loss is associated with long-term sustainment of functional recovery. The 61.1 % reduction of EBW in the present sample appears to be typical of RYGB patients [34], suggesting that the functional results presented here are likely generalizable to the larger female surgical patient population.
Despite being within the 95 % prediction intervals for all gait variables in the Fels sample, however, most of the bariatric samples (seven of nine participants) were below the regression lines for forward velocity and cadence. Thus, as a group, the bariatric sample walked with a lower cadence and slightly more slowly than expected for their current EBW. It may be that some aspects of preoperative gait are retained in forward velocity and cadence, but given the small sample size, this interpretation is preliminary. Another possibility is that age had a confounding effect on the forward velocity/BMI relationship, but there was no correlation between age and velocity in the bariatric sample (P =0.93). Thus, the possibility that patients retain some preoperative gait characteristics, but not others, postsurgically, merits further study.
This study has several strengths, including an extended time frame for observing changes in weight and physical function, but one limitation is the absence of longitudinal gait data. This limitation is partially addressed with the longitudinal evidence for substantial improvements in the SMFA function index and in TGUG times. The change in TGUG time was particularly stark, improving from a preoperative level typical of 80-99-year-old patients at a high risk for falling [35, 36] to a current value within the 95 % confidence interval for healthy individuals aged 19-29 years old [36] despite of a mean age of 48.7 years. Current TGUG time also appears to be positively related to gait forward velocity (Fig. 3), showing an association between functional improvement and current gait.
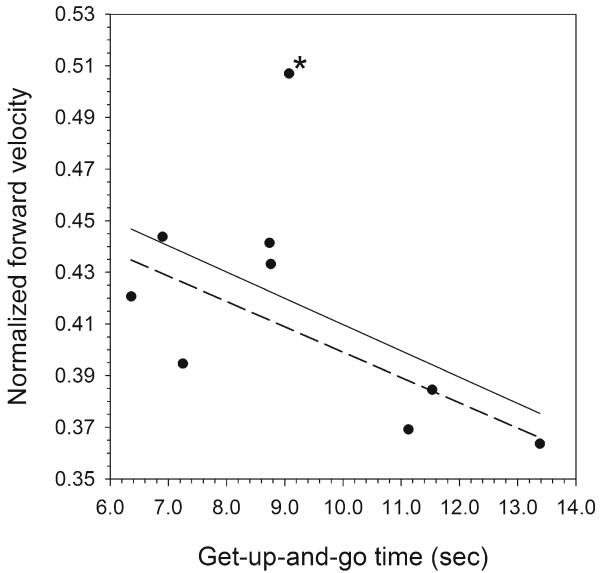
Fig. 3.
Relationship between normalized forward velocity and timed get-up-and-go time in the bariatric sample. Although the initial relationship is not significant (solid line; P =0.14), this result is strongly influenced by one participant (marked with an asterisk) who had the highest current forward velocity and the least improvement in TGUG time (0.7 s vs. a mean of 4.2 s in the remaining sample), suggesting a high degree of functional mobility even before surgery. Excluding that participant, the TGUG/forward velocity relationship is significant (dotted line; P =0.03), and faster walking velocity is associated with shorter TGUG time
In conclusion, this study found a significant improvement in physical function with excess weight loss in nine women 5 years after bariatric surgery. Gait signatures of these women were characteristic of their current weight rather than their presurgical weight, as shown by a comparison with age- and weight-matched peers. Importantly, this finding demonstrates that the musculoskeletal system can recover from the presurgical experience of high adiposity and can sustain that recovery over the long term. Additional work is needed to explore the possibility that some preoperative gait traits are retained despite surgical procedures, despite an improvement in the other aspects of gait and function. Increased knowledge of the gait recovery process will provide clear information to clinicians and prospective patients on long-term postsurgical expectations for improvement of physical function.
Acknowledgments
The authors thank the participants. The study was funded by a grant from the Department of Community Health, Wright State University Boonshoft School of Medicine.
Footnotes
Conflict of Interest None of the authors declare any conflict of interest.
Contributor Information
Andrew W. Froehle, Department of Community Health, Wright State University Boonshoft School of Medicine, 3171 Research Blvd., Kettering, OH 45420, USA andrew.froehle@wright.edu
Richard T. Laughlin, Department of Orthopaedic Surgery, Sports Medicine, and Rehabilitation, Wright State University Boonshoft School of Medicine, 30 E. Apple St., Suite 2200, Dayton, OH 45409, USA, rtlaughlin@mvh.org
Donovan D. Teel, II, Department of Surgery, Wright State University Boonshoft School of Medicine, 128 E. Apple St., Dayton, OH 45409, USA ddteel@mvh.org; Premier Metabolic and Bariatric Associates, Miami Valley Hospital, 30 E. Apple St., Dayton, OH 45409, USA.
Richard J. Sherwood, Department of Community Health, Wright State University Boonshoft School of Medicine, 3171 Research Blvd., Kettering, OH 45420, USA, richard.sherwood@wright.edu; Department of Pediatrics, Wright State University Boonshoft School of Medicine, One Children’s Plaza, Dayton, OH 45404, USA
Dana L. Duren, Department of Community Health, Wright State University Boonshoft School of Medicine, 3171 Research Blvd., Kettering, OH 45420, USA, dana.duren@wright.edu; Department of Orthopaedic Surgery, Sports Medicine, and Rehabilitation, Wright State University Boonshoft School of Medicine, 30 E. Apple St., Suite 2200, Dayton, OH 45409, USA
References
- 1.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–79. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 2.Browning RC, Kram R. Effects of obesity on the biomechanics of walking at different speeds. Med Sci Sports Exerc. 2007;39(9):1632–41. doi: 10.1249/mss.0b013e318076b54b. [DOI] [PubMed] [Google Scholar]
- 3.Ferraro KF, Booth TL. Age, body mass index, and functional illness. J Gerontol Ser B Psychol Sci Soc Sci. 1999;54(6):S339–48. doi: 10.1093/geronb/54b.6.s339. [DOI] [PubMed] [Google Scholar]
- 4.Vincent HK, Ben-David K, Conrad BP, et al. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346–54. doi: 10.1016/j.soard.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2(3):173–82. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Obesity: preventing and managing the global epidemic—introduction. Obesity: preventing and managing the global epidemic. 2000;894:1–253. [PubMed] [Google Scholar]
- 7.Hergenroeder AL, Brach JS, Otto AD, et al. The influence of body mass index on self-report and performance-based measures of physical function in adult women. Cardiopulm Phys Ther J. 2011;22(3):11–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Villareal DT, Apovian CM, Kushner RF, et al. The Obesity Society Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO. Obes Res. 2005;13(11):1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 10.Iossi MF, Konstantakos EK, Teel DD, et al. Musculoskeletal function following bariatric surgery. Obesity. 2013;21(6):1104–10. doi: 10.1002/oby.20155. [DOI] [PubMed] [Google Scholar]
- 11.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132(6):2253–71. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert EW, Wolfe BM. Bariatric surgery for the management of obesity: state of the field. Plast Reconstr Surg. 2012;130(4):948–54. doi: 10.1097/PRS.0b013e318262f566. [DOI] [PubMed] [Google Scholar]
- 13.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232(4):515–26. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Needleman BJ, Happel LC. Bariatric surgery: choosing the optimal procedure. Surg Clin N Am. 2008;88(5):991–1007. doi: 10.1016/j.suc.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Silver HJ, Torquati A, Jensen GL, et al. Weight, dietary and physical activity behaviors two years after gastric bypass. Obes Surg. 2006;16(7):859–64. doi: 10.1381/096089206777822296. [DOI] [PubMed] [Google Scholar]
- 16.Miller GD, Nicklas BJ, You T, et al. Physical function improvements after laparoscopic Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2009;5(5):530–7. doi: 10.1016/j.soard.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hortobagyi T, Herring C, Pories WJ, et al. Massive weight loss-induced mechanical plasticity in obese gait. J Appl Physiol. 2011;111(5):1391–9. doi: 10.1152/japplphysiol.00291.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vartiainen P, Bragge T, Lyytinen T, et al. Kinematic and kinetic changes in obese gait in bariatric surgery-induced weight loss. J Biomech. 2012;45(10):1769–74. doi: 10.1016/j.jbiomech.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Higa K, Ho TC, Tercero F, et al. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7(4):516–25. doi: 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Roche AF. Growth, maturation and body composition: the Fels longitudinal study 1929-1991. Cambridge University Press; Cambridge: 1992. p. 282. [Google Scholar]
- 21.Kovacs CR. Age-related changes in gait and obstacle avoidance capabilities in older adults: a review. J Appl Gerontol. 2005;24(1):21–34. [Google Scholar]
- 22.Felson DT. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996;55(9):668–70. doi: 10.1136/ard.55.9.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Runhaar J, Koes BW, Clockaerts S, et al. A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis. Obes Rev. 2011;12(12):1071–82. doi: 10.1111/j.1467-789X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization reference manual. Human Kinetics; Champaign: 1988. p. vi. [Google Scholar]
- 25.Lorentz FH. Ein neuer Konstitionsinde. Klin Wochenschr. 1929;8:348–51. [Google Scholar]
- 26.Swiontkowski MF, Engelberg R, Martin DP, et al. Short musculoskeletal function assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245–60. doi: 10.2106/00004623-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 28.Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96–101. doi: 10.1191/026921500675545616. [DOI] [PubMed] [Google Scholar]
- 29.Kadaba MP, Ramakrishnan HK, Wootten ME, et al. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthop Res. 1989;7(6):849–60. doi: 10.1002/jor.1100070611. [DOI] [PubMed] [Google Scholar]
- 30.Hof AL. Letter to the editor: scaling gait data to body size. Gait Posture. 1996;4:222–3. [Google Scholar]
- 31.Froehle AW, Nahhas RW, Sherwood RJ, et al. Age-related changes in spatiotemporal characteristics of gait accompany ongoing lower limb linear growth in late childhood and early adolescence. Gait Posture. 2013;38(1):14–9. doi: 10.1016/j.gaitpost.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza SAF, Faintuch J, Valezi AC, et al. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005;15(9):1238–42. doi: 10.1381/096089205774512627. [DOI] [PubMed] [Google Scholar]
- 33.Hergenroeder AL, Wert DM, Hile ES, et al. Association of body mass index with self-report and performance-based measures of balance and mobility. Phys Ther. 2011;91(8):1223–34. doi: 10.2522/ptj.20100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032–40. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 35.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–8. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Wall JC, Bell C, Campbell S, et al. The timed get-up-and-go test revisited: measurement of the component tasks. J Rehabil Res Dev. 2000;37(1):109–13. [PubMed] [Google Scholar]