Abstract
Objective:
To relate serum insulin-like growth factor-1 (IGF-1) to risk of Alzheimer disease (AD) dementia and to brain volumes in a dementia-free community sample spanning middle and older ages.
Methods:
Dementia-free Framingham participants from generation 1 (n = 789, age 79 ± 4 years, 64% women) and generation 2 (n = 2,793, age 61 ± 9 years, 55% women; total = 3,582, age 65 ± 11 years, 57% women) had serum IGF-1 measured in 1990–1994 and 1998–2001, respectively, and were followed prospectively for incident dementia and AD dementia. Brain MRI was obtained in stroke- and dementia-free survivors of both generations 1 (n = 186) and 2 (n = 1,867) during 1999–2005. Baseline IGF-1 was related to risk of incident dementia using Cox models and to total brain and hippocampal volumes using linear regression in multivariable models adjusted for age, sex, APOE ε4, plasma homocysteine, waist-hip ratio, and physical activity.
Results:
Mean IGF-1 levels were 144 ± 60 μg/L in generation 1 and 114 ± 37 μg/L in generation 2. We observed 279 cases of incident dementia (230 AD dementia) over a mean follow-up of 7.4 ± 3.1 years. Persons with IGF-1 in the lowest quartile had a 51% greater risk of AD dementia (hazard ratio = 1.51, 95% confidence interval: 1.14–2.00; p = 0.004). Among persons without dementia, higher IGF-1 levels were associated with greater total brain volumes (β/SD increment in IGF-1 was 0.55 ± 0.24, p = 0.025; and 0.26 ± 0.06, p < 0.001, for generations 1 and 2, respectively).
Conclusion:
Lower serum levels of IGF-1 are associated with an increased risk of developing AD dementia and higher levels with greater brain volumes even among middle-aged community-dwelling participants free of stroke and dementia. Higher levels of IGF-1 may protect against subclinical and clinical neurodegeneration.
Emerging risk factors for dementia include diabetes and its antecedents such as insulin resistance (IR), obesity, physical inactivity, and high caloric intake.1,2 The development of IR and diabetes is preceded by changes in levels of insulin-like growth factor-1 (IGF-1).3 IGF-1 is a hormone induced by physical activity and low caloric intake. This hormone promotes cell survival, prevents apoptosis, and stimulates neurogenesis in the hippocampus—an area affected early in Alzheimer disease (AD).4 IGF-1 also appears to inhibit abnormal tau phosphorylation and β-amyloid deposition in cell cultures and in transgenic mouse models of AD.5 Wild-type mice with low IGF-1, as well as IGF-1–deficient mice, show reduced adult hippocampal neurogenesis and impaired spatial learning.6 In clinical studies, lower IGF-1 levels were associated with greater brain β-amyloid burden within families that have an amyloid precursor protein mutation, and lower IGF-1 levels were also associated with poorer baseline cognition and accelerated cognitive decline in community-based samples of disease-free adults.7–10
In this study, we examined whether lower circulating IGF-1 levels were associated with a higher risk of incident AD dementia and with lower brain volume in the Framingham cohort.
METHODS
Sample.
Participants were recruited from 2 generations in the Framingham community. Recruitment and characterization of the Framingham Study original cohort (generation 1) have been described in prior publications.11 A total of 5,209 participants were enrolled, and these participants were assessed in the Heart Study research clinic once every 2 years, where a detailed medical history was obtained and all traditional cardiovascular risk factors were measured. In 1971, offspring of persons in the original cohort and the spouses of these offspring were enrolled in the offspring cohort (generation 2) and assessed once every 4 years as described elsewhere.12 IGF-1 levels were measured in 789 participants from generation 1 (mean age 79 ± 4 years, 64% women) of a total of 1,005 persons free of dementia who attended the 22nd examination cycle (1990–1994); the remaining persons, most assessed at home visits, did not have sufficient serum drawn to permit IGF-1 assay. IGF-1 levels were also measured in 2,793 participants from generation 2 (mean age 61 ± 9 years, 55% women) of a total of 3,257 persons who attended examination 7 (1998–2001) and were free of dementia.
A total of 219 individuals in generation 1 (of 623 survivors until 1999) and 1,931 participants of generation 2 with available measures of IGF-1 underwent volumetric brain MRI between 1999 and 2005. Those who did not undergo brain MRI declined consent, lived outside the testing area, had a contraindication to brain MRI (such as claustrophobia or a cardiac pacemaker), or died before MRI could be obtained. In addition, we excluded 33 generation 1 and 64 generation 2 participants who had clinical dementia, stroke, or both, as well as other conditions that could affect brain MRI measurements such as a brain tumor. The final MRI sample comprises 186 generation 1 (mean age 77 ± 3 years, 61% women) and 1,867 generation 2 (mean age 61 ± 9 years, 55% women) participants.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the Institutional Review Board at Boston University Medical Center, and all participants gave written informed consent.
IGF-1 assays.
At the baseline examination, each participant had 3 mL of serum drawn. This was obtained in the early afternoon in a supine nonfasting state for generation 1, and in the morning in a supine fasting state for generation 2. Samples were centrifuged and aliquoted immediately for storage at −80°C. The assays in each generation were run in different laboratories because they were funded from different sources. In generation 2, serum IGF-1 was measured by standard immunoassay (R&D Systems Quantikine Human IGF-I DG100, SG100, and PDG100 [R&D Systems, Minneapolis, MN]; ELISA method [assays done by X.H., supervised by T.C.C., laboratory of L.E.B.]) with a lower detection limit of 9.4 ng/mL. The intraassay coefficient of variation (CV) was <4%. The interassay CV was 4.5%. Generation 1 IGF-1 assays were completed at Endocrine Sciences, Inc. (Calabasas Hills, CA) by radioimmunoassay after acid ethanol extraction (laboratory of R.R.); the intraassay CV was <4%. IGF-binding proteins have not been measured in either generation.
Covariates.
IR was estimated using a standardized measure of insulin sensitivity: the homeostasis model assessment of IR (HOMA-IR). The HOMA-IR (mmol/L × µU/mL) formula is defined as fasting insulin (µU/mL) times fasting glucose (mmol/L)/22.5. C-reactive protein (CRP) was assayed with a nephelometer (BN100; Dade Behring, Deerfield, IL). The mean interassay CV was 2.2%. The physical activity index was calculated as a composite score based on information collected from a structured questionnaire.13 Plasma total homocysteine levels were determined by the use of high-performance liquid chromatography with fluorometric detection. The CV of this assay was 9%. HOMA-IR, CRP, physical activity index, and total homocysteine were only measured for the baseline examination in generation 2 and were included as continuous measures. The presence or absence of APOE ε4 alleles was determined by means of isoelectric focusing of the plasma and confirmed by DNA genotyping. Central obesity was measured by waist-hip ratio (WHR) and dichotomized as being in the highest sex-specific quartile (Q4 WHR) or not. Adjustment for the use of antidepressants was dichotomized as yes/no.
Clinical definition of AD dementia.
Participants in the Framingham cohorts undergo periodic surveillance and screening for impaired cognitive function and dementia. These methods have been described earlier.14,15 Persons thought to have possible cognitive decline by objective means or self-, caregiver-, or physician-report were given a detailed evaluation. A consensus review panel including at least one behavioral neurologist and one neuropsychologist determined the probable date of diagnosis and type of dementia. Dementia was defined using DSM-IV criteria.16 The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria were used to classify participants as having definite, probable, or possible AD dementia.15
Brain imaging.
The imaging measures, measurement protocols, and reproducibility of these measurements have been described elsewhere.14 MRIs were obtained with a Siemens Magnetom 1- or 1.5-tesla field strength machine (Siemens AG, Erlangen, Germany) using a double spin-echo coronal imaging sequence of 4-mm contiguous slices from nasion to occiput. Imaging analyses were performed at a central location (C.D.) using a custom-designed image-analysis package, QUANTA 6.2, operating on a Sun Microsystems Ultra 5 workstation (Sun Microsystems, Santa Clara, CA). Experienced clinicians evaluating the brain images were blinded to the participants' demographic, anthropometric, and clinical data including IGF-1 concentrations. Total cerebral brain volume (TCBV) was defined as the ratio of total brain parenchymal volume above the tentorium to total intracranial volume, which therefore adjusted for head size. Hippocampal volume (HPV) was defined using operator-defined, manually traced boundaries. Inter- and intrarater reliability using this method was very good with a κ of 0.96.
Statistical analyses.
Baseline characteristics of participants excluded from and included in the study were compared using age-adjusted linear or logistic regression models. Skewed variables were log-transformed as necessary before analysis. IGF-1 levels were first natural logarithmically transformed and then standardized within each cohort/generation and sex (mean [SD], 0 [1]). Because there did not appear to be effect modification by cohort status, we conducted further analyses on a pooled sample. Cox regression models were used to relate baseline sex-standardized log–IGF-1 levels to the incidence of dementia and AD dementia after confirming that the assumption of proportionality of hazards was met. Age was used as the time scale, because the risk of dementia is more likely to change as a function of age than time. In the analysis relating IGF-1 levels to incident dementia, results from both generations were pooled. Initial analyses were adjusted for age and sex alone (model 1). Model 2 additionally adjusted for the presence or absence of an APOE ε4 allele, a major risk factor for dementia and AD dementia.
In the analysis relating IGF-1 levels to brain volumes, the 2 generations were analyzed separately, given that the mean time interval from baseline IGF-1 measurement to imaging was 0.8 years in generation 2 and 7.7 years in generation 1. Multivariable linear regression was used to relate baseline sex-standardized log–IGF-1 levels to TCBV and HPV. Models 1 and 2 were adjusted for covariates as in the Cox models, with the addition of time to imaging, and in model 3 an additional adjustment for WHR (top sex-specific quartile) was made. In generation 2, model 4 additionally included plasma homocysteine, physical activity index, CRP, and HOMA-IR, the latter having been recently shown to correlate with brain volume.2 We found no effect modification by sex and therefore all analyses were sex-pooled.
RESULTS
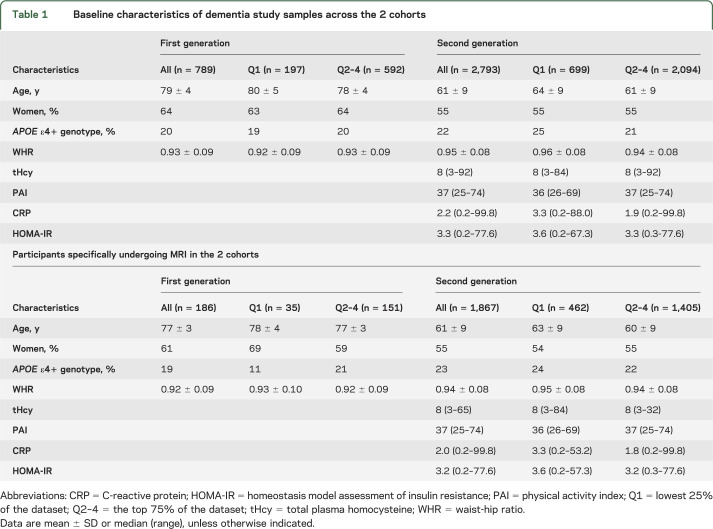
Demographic and clinical characteristics of participants in the generation 1 and 2 cohorts, and of the subset who underwent MRI, are displayed in table 1. Study participants did not differ from persons not enrolled in the study except for being younger (table e-1 on the Neurology® Web site at Neurology.org). Table 2 shows the number of persons who developed incident dementia and AD dementia and the mean values of total brain and HPV measurements in both generations. Sex-specific and cohort-specific quartiles of serum IGF-1 can be found in table e-2.
Table 1.
Baseline characteristics of dementia study samples across the 2 cohorts
Table 2.
Number of cases of incident and AD dementia across the 2 cohorts as well as the number of participants undergoing MRI and respective measurements of precursors to subclinical AD dementia
Association of IGF-1 with incident all-cause dementia and AD dementia.
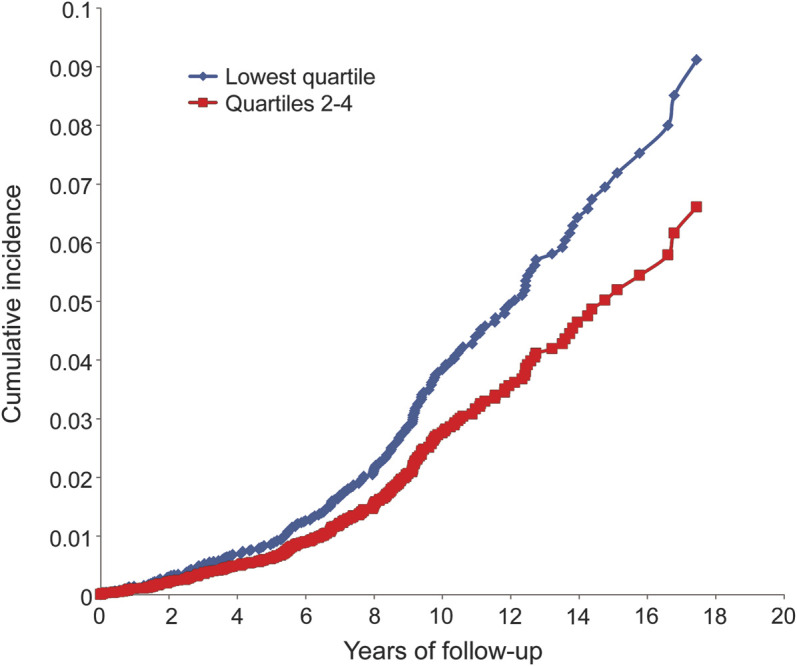
Analysis of pooled data from both generations revealed 279 participants who developed incident dementia; 230 of them were diagnosed with AD dementia. Lower IGF-1 levels showed a trend toward an increased risk of incident all-cause dementia and a definite association with an increased risk of incident AD dementia after adjustment for age, sex, and APOE ε4 (table 3). IGF-1 showed a stronger association with AD dementia than all-cause dementia. The association was nonlinear, and in threshold models, we found a higher risk of AD dementia among persons in the lowest sex-specific quartile than in the upper 3 (table 3; see the figure and table e-2 for IGF-1 quartiles). Adjustment for top quartile WHR (Q4 WHR) did not alter the results nor did the use of antidepressants in generation 1 (data not shown). There was no effect modification by sex.
Table 3.
Association of sex-standardized mean (range) log serum IGF-1 with incident and AD dementia in the pooled generations across quartiles
Figure. Cumulative incidence of Alzheimer disease dementia in the pooled generations: Greatest incidence is observed among persons in the lowest quartile of insulin-like growth factor-1 serum levels.
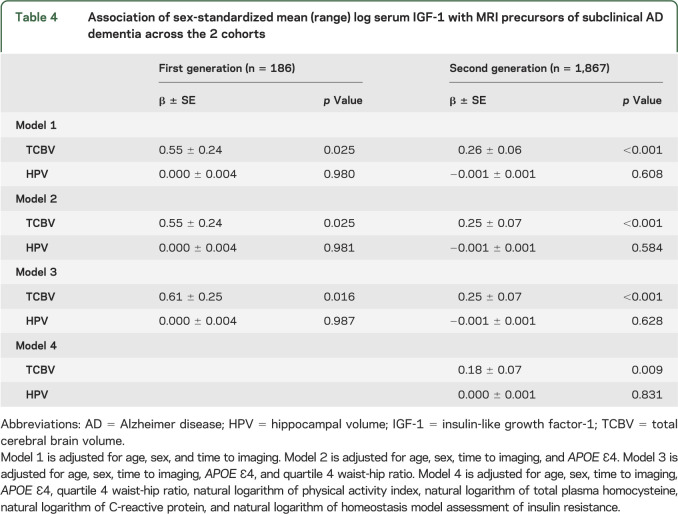
Association of IGF-1 with MRI measures of brain aging.
Higher IGF-1 levels were associated with greater TCBV in both the older and middle-aged participants (table 4). However, we did not observe an association with HPV in either population. Our data suggest a linear relationship with higher IGF-1 levels associated with larger TCBV (β/SD increment in IGF-1 was 0.55 ± 0.24, p = 0.025; and 0.26 ± 0.06, p < 0.001, for generations 1 and 2, respectively) when adjustments were made for age, sex, and time to imaging. Further adjustments did not strengthen or weaken the association in either generation (table 4, models 2–4 including, where available, adjustments for APOE ε4, Q4 WHR, physical activity index, homocysteine, CRP, and HOMA-IR).
Table 4.
Association of sex-standardized mean (range) log serum IGF-1 with MRI precursors of subclinical AD dementia across the 2 cohorts
DISCUSSION
We found that lower serum IGF-1 levels increased the risk of incident AD dementia in older and middle-aged participants. In addition, even among cognitively intact participants, those persons with higher serum IGF-1 levels had greater total brain volumes, as evidenced by MRI. The associations remained significant even after adjustment for several other known risk factors affecting IGF-1 levels and risk of incident AD dementia.
Data relating IGF-1 and cognition have been limited and conflicting, and there have been no prior prospective studies relating serum IGF-1 to the clinical endpoints of incident dementia and AD dementia. Lower serum levels of IGF-1 have been correlated with worse performance on the Mini-Mental State Examination in persons 65 years and older as well as with decline in Mini-Mental State Examination scores over a 2-year follow-up period.10,17 In addition, a further study of 636 men and 899 women showed that higher IGF-1 was associated with better cognitive function, although only in men with dementia in this cohort.9 A study of 75 persons with hypertension also noted an association of lower IGF-1 with poorer performance on tests of orientation, memory, praxis, and frontal functions.18 By contrast, in a large healthy population of 1,318 persons aged 65 to 88 years, only information processing speed was lower among persons within the lowest quintile of IGF-1.8
Our study supports the literature showing that lower IGF-1 levels are associated with a greater risk of AD dementia, and is larger than prior studies (n = 3,582, age = 52–83 years) with a longer follow-up period; furthermore, we used a “hard” endpoint of clinical dementia rather than cognitive testing alone. In an apparently contradictory observation, some small studies have noted an increase in serum IGF-1 levels among persons with AD dementia, compared with controls.19–21 Hippocampal slices from AD brains have been shown to be less responsive to insulin and IGF-1 than slices from control brains.22 Our study had a very small number of persons with prevalent AD dementia at the time that IGF-1 was measured and hence is not sufficiently powered to examine variation in levels among persons with prevalent AD dementia compared with controls. This discrepancy of lower IGF-1 in persons at risk and higher levels in persons with AD dementia could be attributable to a compensatory release of IGF-1 in response to the neurodegeneration that develops in the later stages of AD or from an acquired IGF-1 resistance secondary to alterations in the IGF-1 signaling pathway.21,23
To our knowledge, there have been no prior studies relating IGF-1 to total brain volumes on MRI in healthy persons. One study analyzed brain volume by MRI in exogenously treated patients with human growth hormone deficiency.24 Administration of human growth hormone in this group led to an elevation in serum IGF-1, and initial reports after 3 months showed an increase in brain volume, as scored by 2 experienced neuroradiologists. However reevaluation with a control comparison group did not support this initial report.25 This may have been attributable to several factors, including duration of treatment and time to MRI.
Our study did not show any relationship of circulating IGF-1 to HPV, but the variability caused by measurement error is greater for HPV than for TCBV. A larger sample may be required to show a significant effect on HPV. The temporal horn of the lateral ventricle is adjacent to the hippocampus and shrinks in proportion to the degree of hippocampal atrophy. A prior study relating IGF-1 levels to radial width of the temporal horn on CT also failed to show any association, although this study sample was small (n = 75).18
The discrepancy observed in our study between the effect of IGF-1 on HPV and total brain volume does not necessarily prove that the effect of IGF-1 is restricted to the isocortex; rather, it may be a consequence of technical as well as biological factors. Measurements of HPV have a larger standard error than total brain volume because they are smaller volumes, which amplifies the impact of small measurement errors; furthermore, the intricate shape of the hippocampus increases the complexity of defining precise boundaries. Whereas it is possible that IGF-1 signaling and receptor density might vary across different regions of the brain, both the isocortex and the hippocampus appear sensitive to IGF-1 levels, at least in animal models and in early human development as outlined subsequently. Another explanation for the stronger association with TCBV could also be a greater impact on white matter volumes that would affect TCBV more than HPV. Further studies of gray and white matter volumes and voxel-based morphometric analyses are ongoing.
IGF-1 has been shown to interact with both β-amyloid and tau in vitro and in vivo. In the Tg2567 mouse model, IGF-1 improves β-amyloid clearance.26 In mice deficient of insulin receptor substrate 2, an IGF-1–associated receptor, abnormal cytoplasmic deposits of hyperphosphorylated tau in the hippocampus are seen.27 Higher IGF-1 levels have been associated with larger brains and better memory function in mouse models and in human disease. IGF-1 knockout mice have smaller brain volumes, and specifically a smaller granule cell layer in the dentate gyrus.28 In animal studies, low IGF-1 leads to spatial learning impairment and reduced hippocampal neurogenesis and volume.6 Spatial learning impairment could also be ameliorated with subcutaneous administration of IGF-1.29 Overexpressing IGF-1 in mice results in an increase of brain size and myelination.30 In mouse models, higher IGF-1 promotes learning and memory performance.31 In humans, congenital IGF-1 deficiency causes microcephaly and mental retardation.32 In a family carrying the Swedish amyloid precursor protein 670/671 mutation, persons with AD dementia, when compared with age-matched persons without AD dementia, were observed to have lower plasma IGF-1 levels.7
In light of our findings relating elevated IGF-1 levels to a lower risk of AD dementia and to larger brain volumes, further studies to explore a possible clinical role of medications that increase IGF-1 levels appears warranted. Exogenous administration of human growth hormone as well as secretagogues such as imidazolines may increase serum levels of IGF-1 by up to 30%.33 IGF-1 has also been directly administered in animals with successful CNS uptake after intranasal delivery.34 Administration of intranasal insulin in humans improved cognition and modulated β-amyloid in early AD dementia.35
Furthermore, in rodents, donepezil has been shown to increase IGF-1 in the hippocampus.36 IGF-1 is also increased by administration of monoamine reuptake inhibitors, fluoxetine, and venlafaxine.4,37 In our study, donepezil and other drugs in this class had not been licensed at the time that serum was drawn for IGF-1 measurements in generation 1, and no effect from antidepressant use was observed. Changes in IGF-1 levels may represent one pathway through which lifestyle interventions such as healthier lower caloric diets and increased physical activity might alter the risk of AD dementia.
The large community-based study spanning 2 generations, objective imaging findings, and our rigorous diagnostic evaluations are particular strengths of this study. However, our study is limited to persons of European ancestry, and therefore confirmation in independent samples and in persons of other racial backgrounds is needed at this time.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- CRP
C-reactive protein
- CV
coefficient of variation
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HOMA-IR
homeostasis model of assessment of insulin resistance
- HPV
hippocampal volume
- IGF-1
insulin-like growth factor-1
- IR
insulin resistance
- TCBV
total cerebral brain volume
- WHR
waist-hip ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Westwood, Beiser, Seshadri. Acquisition of data: Au, DeCarli, Harris, Chen, He, Braverman, Roubenoff, Seshadri, Vasan. Analysis and interpretation of data: Beiser, Seshadri. Draft of the manuscript: Westwood, Beiser, Seshadri. Critical revision of the manuscript for important intellectual content: Chen, He, Braverman, Au, Beiser, DeCarli, Harris, Pikula, Roubenoff, Seshadri, Vasan, Westwood, Wolf. Statistical analysis: Beiser. Obtained funding: Seshadri, Vasan, Wolf. Administrative, technical, or material support: Beiser, Seshadri. Supervision: Seshadri, Vasan.
STUDY FUNDING
Supported by the Framingham Heart Study's National Heart, Lung, and Blood Institute (NHLBI) contract (N01-HC-25195) and by grants from the NHLBI (R01 HL089590), the National Institute on Aging (R01 AG16495, AG 033040, AG08122, AG033193, and AG031287, P30AG013846), and the American Heart Association (11CRP4930020). Partial support from BU-CTSI NIH grant UL1-TR000157 is also acknowledged.
DISCLOSURE
A. Westwood, A. Beiser, C. DeCarli, T. Harris, T. Chen, and X. He report no disclosures relevant to the manuscript. R. Roubenoff is a scientific advisor to companies with interests in fatty acids including Monsanto, Unilever, Neptune, Omthera, and GlaxoSmithKline, and has received support for research projects from all except Unilever. He was also a speaker for GlaxoSmithKline. In addition, he is the owner of OmegaQuant, LLC, a company that offers blood fatty acid testing. A. Pikula, R. Au, L. Braverman, P. Wolf, R. Vasan, and S. Seshadri report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Haan MN. Therapy insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol 2006;2:159–166. [DOI] [PubMed] [Google Scholar]
- 2.Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 2011;34:1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunger DB, Ong KK, Sandhu MS. Serum insulin-like growth factor-I levels and potential risk of type 2 diabetes. Horm Res 2003;(60 suppl 3):131–135. [DOI] [PubMed] [Google Scholar]
- 4.Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 2009;42:239–244. [DOI] [PubMed] [Google Scholar]
- 5.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem 1997;272:19547–19553. [DOI] [PubMed] [Google Scholar]
- 6.Llorens-Martín M, Torres-Alemán I, Trejo JL. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol Cell Neurosci 2010;44:109–117. [DOI] [PubMed] [Google Scholar]
- 7.Mustafa A, Lannfelt L, Lilius L, Islam A, Winblad B, Adem A. Decreased plasma insulin-like growth factor-I level in familial Alzheimer's disease patients carrying the Swedish APP 670/671 mutation. Dement Geriatr Cogn Disord 1999;10:446–451. [DOI] [PubMed] [Google Scholar]
- 8.Dik MG, Pluijm SM, Jonker C, Deeg DJ, Lomecky MZ, Lips P. Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol Aging 2003;24:573–581. [DOI] [PubMed] [Google Scholar]
- 9.Al-Delaimy WK, von Muhlen D, Barrett-Connor E. Insulin like growth factor-1, insulin like growth factor binding protein-1, and cognitive function in older men and women. J Am Geriatr Soc 2009;57:1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalmijn S, Janssen JA, Pols HA, Lamberts SW, Breteler MM. A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab 2000;85:4551–4555. [DOI] [PubMed] [Google Scholar]
- 11.Dawber TR, Meadors GF, Moore FE, Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham Study. Arch Intern Med 1979;139:857–861. [PubMed] [Google Scholar]
- 14.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 15.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology 2004;63:1591–1599. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 17.Rollero A, Murialdo G, Fonzi S, et al. Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects. Neuropsychobiology 1998;38:73–79. [DOI] [PubMed] [Google Scholar]
- 18.Angelini A, Bendini C, Neviani F, et al. Insulin-like growth factor-1 (IGF-1): relation with cognitive functioning and neuroimaging marker of brain damage in a sample of hypertensive elderly subjects. Arch Gerontol Geriatr 2009;(49 suppl 1):5–12. [DOI] [PubMed] [Google Scholar]
- 19.Salehi Z, Mashayekhi F, Naji M. Insulin like growth factor-1 and insulin like growth factor binding proteins in the cerebrospinal fluid and serum from patients with Alzheimer's disease. Biofactors 2008;33:99–106. [DOI] [PubMed] [Google Scholar]
- 20.Tham A, Nordberg A, Grissom FE, Carlsson-Skwirut C, Viitanen M, Sara VR. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect 1993;5:165–176. [DOI] [PubMed] [Google Scholar]
- 21.Vardy ER, Rice PJ, Bowie PC, Holmes JD, Grant PJ, Hooper NM. Increased circulating insulin-like growth factor-1 in late-onset Alzheimer's disease. J Alzheimers Dis 2007;12:285–290. [DOI] [PubMed] [Google Scholar]
- 22.Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 2010;31:224–243. [DOI] [PubMed] [Google Scholar]
- 23.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis 2005;8:247–268. [DOI] [PubMed] [Google Scholar]
- 24.Denton ER, Holden M, Christ E, et al. The identification of cerebral volume changes in treated growth hormone-deficient adults using serial 3D MR image processing. J Comput Assist Tomogr 2000;24:139–145. [DOI] [PubMed] [Google Scholar]
- 25.Holden M, Schnabel JA, Hill DL. Quantification of small cerebral ventricular volume changes in treated growth hormone patients using nonrigid registration. IEEE Trans Med Imaging 2002;21:1292–1301. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol 2010;70:384–396. [DOI] [PubMed] [Google Scholar]
- 27.Schubert M, Brazil DP, Burks DJ, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci 2003;23:7084–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 1995;14:717–730. [DOI] [PubMed] [Google Scholar]
- 29.Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res 2003;74:512–523. [DOI] [PubMed] [Google Scholar]
- 30.Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 1993;10:729–740. [DOI] [PubMed] [Google Scholar]
- 31.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature 2010;464:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 1996;335:1363–1367. [DOI] [PubMed] [Google Scholar]
- 33.Carro E, Torres-Aleman I. Insulin-like growth factor I and Alzheimer's disease: therapeutic prospects? Expert Rev Neurother 2004;4:79–86. [DOI] [PubMed] [Google Scholar]
- 34.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci 2001;187:91–97. [DOI] [PubMed] [Google Scholar]
- 35.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008;70:440–448. [DOI] [PubMed] [Google Scholar]
- 36.Narimatsu N, Harada N, Kurihara H, Nakagata N, Sobue K, Okajima K. Donepezil improves cognitive function in mice by increasing the production of insulin-like growth factor-I in the hippocampus. J Pharmacol Exp Ther 2009;330:2–12. [DOI] [PubMed] [Google Scholar]
- 37.Khawaja X, Xu J, Liang JJ, Barrett JE. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: implications for depressive disorders and future therapies. J Neurosci Res 2004;75:451–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.