Abstract
Objective:
We aimed to investigate the relation between apathy symptoms and structural brain changes on MRI, including white matter lesions (WMLs) and atrophy, in a large cohort of older persons.
Methods:
Cross-sectional analyses are based on 4,354 persons without dementia (aged 76 ± 5 years) participating in the population-based Age, Gene/Environment Susceptibility–Reykjavik Study. Apathy symptoms were assessed with 3 items from the 15-item Geriatric Depression Scale. Brain volumes and total WML volume were estimated on 1.5-tesla MRI using an automated segmentation program; regional WML load was calculated using a semiquantitative scale. Regression analyses were adjusted for age, sex, education, intracranial volume, vascular risk factors, physical activity, brain infarcts, depressive symptoms, antidepressants, and cognitive status.
Results:
Compared to those with <2 apathy symptoms, participants with ≥2 apathy symptoms (49% of the cohort) had significantly smaller gray matter volumes (mean adjusted difference −3.6 mL, 95% confidence interval [CI] −6.2 to −1.0), particularly in the frontal and temporal lobes; smaller white matter volumes (mean adjusted difference −1.9 mL, 95% CI −3.6 to −0.3), mainly in the parietal lobe; and smaller thalamus volumes. They were also more likely to have WMLs in the frontal lobe (adjusted odds ratio = 1.08, 95% CI 0.9–1.3). Excluding participants with a depression diagnosis did not change the associations.
Conclusions:
In this older population without dementia, apathy symptoms are associated with a more diffuse loss of both gray and white matter volumes, independent of depression.
A relation between white matter lesions (WMLs) on brain MRI and late-life depression has been consistently reported.1 Disruption of prefrontal cortex–basal ganglia circuits is one mechanism by which WMLs may predispose to late-life depression.2 Symptoms associated with frontal-subcortical dysfunction include anhedonia, energy loss, psychomotor retardation, and executive dysfunction, and have therefore been proposed as characteristic features of “vascular depression.”3,4 These symptoms may also be manifestations of apathy; apathy is not only more prevalent in older age but also presents with symptoms that overlap with depression.5
A relation between apathy and vascular disease has been reported in a community-based study of older persons,6 and it is likely that apathy is also associated with vascular brain changes. In patients with cognitive impairment and Alzheimer disease (AD), apathy has not only been associated with more WMLs in the frontal lobe7,8 but also with atrophy of frontal gray matter, striatum, and thalamus,9–11 suggesting neurodegenerative processes. It remains unclear whether apathy is related to atrophy and WMLs in older persons without dementia. Because apathy differs in prognosis and pharmacologic treatment12,13 and can occur independent of depression,14 it is important to distinguish apathy from late-life depression.
In a large population-based cohort of older persons without dementia, we investigated whether apathy symptoms are associated with WMLs and atrophy in brain regions identified in the literature.9–11 We hypothesized that WMLs and atrophy in frontal matter, striatum, and thalamus are related to apathy symptoms independent of dementia and depression.
METHODS
Participants.
Subjects were from the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, which is a continuation of the Reykjavik Study. The Reykjavik Study was initiated in 1967 by the Icelandic Heart Association, and included men and women born in 1907 to 1935 and living in the Reykjavik area.15 The original cohort of the Reykjavik Study was examined 1 to 6 times according to a schedule that allowed longitudinal and cross-sectional analyses over the 30-year follow-up period. In 2002, 5,764 individuals randomly chosen from the survivors were examined for the AGES-Reykjavik Study. The study design and initial assessments of the cohort have been described elsewhere.15 Examinations were completed within a 4- to 6-week time window, and included blood tests, blood pressure, ECG, anthropometry, physical and cognitive functioning and comprehensive questionnaires (first visit), brain MRI, CT and ultrasonography (second visit), and vision screening, hearing tests, and dementia assessment if indicated (third visit).
Standard protocol approvals, registrations, and patient consents.
The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority, and by the Institutional Review Board for the National Institute on Aging, NIH. Written informed consent was obtained from all participants.
MRI protocol.
All participants without contraindications were eligible for brain MRI scan on a study-dedicated 1.5-tesla Signa TwinSpeed system (General Electric Medical Systems, Waukesha, WI). The image protocol, described in detail elsewhere,16 included the following sequences: axial T1-weighted 3-dimensional spoiled gradient echo; a fluid-attenuated inversion recovery (FLAIR); a proton density/T2-weighted fast spin echo; and a T2*-weighted gradient echo–type echo planar sequence. All images were acquired to give full brain coverage, and slices were angled parallel to the anterior commissure–posterior commissure line.
Brain volumes, WMLs, and infarcts.
Intracranial volume (ICV) and brain parenchyma compartments were segmented automatically with a previously described algorithm modified from the AGES-Reykjavik Study.16 The pipeline is based on a multispectral tissue segmentation method that estimated volumes for 4 tissue classes: gray and white matter regions, WMLs, and CSF. These 4 classes were summed to obtain total ICV. Total brain volume (the sum of gray matter, normal white matter, and WML volumes) was expressed as percent of ICV as brain parenchymal fraction, an indicator of global brain atrophy. Calculation of regional tissue volumes was based on an anatomical atlas and a regional probabilistic atlas, created from a large sample of the AGES cohort (n = 314), which was warped nonlinearly to the T1-weighted images of each study participant.
Cerebral infarcts, identified by trained radiographers, were defined as defects in brain parenchyma with associated hyperintensity on T2 and FLAIR images with a maximal diameter of at least 4 mm. For infarcts in the cerebellum and brainstem or infarcts with cortical involvement, no size criterion was required. Trained radiographers scored the location of subcortical WMLs in frontal, occipitoparietal, and temporal lobes using the Achten Scale, which provides a semiquantitative “volumetric” estimation for WML load by taking into account the lesion size and number.17 WMLs were defined as visible hyperintense lesions on T2-weighted and FLAIR images.
Apathy-related and depression measures.
The 15-item Geriatric Depression Scale (GDS-15) was used to assess depressive symptoms. Because each of the 15 items can be scored as present or absent, the total score ranges from 0 to 15 points; a score ≥6 was used to indicate the presence of clinically relevant depressive symptoms.18
Previously reported factor analyses of the GDS-15 have consistently identified 3 major subdimensions of the GDS-15, including an apathy subdimension consisting of the following 3 items: (1) “Have you dropped many of your activities and interests?” (2) “Do you prefer to stay at home, rather than going out and doing new things?” and (3) “Do you feel full of energy?” For the purpose of this study, and in line with other large, population-based studies in older persons, we distinguished an apathy (range 0–3) and a depression subscale that included the 12 remaining items (table 1). Previous studies have demonstrated a good correlation between this apathy subscale and a clinical diagnosis of apathy.6,19–22 Similar to another recent population-based study, presence of apathy was defined as a score of 2 or 3 vs no apathy (score of 0 or 1).6
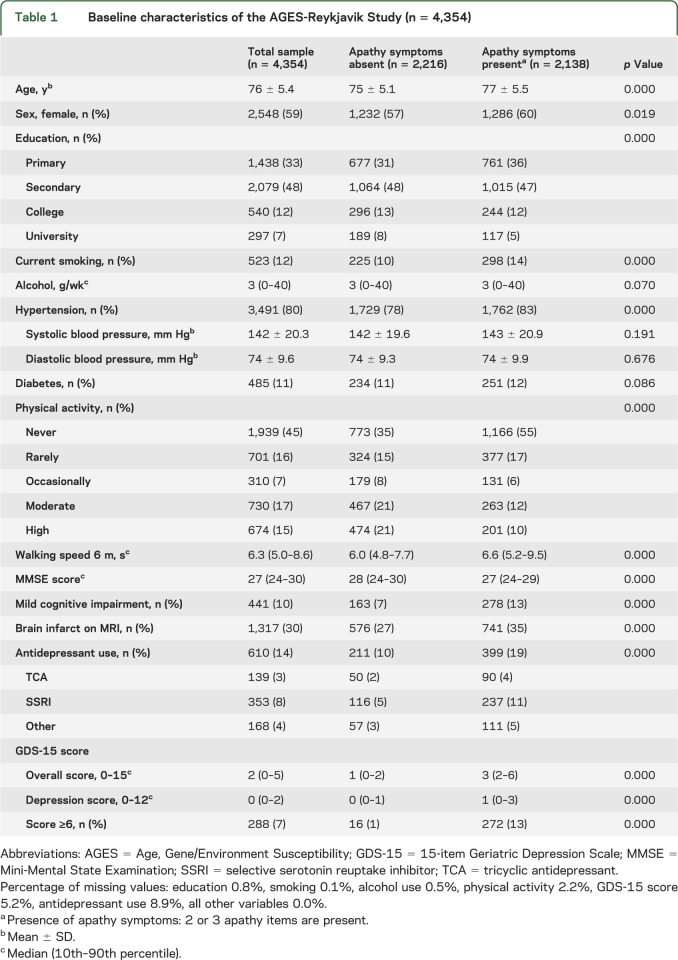
Table 1.
Baseline characteristics of the AGES-Reykjavik Study (n = 4,354)
The presence of major depressive disorder (MDD) in the preceding 2 weeks and in the past was assessed according to DSM-IV criteria using the Mini International Neuropsychiatric Interview.23
Assessment of dementia.
Dementia ascertainment was a 3-step protocol, described previously.15 In brief, all participants were screened using the Mini-Mental State Examination24 and the Digit Symbol Substitution Test.25 Those with positive screen results were administered a diagnostic battery of neuropsychological tests, and, among them, those with positive screen results were examined by a neurologist and a proxy interview was administered regarding medical history, social, cognitive, and daily functioning changes of the participant. A consensus diagnosis according to international guidelines was made by a panel that included a geriatrician, neurologist, neuropsychologist, and neuroradiologist.
Participants were defined as having mild cognitive impairment if they performed at the less than −1.5 SD level on 2 of 8 tests of cognitive function in multiple domains compared with individuals of the same age and level of education, with supporting data collected in the whole cohort, including instrumental type of activities of daily living, depression symptoms, MRI, hearing, and vision.26
Other variables.
Age, sex, education, physical activity, smoking, and alcohol intake (type and number of units per day) were assessed via questionnaires. Smoking history was categorized as current vs nonsmoker. Frequency of moderate or vigorous physical activity in the past 12 months was categorized into moderate/high vs never/rarely/occasionally. Walking speed, a measure of mobility, was evaluated as time in seconds to walk 6 m at usual pace. Total alcohol intake (grams) was calculated as number of units times 14 g (1 unit). Systolic and diastolic blood pressure was measured with a standard mercury sphygmomanometer and the mean of 2 measurements was calculated. Hypertension was defined as self-report plus use of antihypertensive medications, or measured systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. Diabetes mellitus was defined as self-reported history of diabetes, use of blood glucose–lowering drugs, or fasting blood glucose level ≥7.0 mmol/L.
Analytical sample.
Of the 5,764 cohort members, 393 had a diagnosis of dementia and were excluded for this analysis. Of the 5,371 remaining participants, 4,354 had complete data for brain volume analysis.16 Reasons for no MRI included contraindications, refusal, scheduling conflicts, home visit, and a small number did not have all of the sequences necessary for brain segmentation, or there were artifacts in the scan that precluded processing.
Data analysis.
In the analytic sample, the percentage of missing data on covariates used in the analyses varied from 0% to 8.9%. To retain the persons with missing data, we used the statistical program R (version 2.13.1) for multiple imputation (AregImpute)27 (10 datasets). The 10 imputed datasets were pooled and analyzed using PASW version 17.0 (SPSS Inc., Chicago, IL).
We compared baseline characteristics of patients with ≥2 apathy symptoms to those with 0 or 1 symptom. Linear regression analysis was used to investigate associations of apathy symptoms (≥2 vs 0–1, and per symptom increase) to volumes of total brain, total gray matter, total white matter, and total (natural log-transformed) WML volume. Analyses were adjusted for age, sex, education, and ICV in model 1. In model 2, we additionally adjusted for smoking, alcohol intake, hypertension, diabetes mellitus, physical activity, walking speed, depression score, and antidepressant use. Model 3 was additionally adjusted for mild cognitive impairment, brain infarcts on MRI, and total WML volume (gray or white matter volume as dependent variable) or gray and white matter volume (WML volume as dependent variable). Standardized z scores of brain volumes were calculated and used in the regression.
Based on previous studies,7–11 we a priori selected the following quantitatively measured brain regions to examine in association with apathy: lobar gray and white matter regions (frontal, temporal, parietal, and occipital lobes), striatum, hippocampus, amygdala, and thalamus. These regions were entered as dependent variables in separate linear regression models, adjusting for covariates as described above. We chose not to correct for multiple comparisons, because the associations examined in this study and selections of different brain regions were a priori defined and not created based on statistical significance.28 We also examined the association of apathy to semiquantitative measures of lobar WML load (frontal, temporal, parietal, and occipital lobes). Because lobar WML load was skewed, it was dichotomized into the highest quintile vs the 4 lower quintiles of WML load; associations of interest were assessed with logistic regression.
As a final step, we repeated all analyses after excluding participants with a lifetime diagnosis of MDD (n = 189).
RESULTS
The mean age of the study population was 76 ± 5 years, 59% were female, and 6% scored above the cutoff of ≥6 on the GDS-15. The frequency of apathy symptoms ranged from 38% to 58% (table e-1 on the Neurology® Web site at Neurology.org). Of the 2,138 persons with ≥2 apathy symptoms, 13% scored above the GDS-15 cutoff of ≥6, compared with 1% of the persons with 0 or 1 apathy symptom (n = 2,216; table 1). Participants with apathy symptoms were significantly older; were more often female; had lower education, physical activity, and Mini-Mental State Examination scores; had slower walking speed; had more hypertension, mild cognitive impairment, brain infarcts, and antidepressant use; and had higher depression scores compared with those without apathy symptoms (table 1). Apathy and depression scores were significantly correlated (Spearman correlation coefficient 0.326; p < 0.0001). Baseline characteristics for mutually exclusive categories of apathy and depression symptoms are summarized in table e-2.
Apathy symptoms and total brain volumes.
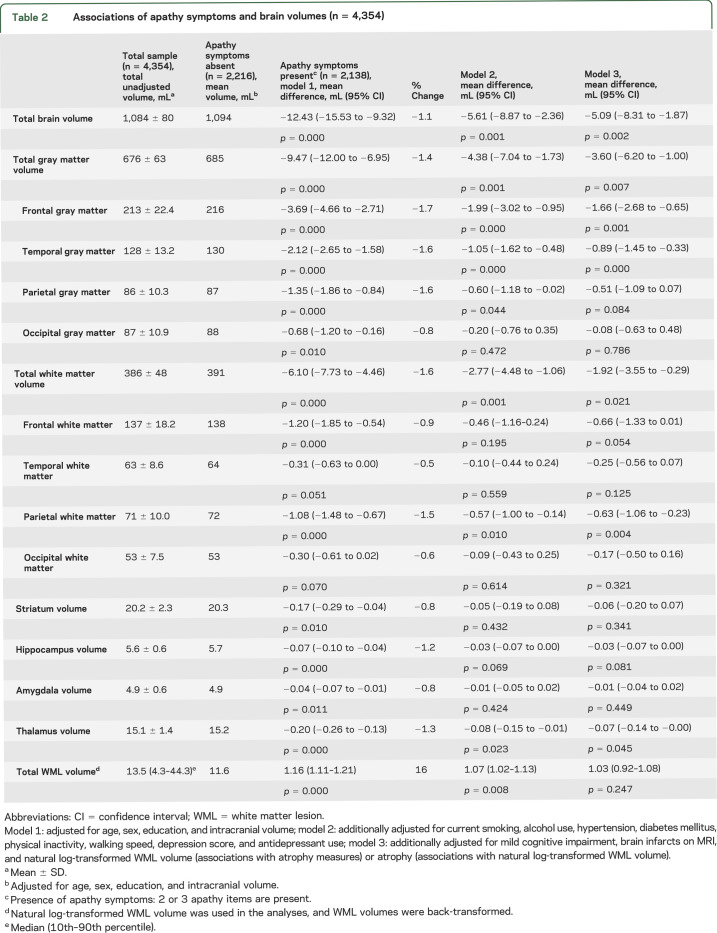
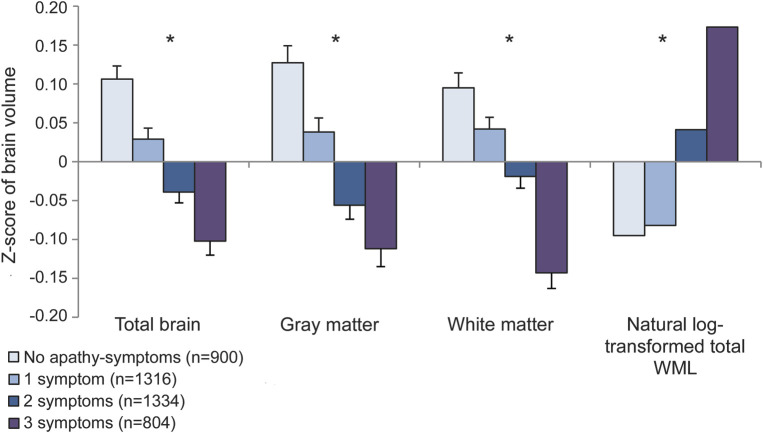
Participants with ≥2 apathy symptoms had significantly smaller total brain volume, total gray matter volume, and total white matter volume compared with those with 0 or 1 symptom (table 2). Part of this association was explained by covariates, although all associations remained significant in the fully adjusted model (table 2). When we investigated the number of apathy symptoms, a significant trend toward smaller total brain volume, total gray matter volume, and total white matter volume was found for an increase in the number of apathy symptoms (p < 0.001; figure 1).
Table 2.
Associations of apathy symptoms and brain volumes (n = 4,354)
Figure 1. Relations between the presence of one or more apathy symptoms and brain volumes.
Shown are the z scores of total brain volume, total gray matter volume, total white matter volume, and natural log-transformed total white matter lesion (WML) volume (mL) for participants with no apathy symptoms (reference) and 1 to 3 apathy symptoms, adjusted for age, sex, education, and intracranial volume. Statistically significant trends (p < 0.001) are indicated with an asterisk.
Apathy symptoms and total WML volume.
Participants with ≥2 apathy symptoms had significantly greater total WML volume compared with persons with 0 or 1 apathy symptom (table 2). This association attenuated somewhat after additional adjustment for covariates in models 2 and 3 but remained significant (table 2). There was a significant trend toward greater total WML volume as the number of apathy symptoms increased (p < 0.001; figure 1).
Apathy symptoms and regional brain volumes.
Participants with ≥2 apathy symptoms had significantly smaller gray matter volumes in all brain lobes, and smaller white matter volumes in the frontal and parietal lobes compared with persons with 0 or 1 apathy symptom (table 2). They also had smaller volumes of hippocampus, amygdala, striatum, and thalamus compared with persons with 0 or 1 apathy symptom (table 2). After additional adjustment for covariates, only associations with smaller frontal and temporal gray matter volumes, parietal white matter volume, and thalamus remained statistically significant (table 2). As the number of apathy symptoms increased, there was a significant trend toward smaller gray matter volume in frontal, parietal, and temporal lobes, normal white matter in frontal and parietal lobes, and hippocampus and thalamus (data not shown).
Apathy symptoms and regional WML load.
Persons with apathy symptoms had significantly greater WML load in the frontal lobe but not elsewhere (figure 2). This association attenuated and was borderline significant in models 2 and 3 (table e-3). Also, there was a significant trend toward greater frontal WML load as the number of apathy symptoms increased (data not shown).
Figure 2. Relations between the presence of 2 or more apathy symptoms and regional white matter lesion load.
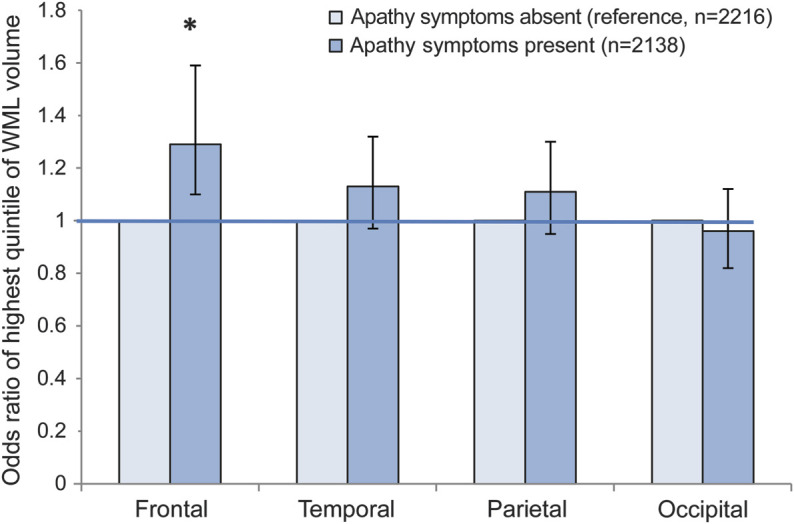
Odds ratios of high white matter lesion (WML) load (highest quintile of WML volume) in different brain regions for participants with 2 or 3 apathy symptoms compared with those with 0 or 1 symptom (reference), adjusted for age, sex, education, and intracranial volume. Error bars indicate 95% confidence intervals. Statistically significant associations (p < 0.050) are indicated with an asterisk.
All associations were independent of concurrent depressive symptoms (GDS-12 score). Excluding participants with a lifetime diagnosis of MDD (n = 198) did not materially change any effect estimates or significance levels (data not shown).
DISCUSSION
In this large-scale population-based cohort study of older persons without dementia, we observed that apathy symptoms were associated with volume reductions in both gray and white matter regions and to a lesser extent with WML load in the frontal lobe, and that these relations were independent of depression.
Important strengths of our study include the population-based design, the large sample size, the standardized assessment and exclusion of dementia and MDD, the volumetric measurements of total as well as regional gray and white matter volumes and WMLs, and the extensive assessment of vascular and behavioral factors for which we could adjust in our analyses.
With this cross-sectional design, we cannot investigate whether brain atrophy and lesions precede apathy symptoms or whether they both result from a common underlying mechanism. In addition, we did not have a formal diagnosis of apathy and can only speak of apathy symptoms. However, the GDS-15 apathy subscale has been shown to have a consistent correlation with a formal diagnosis of apathy19–21 and has been used as a measure of apathy in other large population-based studies.6,22
In line with other population-based studies in older persons, we found that apathy symptoms were the most frequently reported symptoms in the GDS-15 questionnaire.6,19,29 Absolute percentages of reported apathy symptoms vary greatly among different community-based studies, and although our prevalence is higher than some,6,22 it is similar to others.19,29 The majority of persons with apathy symptoms scored below the traditional screening threshold on the GDS-15, indicating that these persons would remain undetected if a conventional cutoff was used. Moreover, 58% of participants stated that they preferred to stay at home, suggesting that these persons may be less likely to seek help, further contributing to an underdetection of apathy symptoms in clinical practice.
Several studies in patients with AD dementia found an association of apathy with WMLs in the frontal region.7,8,30 Because WMLs are also strongly related to cognitive performance,31 findings in persons with dementia may not altogether be generalizable to older persons without dementia. The only other study in a population-based sample without dementia found a relation between deep WMLs and apathy.32 Similarly, 2 other studies found deep WML volume to be associated with motivational symptoms.33,34
Several studies in patients with AD dementia found associations between apathy and gray matter atrophy in medial frontal regions,9,11 striatum, and thalamus.10 Our study adds to these findings in showing that brain atrophy and lesions associated with apathy are already present in older community-dwelling persons without dementia. Nevertheless, regions of gray and white matter loss associated with apathy symptoms in our study resemble those identified in patients with AD dementia, suggesting that impairments in cognitive functioning may have a role. Although the relation between apathy symptoms and brain atrophy in our cohort was independent of mild cognitive impairment, it is still possible that apathy is a subclinical symptom of mild cognitive impairment or dementia. This hypothesis is supported by studies that identified apathy as an early neuropsychiatric symptom in patients with mild cognitive impairment and dementia,35 but also as a risk factor for progression to dementia in patients with subjective memory problems and mild cognitive impairment.36,37 Clearly, prospective studies are needed to examine this further.
In our study, apathy symptoms were associated with greater WML load in the frontal region. This finding is in line with the concept of disruption of frontal-subcortical pathways as an underlying mechanism of late-life depressive symptoms and could be interpreted as support for the “vascular depression” hypothesis. Nevertheless, an important finding was that the relation between apathy symptoms and frontal WML load was independent of depressive symptoms and major depression, providing support for the existence of “vascular apathy” rather than “vascular depression.”38,39 If true, this could have important therapeutic consequences. Whereas serotonergic drugs are frequently prescribed and may be effective to relieve depression, these can cause or aggravate apathy symptoms. In contrast, although experimental, dopaminergic agents might have a role in the treatment of apathy but are ineffective as antidepressant.14 The finding that 10% to 15% of participants in our study used selective serotonin reuptake inhibitors (SSRIs), also in absence of depressive symptoms, may either indicate that mood symptoms were effectively treated or suggest that SSRIs were prescribed for indications other than depression, such as preclinical dementia symptoms. Because we do not have information on prescription indications, we cannot distinguish whether SSRI use may have aggravated apathy symptoms or whether serotonergic drugs may sometimes be prescribed to persons with apathy in whom other drug classes are more appropriate.
Apathy symptoms are highly common in community-dwelling older persons without dementia and often remain unrecognized if a traditional depression screening tool is used. Because treatments for depression and apathy differ, this can have significant clinical implications. Apathy symptoms are associated with a reduction of both gray and white matter volume and to a lesser extent with frontal WMLs, suggesting that both neurodegeneration and cerebral small-vessel disease contribute to apathy in older people without dementia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all of the employees of the Icelandic Heart Preventive Clinic (Hjartavernd) for their skillful contribution to the data collection. The researchers are indebted to the participants for their willingness to participate in the study.
GLOSSARY
- AD
Alzheimer disease
- AGES
Age, Gene/Environment Susceptibility
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FLAIR
fluid-attenuated inversion recovery
- GDS-15
15-item Geriatric Depression Scale
- ICV
intracranial volume
- MDD
major depressive disorder
- SSRI
selective serotonin reuptake inhibitor
- WML
white matter lesion
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. A.M. Grool was involved in the analysis and interpretation of data, and writing of the manuscript. Dr. M.I. Geerlings was involved in the analysis and interpretation of data, and writing of the final manuscript. Dr. S. Sigurdsson and Dr. T.B. Harris were involved in the study design, in the collection of data, and review of the final manuscript. Dr. G. Eiriksdottir, Dr. P.V. Jonsson, Dr. M.E. Garcia, Dr. K. Siggeirsdottir, Dr. T. Sigmundsson, and Dr. V. Gudnason were involved in the interpretation of data, and review of the final manuscript. Dr. L.J. Launer was involved in the study design, in the collection, analysis, and interpretation of data; and writing of the final manuscript.
STUDY FUNDING
The AGES-Reykjavik Study is funded by NIH contract N01-AG-12100, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and Althingi (the Icelandic Parliament).
DISCLOSURE
A. Grool was supported by a grant from the Dutch Heart Foundation (2007B027). M. Geerlings was supported by a grant from the Netherlands Organization for Scientific Research (917-66-311). S. Sigurdsson, G. Eiriksdottir, P. Jonsson, M. Garcia, K. Siggeirsdottir, T. Harris, T. Sigmundsson, V. Gudnason, and L. Launer report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry 2008;79:619–624. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. “Vascular depression” hypothesis. Arch Gen Psychiatry 2007;54:915–922. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry 2002;10:98–106. [PubMed] [Google Scholar]
- 4.Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry 2004;55:390–397. [DOI] [PubMed] [Google Scholar]
- 5.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991;3:243–254. [DOI] [PubMed] [Google Scholar]
- 6.Ligthart SA, Richard E, Fransen NL, et al. Vascular factors are associated with apathy in community-dwelling elderly. Arch Gen Psychiatry 2012;69:636–642. [DOI] [PubMed] [Google Scholar]
- 7.Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD. Neuroimaging correlates of apathy and depression in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 2009;21:259–265. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson M, Edman A, Lind K, Rolstad S, Sjogren M, Wallin A. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. Int J Geriatr Psychiatry 2010;25:588–595. [DOI] [PubMed] [Google Scholar]
- 9.Apostolova LG, Akopyan GG, Partiali N, et al. Structural correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord 2007;24:91–97. [DOI] [PubMed] [Google Scholar]
- 10.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain 2008;131:2455–2463. [DOI] [PubMed] [Google Scholar]
- 11.Tunnard C, Whitehead D, Hurt C, et al. Apathy and cortical atrophy in Alzheimer's disease. Int J Geriatr Psychiatry 2011;26:741–748. [DOI] [PubMed] [Google Scholar]
- 12.Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. J Nerv Ment Dis 1994;182:235–239. [DOI] [PubMed] [Google Scholar]
- 13.Drijgers RL, Aalten P, Leentjens AF, Verhey FR. Apathy: from symptom to syndrome [in Dutch]. Tijdschr Psychiatr 2010;52:397–405. [PubMed] [Google Scholar]
- 14.Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998;10:314–319. [DOI] [PubMed] [Google Scholar]
- 15.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik Study. Neuroimage 2012;59:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145–151. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 19.Adams KB. Depressive symptoms, depletion, or developmental change? Withdrawal, apathy, and lack of vigor in the Geriatric Depression Scale. Gerontologist 2001;41:768–777. [DOI] [PubMed] [Google Scholar]
- 20.Malakouti SK, Fatollahi P, Mirabzadeh A, Salavati M, Zandi T. Reliability, validity and factor structure of the GDS-15 in Iranian elderly. Int J Geriatr Psychiatry 2006;21:588–593. [DOI] [PubMed] [Google Scholar]
- 21.Cheng ST, Chan AC. Withdrawal, apathy and lack of vigor in late life depression: factorial validity and relationship to diagnosis. Aging Ment Health 2007;11:532–537. [DOI] [PubMed] [Google Scholar]
- 22.van der Mast RC, Vinkers DJ, Stek ML, et al. Vascular disease and apathy in old age: the Leiden 85-Plus Study. Int J Geriatr Psychiatry 2008;23:266–271. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:S22–S33. [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler DW. Adult Intelligence Scale Manual. New York: The Psychological Corporation; 1955. [Google Scholar]
- 26.Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry 2006;77:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585–598. [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- 29.Onishi J, Suzuki Y, Umegaki H, Endo H, Kawamura T, Iguchi A. A comparison of depressive mood of older adults in a community, nursing homes, and a geriatric hospital: factor analysis of Geriatric Depression Scale. J Geriatr Psychiatry Neurol 2006;19:26–31. [DOI] [PubMed] [Google Scholar]
- 30.Starkstein SE, Sabe L, Vazquez S, et al. Neuropsychological, psychiatric, and cerebral perfusion correlates of leukoaraiosis in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1997;63:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shim YS, Youn YC, Na DL, et al. Effects of medial temporal atrophy and white matter hyperintensities on the cognitive functions in patients with Alzheimer's disease. Eur Neurol 2011;66:75–82. [DOI] [PubMed] [Google Scholar]
- 32.Yao H, Takashima Y, Mori T, et al. Hypertension and white matter lesions are independently associated with apathetic behavior in healthy elderly subjects: the Sefuri brain MRI study. Hypertens Res 2009;32:586–590. [DOI] [PubMed] [Google Scholar]
- 33.Nebes RD, Vora IJ, Meltzer CC, et al. Relationship of deep white matter hyperintensities and apolipoprotein E genotype to depressive symptoms in older adults without clinical depression. Am J Psychiatry 2001;158:878–884. [DOI] [PubMed] [Google Scholar]
- 34.Grool AM, van der Graaf Y, Mali WP, Witkamp TD, Vincken KL, Geerlings MI. Location and progression of cerebral small-vessel disease and atrophy, and depressive symptom profiles: the Second Manifestations of ARTerial disease (SMART)-Medea Study. Psychol Med 2012;42:359–370. [DOI] [PubMed] [Google Scholar]
- 35.Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer's disease. Am J Geriatr Psychiatry 2005;13:460–468. [DOI] [PubMed] [Google Scholar]
- 36.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer's disease. Dement Geriatr Cogn Disord 2007;24:253–259. [DOI] [PubMed] [Google Scholar]
- 37.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis 2010;20:175–183. [DOI] [PubMed] [Google Scholar]
- 38.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol 1993;50:873–880. [DOI] [PubMed] [Google Scholar]
- 39.Levy R, Czernecki V. Apathy and the basal ganglia. J Neurol 2006;253:VII54–VII61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.