Abstract
Arsenic-contaminated groundwater is a global environmental health concern. Inorganic arsenic is a known carcinogen, and epidemiologic studies suggest that persons with impaired arsenic metabolism are at increased risk for certain cancers, including skin and bladder carcinoma. Arsenic metabolism involves methylation to monomethylarsonic acid and dimethylarsinic acid (DMA) by a folate-dependent process. Persons possessing polymorphisms in certain genes involved in folate metabolism excrete a lower proportion of urinary arsenic as DMA, which may influence susceptibility to arsenic toxicity. A double-blind placebo-controlled trial in a population with low plasma folate observed that after 12 weeks of folic acid supplementation, the proportion of total urinary arsenic excreted as DMA increased and blood arsenic concentration decreased, suggesting an improvement in arsenic metabolism. Although no studies have directly shown that high folate intake reduces the risk of arsenic toxicity, these findings provide evidence to support an interaction between folate and arsenic metabolism.
Keywords: arsenic, cancer, epigenetic, folate
INTRODUCTION
Inorganic arsenic is ubiquitous in the environment and is a global environmental health concern. Individuals can be exposed to inorganic arsenic from mining and smelting metal ores, pesticide manufacturing and application, wood preservatives, medicines, and homeopathic remedies. In non-occupationally exposed populations, ingestion of arsenic-contaminated food and drinking water is the primary route of exposure.1 Currently, the Ganges Delta is the most heavily affected region with over 60 million people exposed to elevated arsenic in their drinking water, although other countries, including Mexico, Chile, Argentina, and the United States, use groundwater contaminated with naturally occurring inorganic arsenic as a potable source.1
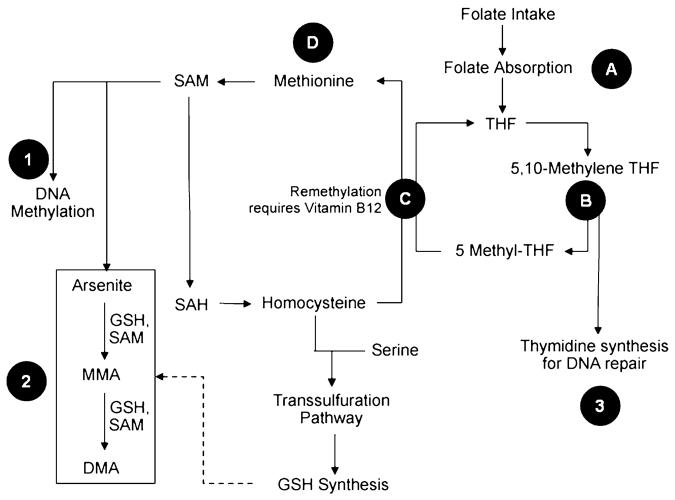
Once ingested, inorganic arsenic is methylated through a series of reduction and oxidation reactions to form monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). S-adenosylmethionine (SAM), produced through the methionine cycle (Figure 1),acts as the methyl donor. Arsenic metabolism varies substantially among species, with humans excreting much more of the intermediate metabolite, MMA, than do other species.2 The percentage of each arsenic metabolite measured in urine, a marker of methylation efficiency, has been shown to vary widely among individuals.3 These findings have led to speculation that certain subpopulations may be genetically more susceptible to the toxic effects of arsenic exposure. Chronic exposure to inorganic arsenic in humans is associated with increased risk of cancer and diabetes as well as reproductive, neurological, cardiovascular, respiratory, hepatic, and hematological effects.1
Figure 1. Folate and arsenic metabolism.
Insufficient folate and vitamin B12 intake could influence arsenic toxicity through aberrant gene expression due to altered DNA methylation (1), decreased arsenic methylation (2), or impaired DNA repair (3). Genetic polymorphisms in the reduced folate carrier gene (RFC-1) can result in lower serum folate levels (A). Two genetic variants (MTHFR C667T and MTHFR A1298C) in methylenetetrahydrofolate reductase (MTHFR) (B) decrease the synthesis of 5-methyl-THF. 5-methyl-THF, along with vitamin B12, is required by the methionine cycle to remethylate homocysteine to methionine (C), in a reaction catalyzed by methionine synthetase (MS, also known as 5-methyltetrahydrofolate-homocysteine methyltransferase, MTR) and to generate S-adenosylmethionine (SAM), in a reaction catalyzed by methionine adenosyltransferase (D).
ARSENIC AND CANCER
Arsenic is a known human carcinogen and chronic exposure to inorganic arsenic has been shown to increase the risk of bladder, liver, lung, and skin cancer.4 Some epidemiologic evidence suggests that the ability to metabolize inorganic arsenic may be related to the risk of developing arsenic-induced cancers. For example, three studies conducted in a region of Taiwan that historically used heavily arsenic-contaminated artesian wells as a source of drinking water observed that persons who excreted a higher percentage of MMA relative to DMA in urine had a greater risk (OR 5.5; 95% CI 1.2–24.8) of arsenic-induced skin lesions5 and increased risk (OR 7.48; 95% CI 1.7–34.0) of skin6 and bladder7 cancers (OR 4.23; 95% CI 1.1–16.0). Each of these studies used a hospital-based case-control design that compared cases (26 cases of skin lesions; 76 cases of skin cancer; and 49 cases of bladder cancer, respectively) with gender- and aged-matched controls, with all study subjects coming from regions that had used arsenic-contaminated artesian wells.
Although these studies are widely cited as evidence that an individual’s ability to metabolize inorganic arsenic influences disease susceptibility, the mode of action for arsenic-induced carcinogenesis has been difficult to determine. Arsenic is not a potent mutagen and when administered alone does not produce tumors in traditional animal models; however, an exception was noted in a transplacental model in mice, where short-term exposure to inorganic arsenic in utero produced liver, lung, ovary, and adrenal tumors in the offspring when they reached adulthood.8 There is also growing evidence that inorganic arsenic acts through epigenetic mechanisms by altering DNA methylation,9 which is catalyzed by DNA methyltransferases that covalently bond the methyl group from SAM to cytosine located 5′ to a guanosine. Typically under-represented in the genome, CpG dinucleotides cluster in the promoter region and intronic regions of genes and are an important mechanism for modulating gene expression.10 Abnormal patterns of DNA methylation, including global DNA hypomethylation and promoter region hypermethylation, have been observed in almost every type of human neoplasia and are believed to be an early event in carcinogenesis.11
Chronic inorganic arsenic exposure has been associated with dose-dependent increases in DNA methylation in the promoter region of tumor suppressor genes, including p53 in human lung adenocarcinoma cell cultures.12 Arsenic has also been associated with increased DNA methylation of p53 and p16 in peripheral blood DNA in humans chronically exposed to arsenic-contaminated water.13 Altered DNA methylation of promoter regions of tumor suppressor genes could result in the silencing of these genes and the loss of their tumor suppressor function. Furthermore, Pilsner et al.14 assessed genomic methylation of peripheral blood leukocyte DNA in Bangladeshi adults and also observed that chronic exposure to inorganic arsenic was associated with increased genomic DNA methylation. The consequences of genomic DNA hypermethylation are still unclear, but it may contribute to inorganic arsenic genotoxicity because 5-methylcytosines deaminate more frequently than cytosine, leading to an increased rate of cytosine to thymine transitions.
FOLATE STATUS, ARSENIC METABOLISM, AND DISEASE RISK
Folate, a water-soluble B vitamin found in green leafy vegetables, citrus fruit, and legumes, serves as the initial methyl donor in methionine biosynthesis, a process that also involves vitamin B12 (Figure 1). Methionine is subsequently activated by ATP to generate S-adenosylmethionine (SAM), the universal methyl donor involved in both arsenic metabolism and DNA methylation. Folate deficiency may influence the risk of cancer by causing epigenetic changes, such as aberrant DNA methylation, resulting in altered expression of oncogenes or tumor suppressor genes.15 Susceptibility to arsenic-induced cancers may be modified by dietary folate intake since the generation of SAM is dependent on folate availability. For instance, Pilsner et al.14 found that the positive association between arsenic exposure and genomic DNA methylation in peripheral blood leukocytes was evident only in persons with folate concentrations of 9 nmol/L or more. Several well-characterized functional genetic polymorphisms that influence folate metabolism may also modify susceptibility to arsenic.16
Animal studies have shown that folate status influences arsenic metabolism and distribution in tissues, but only recently has evidence emerged suggesting that folate status may influence arsenic metabolism in humans. The evidence of such an association appears more convincing when folate status is assessed by measuring plasma folate concentration, rather than by estimating dietary folate intake. Gamble et al.17 conducted a cross-sectional study among 1,650 men and women in Bangladesh to assess the relationship between biomarkers of nutritional status and arsenic metabolism. Folate, vitamin B12, and homocysteine were measured in plasma; urinary arsenic metabolites were measured in a subset of 300 persons. The results indicated the amount of DMA in urine was positively associated with plasma folate and negatively associated with homocysteine. In addition, the percentages of both DMA and inorganic arsenic were inversely associated with plasma folate. These findings suggest that folate status may influence arsenic metabolism.
A subsequent analysis by Heck et al.18 examined the association between dietary intake of folate and other compounds involved in methylation reactions and urinary excretion of arsenic metabolites. In this study, folate intake was not associated with urinary arsenic metabolites. However, higher intakes of several dietary compounds, including protein, cysteine, methionine, and vitamin B12 were associated with lower excretion of inorganic arsenic and higher ratios of MMA to inorganic arsenic in urine. Higher intakes of choline and niacin were also associated with a higher ratio of DMA to MMA. Another study, conducted in the United States, also found no association between urinary methylated arsenic metabolites and folate intake, although it did find that persons with the lowest protein intake excreted less DMA than those with the highest protein intake.19 While these studies do not support a direct link between folate intake and arsenic metabolism, they do suggest a possible association between arsenic metabolism and nutritional factors, including folate and other compounds involved in methylation reactions.
The strongest evidence supporting a specific role for folate in arsenic metabolism comes from a double-blind, placebo-controlled, 12-week folic acid supplementation trial conducted in 200 adults recruited from rural Bangladesh.20 All participants, known to have low plasma folate concentrations at the beginning of the study, were randomly assigned to receive either folic acid supplementation (400 μg/d) or a placebo; compliance with the study regimen was confirmed through direct observation. At the end of the trial, the folic acid-supplemented group excreted significantly more urinary arsenic in the form of DMA than did the control group. In addition, blood levels of both total arsenic and MMA were significantly lower in the folic acid-supplemented group than in the control group. 21 These findings further support a role for folate in arsenic metabolism, and they suggest that folic acid supplementation can favorably alter arsenic metabolism in this population.
Although folate and other nutrients may be important in arsenic metabolism, epidemiologic evidence linking folate status with the risk of arsenic-induced disease is sparse. One study in West Bengal, India, where arsenic exposure in drinking water is high, reported a modest increase in the risk of arsenic-induced skin lesions for persons in the lowest quintiles of animal protein (RR 1.94; 95% CI 1.05–3.59) and folate intake (RR 1.67; 95% CI 0.87–3.2).22 However, no associations were observed between nutritional status and skin lesions when folate and other micronutrients were measured in serum.23 While these results appear to be contradictory, they could reflect exposure misclassification. For some nutrients such as folate, serum levels, while more quantitative, reflect recent (less than 1 month) nutrient intake. Considering that the latency period for arsenic-induced skin lesions is on the order of years, a food questionnaire with a longer recall period may be a more appropriate method for capturing long-term nutrient intake than methods based on recent dietary habits.
GENETIC POLYMORPHISMS AND DISEASE RISK
Besides inadequate dietary folate intake, several genetic polymorphisms in folate-metabolizing genes can impair the methionine cycle (Figure 1) and the generation of SAM, thereby potentially influencing arsenic methylation. To become metabolically active, folic acid must be reduced and methylated, forming 5,10-methylenetetrahydrofolate as an intermediate. This compound is then converted by 5,10-methylenetetrahydrofolate reductase (MTHFR) to 5-methyltetrahydrofolate, which is the metabolically active form of folate found in blood and utilized by tissues.
Two commonly occurring polymorphisms of the MTHFR gene, C677T and A1298C, have been studied widely. Heterozygotes (CT) and homozygotes (TT) for the C677T polymorphism have approximately 65% and 30%, respectively, of the MTHFR activity of persons with the wild type (CC). As a result, TT homozygotes generally have lower plasma folate levels.24 Persons who are homozygotes (CC) for the A1298C polymorphism have approximately 60% of normal MTHFR activity, but it is unclear whether this MTHFR polymorphism results in lower plasma folate levels. Two studies, one conducted in Argentina and another in central Europe, observed that genetic polymorphisms in MTHFR influenced arsenic metabolism. In Argentina, persons with the TT/AA haplotype MTHFR 677/1298, excreted a significantly higher proportion of ingested arsenic as inorganic arsenic and a lower proportion as DMA.25 However, in the central European population, the TT allele for the C677T MTHFR polymorphism was associated with a higher percentage of arsenic excreted as MMA and a lower percentage as DMA compared to the wild-type allele.26 While these outcomes differ slightly, they support the hypothesis that functional polymorphisms in MTHFR influence inorganic arsenic metabolism.
In addition to MTHFR, polymorphisms in other genes involved in folate metabolism, including the folate-binding protein that transports 5-methyltetrahydrofolate to the cell interior, could also influence the relationship between folate status and arsenic-associated disease risk. A study using knock-out mice showed that animals deficient in folate-binding protein 2 were more susceptible to arsenic-induced neural tube defects than were wild-type mice and that the detrimental effects of arsenic were increased by low-folate diets.27 However, no changes in arsenic metabolism were evident in this study, and the authors concluded that the observed neural tube defects were not due to impaired arsenic methylation.
CONCLUSION
While there is mounting evidence that folate influences arsenic metabolism, further research is needed to determine whether poor dietary folate intake is associated with an increased risk of arsenic-induced cancers. Improved understanding of this relationship would help to inform public health campaigns in arsenic-endemic regions, where programs to increase folate intake through supplementation or food fortification with folic acid might help to ameliorate the negative health effects of chronic arsenic exposure. In addition, increased understanding of the effect of genetic polymorphisms in genes involved in folate and arsenic metabolism could be used to help define appropriate folate intakes. A better understanding of the relationship between arsenic and epigenetic events could lead to new treatments for chronic inorganic arsenic exposure that would help reduce the burden of arsenic-associated disease.
Contributor Information
Molly L Kile, Department of Environmental Health, Harvard School of Public Health, 665 Huntington Avenue, Boston, Massachsetts, USA.
Alayne G Ronnenberg, Department of Nutrition, University of Massachusetts Amherst, 209 Chenoweth Laboratory, Amherst, Massachusetts, USA.
REFERENCES
- 1.Mukherjee A, Sengupta MK, Hossain MA, et al. Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr. 2006;24:142–163. [PubMed] [Google Scholar]
- 2.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vahter M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett. 2000;112-113:209–217. doi: 10.1016/s0378-4274(99)00271-4. [DOI] [PubMed] [Google Scholar]
- 4.Bates MN, Smith AH, Hopenhayn-Rich C. Arsenic ingestion and internal cancers: a review. Am J Epidemiol. 1992;135:462–476. doi: 10.1093/oxfordjournals.aje.a116313. [DOI] [PubMed] [Google Scholar]
- 5.Yu RC, Hsu KH, Chen CJ, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1259–1262. [PubMed] [Google Scholar]
- 6.Chen YC, Guo YL, Su HJ, et al. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- 7.Chen YC, Su HJ, Guo YL, et al. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003;14:303–310. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- 8.Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol Appl Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 9.Reichard JF, Schnekenburger M, Puga A. Long-term low-dose arsenic exposure induces loss of DNA methylation. Biochem Biophys Res Commun. 2007;352:188–192. doi: 10.1016/j.bbrc.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird AP. CPG-Rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 11.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Gen. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 12.Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor p53 in human lung cells: a model for a mechanism of carcinogenesis. Mut Res. 1997;386:263–277. doi: 10.1016/s1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]
- 13.Chanda S, Dasgupta UB, GuhaMazumder D, et al. DNA hypermethylation of promoter of gene p53 and p16 in arsenicexposed people with and without malignancy. Toxicol Sci. 2006;89:431–437. doi: 10.1093/toxsci/kfj030. [DOI] [PubMed] [Google Scholar]
- 14.Pilsner JR, Liu X, Ahsan H, et al. Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. Am J Clin Nutr. 2007;86:1179–1186. doi: 10.1093/ajcn/86.4.1179. [DOI] [PubMed] [Google Scholar]
- 15.Kim YI, Pogribny IP, Basnakian AG, et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65:46–52. doi: 10.1093/ajcn/65.1.46. [DOI] [PubMed] [Google Scholar]
- 16.Steinmaus C, Moore LE, Shipp M, et al. Genetic polymorphisms in MTHFR 677 and 1298, GSTM1 and T1, and metabolism of arsenic. J Toxicol Environ Health A. 2007;70:159–170. doi: 10.1080/15287390600755240. [DOI] [PubMed] [Google Scholar]
- 17.Gamble MV, Liu X, Ahsan H, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heck JE, Gamble MV, Chen Y, et al. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85:1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- 19.Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005;113:1153–1159. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamble MV, Liu X, Ahsan H, et al. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84:1093–1101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamble MV, Liu X, Slavkovich V, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86:1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitra SR, Mazumder DN, Basu A, et al. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect. 2004;112:1104–1109. doi: 10.1289/ehp.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung JS, Haque R, Guha Mazumder DN, et al. Blood concentrations of methionine, selenium, beta-carotene, and other micronutrients in a case-control study of arsenic-induced skin lesions in West Bengal, India. Environ Res. 2006;101:230–237. doi: 10.1016/j.envres.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Jacques PF, Bostom AG, Williams RR, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Schlawicke Engstrom K, Broberg K, Concha G, Nermell B, Warholm M, Vahter M. Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect. 2007;115:599–605. doi: 10.1289/ehp.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg AL, Kumar R, Goessler W, et al. Metabolism of low-dose inorganic arsenic in Europe. Environ Health Perspect. 2007;115:1081–1086. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wlodarczyk B, Spiegelstein O, Gelineau-van Waes J, et al. Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicol Appl Pharmacol. 2001;177:238–246. doi: 10.1006/taap.2001.9303. [DOI] [PubMed] [Google Scholar]