ABSTRACT
We demonstrated previously that a knockout (KO) of the lactate dehydrogenase type C (Ldhc) gene disrupted male fertility and caused a considerable reduction in sperm glucose consumption, ATP production, and motility. While that study used mice with a mixed genetic background, the present study used C57BL/6 (B6) and 129S6 (129) Ldhc KO mice. We found that B6 KO males were subfertile and 129 KO males were infertile. Sperm from 129 wild-type (WT) mice have a lower glycolytic rate than sperm from B6 WT mice, resulting in a greater reduction in ATP production in 129 KO sperm than in B6 KO sperm. The lower glycolytic rate in 129 sperm offered a novel opportunity to examine the role of mitochondrial respiration in sperm ATP production and motility. We observed that in media containing a mitochondrial substrate (pyruvate or lactate) as the sole energy source, ATP levels and progressive motility in 129 KO sperm were similar to those in 129 WT sperm. However, when glucose was added, lactate was unable to maintain ATP levels or progressive motility in 129 KO sperm. The rate of respiration (ZO2) was high when 129 KO or WT sperm were incubated with lactate alone, but addition of glucose caused a reduction in ZO2. These results indicate that in the absence of glucose, 129 sperm can produce ATP via oxidative phosphorylation, but in the presence of glucose, oxidative phosphorylation is suppressed and the sperm utilize aerobic glycolysis, a phenomenon known as the Crabtree effect.
Keywords: capacitation, energy metabolism, hyperactivation, sperm motility
Mouse sperm mitochondrial respiration is suppressed and glycolysis is favored in the presence of glucose.
INTRODUCTION
Sperm produce high levels of ATP, 70% of which is utilized by axonemal dynein to drive sperm motility [1]. However, ATP also is required for capacitation, a process involving multiple biochemical changes and signaling events that are essential for fertilization [2–6]. Although the precise underlying molecular processes are unclear, the involvement of cAMP/PKA-dependent signaling pathways is important. An increase in intracellular Ca2+ and bicarbonate (HCO3−) activates the production of cyclic adenylate monophosphate (cAMP) by a soluble adenylate cyclase (ADCY10), which in turn activates protein kinase A to phosphorylate certain proteins [7–11]. In addition to being required for cAMP production, ATP is an essential phosphate donor for kinase reactions.
While ATP is generated in animal cells by two principal processes, glycolysis and mitochondrial oxidative phosphorylation, their relative roles in sperm have been debated [12, 13]. Mitochondrial activity correlates with sperm motility and quality [14, 15], and the efficiency of mitochondrial respiration would suggest that sperm rely on this mechanism for ATP production. However, sperm mitochondria are localized in the middle piece of the flagellum adjacent to the sperm head, and it is unlikely that ATP can distribute rapidly enough over the length of the flagellum by simple diffusion to support motility [16, 17]. In addition, sperm motility is maintained in the presence of inhibitors of mitochondrial oxidative phosphorylation, but not in the presence of inhibitors of glycolysis [18, 19].
Multiple glycolytic enzymes are localized in the principal piece region of the flagellum in close proximity to the axonemal motor apparatus and often are products of novel spermatogenic cell-specific genes [20]. Glycolysis was shown to be essential for ATP production in sperm by using gene targeting to disrupt some of these genes. Mice with a knock out (KO) of the Gapdhs (glyceraldehyde 3-phosphate dehydrogenase, spermatogenic) gene [21] were infertile, and male mice with a KO of the Pgk2 (phosphoglycerate kinase 2) gene were severely subfertile [22] due to impairment of sperm motility and low ATP levels. In contrast, mice with a KO of the Cyct (cytochrome c, testis) gene encoding a protein essential for mitochondria respiration were fertile [23].
The gene for the glycolytic enzyme lactate dehydrogenase C (Ldhc) also is expressed predominantly in spermatogenic cells, and LDHC is abundant in sperm [24, 25]. We reported previously the generation of LDHC-null (KO) mice using 129SvEvTac embryonic stem cells and the phenotype of males from the third and fifth generations backcrossed with C57BL/6 (B6) wild-type (WT) mice [26]. On this mixed genetic background, the male LDHC KO mice were subfertile or infertile and the motility rates and ATP levels of their sperm declined more rapidly in vitro than occurred for WT sperm [27]. In the present study, we used mice that resulted from backcrossing heterozygous Ldhc mice with WT B6 and 129 (129) mice for ten generations to determine the effects of the two different genetic backgrounds on sperm from Ldhc KO mice. Because 129 mice have a Pgk2 allelic variant resulting in low PGK2 activity [28, 29], this also provided a unique opportunity to utilize 129 Ldhc KO sperm with severely inhibited glycolysis as a model to examine the role of mitochondrial respiration in sperm motility.
MATERIALS AND METHODS
Materials and Reagents
Materials and reagents were of the highest quality available and purchased from Sigma-Aldrich (St. Louis, MO) or Mallinckrodt Baker (Phillipsburg, NJ), unless indicated otherwise.
Animal Procedures
Animal procedures were performed in accordance with the National Institute of Health (NIH) Guide and approved prior to use by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences, NIH. Mice were euthanized by CO2 asphyxiation followed by cervical dislocation. Adult mice were 3 to 5 mo old; aged mice were a minimum of 16 mo old. Inbred C57BL/6Ncrl (B6) WT mice were from Charles River (Raleigh, NC), and 129S6SvEvTac (129) WT mice were from Taconic (Germantown, NY). This study was performed with animals from the tenth backcrossed generation. The first generation of mice was generated with TC1 129S6SvEvTac ES cells and C57BL/6Ncrl blastocysts as described previously [26]. Backcrosses were carried out using heterozygous (Ldhc+/−) mutant males and B6 or 129 WT females.
To assess male fertility, individual B6 (n = 8) and 129 (n = 10) Ldhc KO males were mated continuously for 4 wk with two WT females of the same genetic background. The females were replaced with two females for an additional 4 wk. Individual Ldhc KO females were mated with one WT male for 8 wk. All the females were maintained for 4 wk after mating to assess possible pregnancy.
Sperm Preparation
Sperm were isolated from the cauda epididymis by swim-out in 1 ml 1 × PBS (Ca2+/Mg2+-free) and washed in 13 ml 1 × PBS followed by gentle centrifugation (10 min at 300 × g) to remove energy substrates present in epididymal fluid.
The number of spermatozoa was determined using a hemocytometer. Sperm were diluted in TYH medium (119.37 mM NaCl, 4.78 mM KCl, 1.71 mM CaCl2.2H2O, 1.19 mM MgSO4.7H2O, 1.19 mM KH2PO4, 25 mM NaHCO3) [30] containing 5 mg/ml bovine serum albumin, supplemented with various substrates, and incubated for selected lengths of time at 37°C in 5% CO2 in humidified air.
Sperm Motility and ATP Level Assessment
Sperm motility was determined by computer-assisted sperm analysis (CASA) (Hamilton Thorne Research, Beverly, MA) as described previously [27]. Sperm were counted as motile with any type of movement and as progressively motile when smoothed path velocity > 50 μm/sec and straightness > 50%. Hyperactive sperm were identified with the sort fraction function of the CASA analyzer by using these criteria: track velocity > 240 μm/sec, amplitude of lateral head displacement > 18 μm, and beat cross frequency < 40 Hz [31]. Median values of each of the kinematic parameters were obtained for each sample. The remaining sperm were centrifuged at 1000 × g for 5 min, resuspended in 100 mM Tris-HCl, 4 mM ethylenediaminetetraacetic acid, pH 7.8, and boiled for 5 min to determine the ATP level using a luciferase bioluminescence assay according to the manufacturer's protocol (ATP Bioluminescence Assay kit CLS II; Roche Applied Science, Indianapolis, IN).
Nuclear Magnetic Resonance Spectroscopy
Metabolism of [1-13C]-glucose was assessed by nuclear magnetic resonance (NMR) as described previously [27]. Briefly, 15 × 106 viable sperm from the cauda epididymis were collected in HEPES-TYH without substrates. Six millimolar [1-13C]-glucose (Isotec/Sigma-Aldrich) was added, and NMR acquisition quickly initiated using a Varian INOVA 500 NMR spectrometer. Data were collected in 1 h blocks of time and followed for 20 h.
Activity of Glycolytic Enzymes
Glycolytic enzyme activity was assayed as described previously [27]. Enzymatic activity was assayed in solution by monitoring the NADH or NADPH concentrations at 340 nm by spectrophotometry over a period of 1 to 30 min as described previously [32].
Oxygen Consumption
Oxygen consumption (ZO2) by sperm was monitored using a BD Oxygen Biosensor 96-well plate system (BD Biosciences, San Jose, CA) as described [33]. Each experiment was performed in room air at room temperature (RT). To reduce pH variability, the sperm were isolated and resuspended in TYH medium supplemented with 10 mM HEPES. Fluorescence was monitored in a SpectraMax Gemini EM microplate reader (Molecular Device, Sunnyvale, CA) at 485 nm excitation and 630 nm emission from the base of the plate. The oxygen biosensor plate (BD Biosciences) was kept at RT, and a first read of the empty plate was performed to allow each well to be referenced against its own initial signal. Controls of 0% oxygen (300 μl 100 mM Na2SO3, freshly prepared) and medium without spermatozoa (as indicator of passive changes in oxygen level) were loaded each time in duplicate. Wells contained 200 μl medium and were allowed to equilibrate at RT for at least 1 h before 100 μl of membrane-intact cauda sperm were added (5 × 106 per well). Serial readings were taken every 5 min for 1 h. The results are expressed as the concentration of O2 consumed ([O2] = [O2]t − [O2]t0 mmHg/107 sperm/min).
Data Analysis
All the results are represented as the mean values of each group ± SEM. The significance of the results was determined using one-way ANOVA followed by a Bonferroni multiple comparison test. Paired t-test was used to compare mean differences from samples treated under different conditions, with these samples derived from the same pool of sperm. Differences were considered significant at P < 0.05.
RESULTS
Fertility Is Different in B6 and 129 KO Male Mice
After backcrossing heterozygous (Ldhc+/−) male mice for 10 generations with female WT B6 or 129 mice, the resulting males were estimated to contain an average of 0.098% residual amount of the genome of the opposite strain. By comparison, mice used in our previous studies backcrossed for three generations with B6 mice [26, 27] contained an average of 12.5% of the 129 genome and 87.5% of the B6 genome. No differences were seen in body weight, reproductive organ weights, or sperm counts for wild type (WT), heterozygous (Ldhc+/−), and KO (Ldhc−/−) mice within the B6 and 129 strains (Tables 1 and 2). However, significant differences were seen between B6 and 129 mice, with higher body weight, lower testis weight, and higher sperm counts in B6 mice. No other differences were apparent between adults (3 to 5 mo of age) and older males (16 mo or older) (Tables 1 and 2).
TABLE 1.
Phenotypes of wild-type (WT), Ldhc+/− (HET), and Ldhc-null (KO) male mice in the tenth backcrossed generation onto the C57BL/6 or 129S6 generation backgrounds, 3 to 5 mo old.a
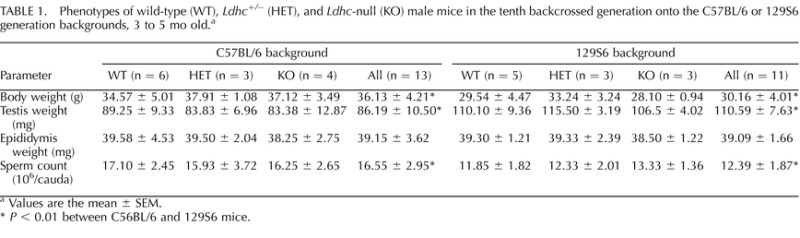
Values are the mean ± SEM.
P < 0.01 between C56BL/6 and 129S6 mice.
TABLE 2.
Phenotypes of wild-type (WT), Ldhc+/− (HET), and Ldhc-null (KO) male mice in the tenth backcrossed generation onto the C57BL/6 or 129S6 generation backgrounds, more than 16 mo old.a
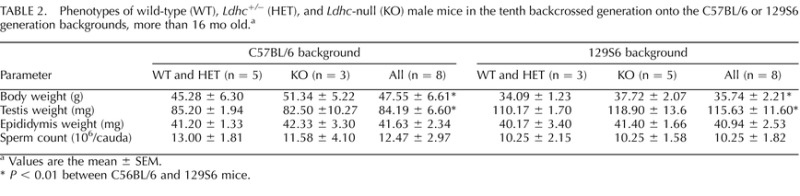
Values are the mean ± SEM.
P < 0.01 between C56BL/6 and 129S6 mice.
The 2 mo mating studies showed that B6 KO males (n = 8) were subfertile, with six males siring no pups and two siring a litter of one pup and three pups, respectively. However, no pups were sired by 129 KO males (n = 10) in the mating study. The KO females from both backgrounds were fertile. There were no significant differences in the fertility of WT and heterozygous B6 and 129 males.
Glycolysis Is Different in Sperm from B6 and 129 Mice
We demonstrated previously that the infertility of Ldhc KO males was associated with a defect in sperm energy metabolism. To explore this further, we compared ATP levels and progressive motility in KO and WT sperm from B6 and 129 mice incubated for 2 h under three conditions (Fig. 1): 1) in medium with 1 mM glucose as the energy substrate, 2) in medium with no substrate, and 3) in medium with 1 mM glucose plus 10 μM iodoacetate (IA) as a selective inhibitor of glyceraldehyde-3-phosphate dehydrogenase and glycolysis [34–36].
FIG. 1.
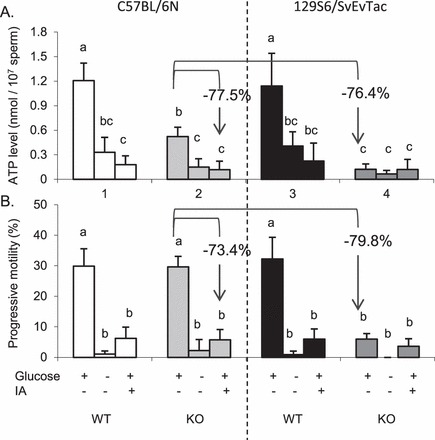
ATP levels (A) and progressive motility (B) of sperm from WT and KO C57BL/6 (B6) and 129S6 (129) mice. Sperm were incubated for 2 h in capacitating medium at 37°C in 5% CO2 in humidified air with 1 mM glucose (+), no substrates (−) or 1 mM glucose (+) plus 10 μM sodium iodoacetate (IA). The percentages of decrease between B6 KO sperm in medium with glucose and with IA, and between B6 KO and 129 KO sperm are noted. Letters (a, b, c) correspond to statistical multiple comparison tests and denote no significant difference if identical or a significant difference if not identical, P < 0.05.
In medium with glucose, ATP levels (Fig. 1A) were lower in KO compared to WT in both strains. However, the decrease in KO versus WT sperm was more pronounced in the 129 sperm (−89.2%), with a similar decrease being observed in progressive motility (−81.4%; Fig. 1B) compared to B6 sperm (−56.7% for the ATP level, and no significant difference for the progressive motility). A further comparison between B6 and 129 sperm showed that these parameters were comparable for 129 WT and B6 WT sperm, but lower in 129 KO than in B6 KO sperm (−76.4% for the ATP level and −79.8% for the progressive motility). These results demonstrate that ATP levels are lower in 129 WT and KO sperm than in B6 WT and KO sperm incubated in medium containing glucose and that lack of LDHC is considerably more detrimental to progressive motility in 129 than in B6 sperm.
In medium without glucose, the ATP levels (Fig. 1A) in B6 KO sperm were lower than in medium with glucose (71.4%), but not significantly different in 129 KO sperm. In medium with glucose plus IA, the ATP levels in B6 KO sperm were lower than in medium with glucose alone (77.5%), but not significantly different in 129 KO sperm. Similar results were observed for reductions in progressive motility (Fig. 1B) for B6 KO sperm in medium without glucose or in medium with glucose plus IA (92.5% and 80.7%, respectively). These data indicate that while ATP production by glycolysis is reduced substantially in B6 KO sperm, they produce significantly more ATP by glycolysis than 129 KO sperm.
Glucose Consumption and Glycolytic Enzyme Activities Are Different in Sperm from B6 and 129 Mice
Glucose consumption by B6 WT and 129 WT sperm was assayed using [1-13C]-glucose and NMR spectroscopy (Fig. 2A). Glucose consumption by B6 WT sperm was substantially higher than the consumption for 129 WT sperm during the period assayed. The rate of glucose consumption for B6 WT sperm was 12.93 ± 3.48 nmol/h/106 sperm and for 129 WT sperm was 3.84 ± 1.15 nmol/h/106 sperm.
FIG. 2.
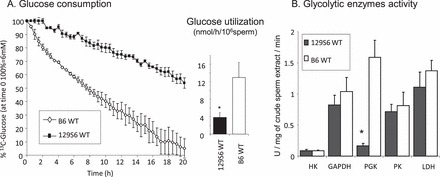
A) [1-13C]-glucose consumption by 129 and B6 WT sperm measured by NMR spectroscopy. At time 0, [1-13C]-glucose was added to 15 × 106 living sperm and utilization of [1-13C]-glucose was monitored every hour for 20 h. Columns correspond to the calculated nanomoles of glucose consumed per hour per 106 sperm. B) Activity of different glycolytic enzymes was measured in 129 WT and B6 WT sperm extracts (HK, hexokinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; PK, pyruvate kinase; LDH, lactate dehydrogenase). Values are the mean ± SEM for 129 versus B6, n = 3 minimum, *P < 0.01.
The activities of five glycolytic enzymes were measured in B6 WT and 129 WT crude sperm extracts to identify a possible explanation for the differences in glucose consumption (Fig. 2B). We found that the PGK activity was approximately 10 times higher in B6 WT than in 129 WT sperm, while the activities of the other glycolytic enzymes were not significantly different.
Mitochondrial Substrates Are Able to Rescue ATP Levels and Progressive Motility in 129 KO Sperm
After washing to remove energy substrates in epididymal fluid, 129 WT and KO sperm were incubated for 2 h in medium containing selected substrates at 1 mM each (Fig. 3): 1) glucose as a substrate for glycolysis, 2) pyruvate or lactate as substrates for mitochondrial oxidative phosphorylation, or 3) a combination of glucose and lactate. The proton gradient uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP; 10 μM) was added in parallel incubations with these substrates to inhibit sperm mitochondrial activity. The effects of these substrates on ATP levels, progressive motility, and hyperactivated motility were determined.
FIG. 3.
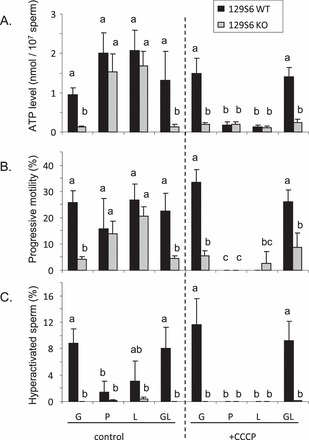
ATP levels (A), progressive motility (B), and hyperactivated motility (C) measured in sperm from 129 WT and KO mice. Sperm incubated for 2 h in capacitating medium at 37°C in 5% CO2 in humidified air with 1 mM glucose (G), pyruvate (P) or lactate (L), and glucose plus lactate (GL). CCCP (carbonyl cyanide m-chlorophenylhydrazone) was added to inhibit mitochondria activity. Values are the mean ± SEM, n = 3. Letters (a, b, c) denote no significant difference if identical or significant difference if not identical, P < 0.05.
The 129 WT sperm in medium containing glucose or glucose plus CCCP, had similar ATP levels (Fig. 3A), progressive motility (Fig. 3B) and hyperactive motility (Fig. 3C). The lack of effect of CCCP on sperm motility in the presence of glucose was consistent with earlier reports [18, 19] that glycolysis is sufficient to maintain mouse sperm motility. However, 129 KO sperm had markedly lower levels of ATP and motility in both media, presumably due to compromised glycolysis in the absence of LDHC (Fig. 1A) and their low PGK activity level (Fig. 2B).
In medium containing pyruvate or lactate, 129 WT and 129 KO sperm showed similar levels of ATP (Fig. 3A) and progressive motility (Fig. 3B), but these were reduced significantly by addition of CCCP. However, pyruvate or lactate did not support development of hyperactivated motility (Fig. 3C). These results show that even when the glycolytic pathway is disrupted by a lack of LDHC, the production of ATP through mitochondrial oxidative phosphorylation is sufficient to maintain progressive motility but not hyperactivated motility.
In medium containing glucose plus lactate, 129 WT sperm showed normal levels of ATP (Fig. 3A) and motility (Fig. 3B), and these levels were not reduced by addition of CCCP. However, 129 KO sperm in medium containing glucose and lactate (GL) had extremely low levels of ATP (Fig. 3A) and motility (Fig. 3B), comparable to the levels in 129 KO sperm incubated in medium with glucose alone. This was unexpected because 129 KO sperm were able to utilize lactate to produce ATP (Fig. 3A). These results suggest that in 129 KO sperm lacking LDHC activity and with low PGK2 activity, the utilization of lactate by oxidative phosphorylation is disrupted by the presence of glucose.
Glucose Inhibits the Utilization of Lactate and ZO2 by Mitochondria
To examine further the effect of glucose on lactate utilization, sperm from 129 WT and 129 KO mice were incubated for 2 h in medium containing 1 mM lactate and different concentrations of glucose (0.001 to 1 mM). As a control, sperm were incubated in medium without lactate and containing 1 mM glucose (Fig. 4A). There was an increase in the percentage of hyperactivated 129 WT sperm with increasing glucose concentrations but no differences in progressive motility or ATP levels. In contrast, ATP levels and progressive motility of 129 KO sperm decreased significantly with the addition of glucose, confirming that glucose had a negative effect on lactate utilization by 129 KO sperm.
FIG. 4.
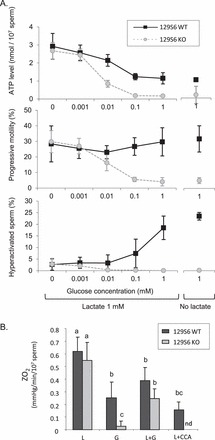
A) Influence of an increase in glucose concentration on ATP levels, the percentage of progressive motility and hyperactivated motility of sperm from 129 WT and 129 KO mice. Sperm were incubated in capacitating medium at 37°C in 5% CO2 in humidified air with 1 mM lactate. One measurement was performed in medium containing 1 mM glucose and no lactate. B) Oxygen consumption (ZO2) was measured using an Oxygen Biosensor System. The 129 WT and KO sperm were incubated 2 h at room temperature in the dark with 1 mM lactate (L), 1 mM glucose (G), or 1 mM lactate plus 1 mM glucose (L+G), and fluorescence emission indicating the level of oxygen present in the medium was monitored every 5 min for 1 h. ZO2 were calculated in mmHg per min per 107 spermatozoa. The 129 WT sperm were treated with CCA (α-cyano-4-hydroxycinnamic acid, 10 mM), an inhibitor of mitochondrial lactate and pyruvate transport, as a negative control (nd = not determined in 129S6 sperm). Values are the mean ± SEM, n = 3 minimum. Letters (a, b, c) denote no significant difference if identical or significant difference if not identical, P < 0.05.
The effect of different energy substrates on mitochondrial respiration was determined by measuring ZO2. Sperm from 129 KO and 129 WT mice were incubated in medium with 1 mM lactate, 1 mM glucose, or 1 mM lactate plus 1 mM glucose (Fig. 4B). As a negative control, 129 WT sperm were incubated in medium with 1 mM lactate plus 10 mM α-cyano-4-hydroxycinnamic acid (L+CCA), an inhibitor of mitochondrial lactate and pyruvate transport [37]. With lactate, ZO2 was highest in 129 WT and 129 KO sperm. With glucose, ZO2 was partially reduced in 129 WT sperm and considerably reduced in 129 KO sperm. With glucose plus lactate, ZO2 was higher in 129 WT and 129 KO sperm than with glucose, but lower than with lactate. ZO2 was lowest in 129 WT sperm in medium with L+CCA. These results indicate mitochondrial respiration is reduced in the presence of glucose, particularly in 129 KO sperm, but this effect can be alleviated partially by the presence of lactate in the medium.
DISCUSSION
We demonstrated previously that some Ldhc KO male mice with the mutation on a mixed B6 and 129 genetic background were subfertile, while others were infertile [26, 27]. The current study found that B6 KO male mice were subfertile and 129 KO male mice were infertile. These differences correlated with the findings that ATP levels and progressive motility levels in 129 KO sperm were lower than in B6 KO sperm. Although other genetic factors may be involved, this probably is due mainly to the effect on glycolysis of the lower level of PGK activity in 129 sperm than in B6 sperm (Fig. 2B). A novel PGK isozyme (PGK2) is expressed specifically in male germ cells, and three mouse strain-specific allelic variants of PGK2 have been observed. A low activity variant is present in 129/Sv strain mice [28, 29]. It was shown that complete disruption of the Pgk2 gene resulted in impaired sperm function and a severe reduction in male fertility [22]. However, there were no significant differences in fertility of 129 WT and B6 WT males, indicating that while glycolysis is essential for sperm function, the presence of a higher activity variant of PGK2 does not result in higher sperm fertilizing ability in mice.
The current study used sperm from 129 Ldhc KO mice with both low PGK2 activity and lacking LDHC, resulting in substantially diminished sperm glycolytic activity. We found that 129 KO sperm incubated in medium containing the nonglycolysable substrates pyruvate or lactate as the sole energy source were able to generate sufficient ATP to maintain progressive motility. The CASAnova program for defining slow and weak categories of progressive motility [34] was not available at the time of this study. However it was clear that lactate or pyruvate were not able to induce hyperactivated motility (Fig. 3C). The addition of CCCP, an uncoupler of oxidative phosphorylation, blocked the production of ATP by 129 KO sperm in these media, showing that the mitochondrial oxidative phosphorylation pathway was responsible for this effect. While there are likely to be strain differences, these results are consistent with other studies showing that ATP in sperm is produced both by glycolysis and oxidative phosphorylation, but that glycolysis is required for sperm to achieve hyperactivated motility and the changes in tyrosine phosphorylation associated with capacitation [19].
Although sperm are able to metabolize pyruvate to generate ATP by oxidative phosphorylation and WT sperm can utilize LDHC to convert lactate to pyruvate, it is not clear how LDHC-null sperm are able to utilize lactate to generate ATP. Earlier studies reported that LDHC is present in the sperm mitochondrial matrix [35–37], suggesting a role for LDHC in mitochondrial activity as a potential lactate shuttle. This led us to determine if mitochondrial respiration was able to compensate for the impairment in energy production in LDHC KO sperm. However, our results showed that mitochondrial respiration is intact in LDHC-null sperm, indicating that LDHC is not essential for mitochondrial respiration, but this does not rule out the possibility that another LDH is involved. Indeed, LDHA is present in sperm flagellum [38] and might be also present in sperm mitochondria. There is also the existence of a new isoform LDHAl6B, and some evidence suggests a mitochondrial localization of LDHAl6B [39]. In addition, our results confirmed that mitochondria respiration does not require active glycolysis to support sperm motility [13, 40].
An unexpected finding was that ATP and progressive motility levels were lower in 129 KO sperm in medium containing lactate plus glucose than in medium with lactate alone. Similar results were observed with inhibitors of glycolysis in mouse sperm [18] and in rat or boar sperm [40, 41]. Those studies showed that in medium containing pyruvate or lactate, the addition of glycolytic inhibitors (deoxy-glucose or α-chlorohydrin) did not have a significant effect on motility or ATP levels when glucose was absent, but both were reduced to very low levels when glucose was present. We also observed that the respiration rate, as measured by ZO2, was higher when 129 WT or 129 KO sperm were incubated in medium with lactate alone than in medium with glucose or with lactate plus glucose. The presence of glucose induced a decrease in the respiration rate even in aerobic conditions and in the presence of a mitochondrial substrate. This effect was dose dependent, indicating that residual amounts of energy substrates in epididymal fluid could lead to differences in measurements of sperm energy production and function in vitro.
The phenomenon whereby cells produce ATP by aerobic glycolysis in the presence of glucose rather than by oxidative phosphorylation is known as the Crabtree effect [42]. Although not reported previously in mouse sperm, it has been observed in bull [43] and guinea pig sperm [44, 45]. The sperm in these two species appear to rely largely on mitochondrial respiration for energy production, and sperm capacitation is inhibited by glucose. It remains to be determined if this phenomenon occurs in sperm from species thought to rely primarily on glycolysis, including boar and human [19, 46]. Although it was first described over 80 years ago, the mechanisms underlying the Crabtree effect remain unclear. Hypotheses that have been proposed include competition between oxidative phosphorylation and glycolysis for ADP and inorganic phosphate [13] and glucose acting as a signaling molecule to enhance Ca2+ uptake by the mitochondria, leading to inhibition of ATP synthase [47]. However, the former hypothesis seems unlikely in 129 KO mouse sperm where glucose is not metabolized [27]. A recent study showed that fructose acting as a fuel of glycolysis was not sufficient to induce hyperactivation [48] but could support hyperactivation when mitochondrial function was uncoupled with CCCP. This might suggest that fructose cannot induce a Crabtree effect, leading to its inability to induce hyperactivation. Although our studies with 129 KO mouse sperm have been informative about the relationship between glycolysis and oxidative phosphorylation in mouse sperm, further studies will be required to determine the mechanisms involved in producing the Crabtree effect in mouse sperm.
ACKNOWLEDGMENT
The authors thank Drs. Deborah O'Brien and Summer Goodson for their helpful discussions of the data, Linwood Koonce for the excellent animal care, and Polina Danshina and Paula Brown for critically reading this manuscript.
Footnotes
Supported by the Intramural Research Program of the NIH, National Institutes of Environmental Health Sciences (E.M.E., ES070076).
REFERENCES
- Bohnensack R, Halangk W. Control of respiration and of motility in ejaculated bull spermatozoa. Biochim Biophys Acta 1986; 850: 72 79. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature 1952; 170: 326. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951; 168: 697 698. [DOI] [PubMed] [Google Scholar]
- Chang MC. The meaning of sperm capacitation. A historical perspective. J Androl 1984; 5: 45 50. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Sperm capacitation and gamete interaction. J Reprod Fertil Suppl 1989; 38: 27 33. [PubMed] [Google Scholar]
- Tulsiani DR, Zeng HT, Abou-Haila A. Biology of sperm capacitation: evidence for multiple signalling pathways. Soc Reprod Fertil Suppl 2007; 63: 257 272. [PubMed] [Google Scholar]
- Carlson AE, Hille B, Babcock DF. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol 2007; 312: 183 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 2004; 101: 2993 2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 2006; 296: 353 362. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129 1137. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995; 121: 1139 1150. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Diez-Sanchez C, Lopez-Perez MJ, Enriquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol 2007; 77: 3 19. [DOI] [PubMed] [Google Scholar]
- Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 2006; 12: 269 274. [DOI] [PubMed] [Google Scholar]
- Ferramosca A, Focarelli R, Piomboni P, Coppola L, Zara V. Oxygen uptake by mitochondria in demembranated human spermatozoa: a reliable tool for the evaluation of sperm respiratory efficiency. Int J Androl 2008; 31: 337 345. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan K, Padwal V, D'Souza S, Shah R. Severe asthenozoospermia: a structural and functional study. Int J Androl 1995; 18 (Suppl 1): 67 74. [DOI] [PubMed] [Google Scholar]
- Nevo AC, Rikmenspoel R. Diffusion of ATP in sperm flagella. J Theor Biol 1970; 26: 11 18. [DOI] [PubMed] [Google Scholar]
- Takao D, Kamimura S. FRAP analysis of molecular diffusion inside sea-urchin spermatozoa. J Exp Biol 2008; 211: 3594 3600. [DOI] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71: 540 547. [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001; 22: 680 695. [PubMed] [Google Scholar]
- Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl 2007; 65: 309 325. [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA 2004; 101: 16501 16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, McCarrey JR, Eddy EM, O'Brien DA. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod 2009; 82: 136 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC, Millan JL. Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol Cell Biol 2002; 22: 5554 5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. Reproductive implications of LDH-C4 and other testis-specific isozymes. Exp Clin Immunogenet 1985; 2: 120 124. [PubMed] [Google Scholar]
- Goldberg E, Eddy EM, Duan C, Odet F. LDHC: the ultimate testis-specific gene. J Androl 2010; 31: 86 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F, Duan C, Willis WD, Goulding EH, Kung A, Eddy EM, Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod 2008; 79: 26 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F, Gabel SA, Williams J, London RE, Goldberg E, Eddy EM. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol Reprod 2011; 85: 556 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeberg JL, Blohm SV. An allelic isozyme of mouse PGK-2 with low activity. J Exp Zool 1977; 201: 479 483. [DOI] [PubMed] [Google Scholar]
- VandeBerg JL, Cooper DW, Close PJ. Testis specific phosphoglycerate kinase B in mouse. J Exp Zool 1976; 198: 231 240. [DOI] [PubMed] [Google Scholar]
- Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod 1986; 34: 349 356. [DOI] [PubMed] [Google Scholar]
- Mortimer ST. CASA—practical aspects. J Androl 2000; 21: 515 524. [PubMed] [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis, Verlag Chemie, vol. 1. Weinheim: Academic Press; 1974. [Google Scholar]
- Garrett LJ, Revell SG, Leese HJ. Adenosine triphosphate production by bovine spermatozoa and its relationship to semen fertilizing ability. J Androl 2008; 29: 449 458. [DOI] [PubMed] [Google Scholar]
- Goodson SG, Zhang Z, Tsuruta JK, Wang W, O'Brien DA. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol Reprod 2011; 84: 1207 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos C, Maldonado C, Gerez de Burgos NM, Aoki A, Blanco A. Intracellular localization of the testicular and sperm-specific lactate dehydrogenase isozyme C4 in mice. Biol Reprod 1995; 53: 84 92. [DOI] [PubMed] [Google Scholar]
- Montamat EE, Vermouth NT, Blanco A. Subcellular localization of branched-chain amino acid aminotransferase and lactate dehydrogenase C4 in rat and mouse spermatozoa. Biochem J 1988; 255: 1053 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Takahashi T, Iguchi N, Kitamura K, Miyagawa Y, Tsujimura A, Matsumiya K, Okuyama A, Nishimune Y. Ketone bodies could support the motility but not the acrosome reaction of mouse sperm. Int J Androl 2004; 27: 172 177. [DOI] [PubMed] [Google Scholar]
- Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod 2006; 75: 270 278. [DOI] [PubMed] [Google Scholar]
- Holmes RS, Goldberg E. Computational analyses of mammalian lactate dehydrogenases: human, mouse, opossum and platypus LDHs. Comput Biol Chem 2009; 33: 379 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford WC, Harrison A. The concerted effect of alpha-chlorohydrin and glucose on the ATP concentration in spermatozoa is associated with the accumulation of glycolytic intermediates. J Reprod Fertil 1986; 77: 537 545. [DOI] [PubMed] [Google Scholar]
- Ford WC, Harrison A. Futile substrate cycles in the glycolytic pathway of boar and rat spermatozoa and the effect of alpha-chlorohydrin. J Reprod Fertil 1987; 79: 21 32. [DOI] [PubMed] [Google Scholar]
- Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochem J 1929; 23: 536 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardy HA, Phillips PH. The interrelation of oxidative and glycolytic processes as sources of energy for bull spermatozoa. Am J Physiol 1941; 133: 602 609. [Google Scholar]
- Mujica A, Moreno-Rodriguez R, Naciff J, Neri L, Tash JS. Glucose regulation of guinea-pig sperm motility. J Reprod Fertil 1991; 92: 75 87. [DOI] [PubMed] [Google Scholar]
- Rogers BJ, Chang L, Yanagimachi R. Glucose effect on respiration: possible mechanism for capacitation in guinea pig spermatozoa. J Exp Zool 1979; 207: 107 112. [DOI] [PubMed] [Google Scholar]
- Marin S, Chiang K, Bassilian S, Lee WN, Boros LG, Fernandez-Novell JM, Centelles JJ, Medrano A, Rodriguez-Gil JE, Cascante M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett 2003; 554: 342 346. [DOI] [PubMed] [Google Scholar]
- Wojtczak L. The Crabtree effect: a new look at the old problem. Acta Biochim Pol 1996; 43: 361 368. [PubMed] [Google Scholar]
- Goodson SG, Qiu Y, Sutton KA, Xie G, Jia W, O'Brien DA. Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol Reprod 2012; 87 (3): , 1 15. [DOI] [PMC free article] [PubMed] [Google Scholar]


