ABSTRACT
The gonadotropin surge is the essential trigger to stimulate ovulation and luteinization of ovarian follicles. While the hormone signals from the brain that initiate ovulation are known, the specific targets which regulate this process are not well known. In this study, we assessed the suitability of the Rhox homeobox gene cluster to serve as the master regulators of folliculogenesis. In superovulated (equine chorionic gonadotropin [eCG]/human chorionic gonadotropin [hCG]) mice, the Rhox genes exhibited four distinct windows of peak expression, suggesting that these genes may regulate specific events during the ovulatory cycle. Like many members of the cluster, Rhox8 mRNA and protein were induced by follicle stimulating hormone [FSH]/eCG in granulosa cells. However, Rhox8 displayed unique peak expression at 8 h post-hCG administration, implying it might be the lone member of the cluster regulated by progesterone. Subsequent promoter analysis in granulosa cells revealed relevant homeobox binding and progesterone response elements within Rhox8's 5′-flanking region. In superovulated mice, progesterone receptor (PGR) is recruited to the Rhox8 promoter, as assessed by chromatin immunoprecipitation. In Rhox5-null mice, Rhox8 mRNA was reduced at 2 h and 4 h post-hCG administration but recovered once the follicles passed the antral stage of development. Conversely, in progesterone receptor knockout mice, Rhox8 exhibited normal stimulation by eCG but failed to reach its peak mRNA level at 8 h post-hCG found in wild-type mice. This suggests a model in which Rhox8 transcription is dependent upon RHOX5 during early folliculogenesis and upon progesterone during the periovulatory window when RHOX5 normally wanes. In support of this model, transfection of RHOX5 and PGR expression plasmids stimulated, whereas dominant negative and mutant constructs inhibited, Rhox8 promoter activity.
Keywords: gene regulation, granulosa cells, progesterone, progesterone receptor, transcriptional regulation
The Rhox homeobox gene cluster is differentially expressed during folliculogenesis in mice; RHOX8 is a potential candidate for translating progesterone signaling into successful ovulation.
INTRODUCTION
Mammalian ovulation is a complex, hormone-dependent developmental program that requires the coordinated expression of many genes that must be turned on and off in the right place at the right time for proper development of the follicle. In periovulatory follicles, rapid translation of the luteinizing hormone (LH) surge in granulosa cells is necessary for successful ovulation and differentiation of luteal cells [1]. Progesterone signaling is established following the LH surge and is critical for ovulation but less so for luteinization [2, 3]. Many studies have identified promising candidate target genes (e.g., Adamts1, Ctsl) that respond to nuclear progesterone receptor (PGR) and promote ovulation and that encode proteinases, inflammatory modulators, and kinases [4–6]. Interestingly, secondary analyses of the promoter regions of these genes have failed to identify progesterone response elements [4, 7–9], suggesting that a progesterone-induced transcription factor or signaling pathway is necessary to bridge the gap between PGR and complete ovulation.
Homeobox genes encode DNA-binding proteins that are master regulators of many developmental programs, best characterized in the embryo [10, 11], but reports of their relevance in governing cellular differentiation, proliferation, and survival postnatally are increasing [12, 13]. Approximately 200 homeobox genes have been characterized in rodents, and ∼33% are known to be expressed in the gonads [12, 14]. However, a clear function for most of them has yet to be identified as their ablation often results in malformation of a key system, leading to pre- or perinatal lethality that precludes examination of their role in fertility [12]. The Arx, Emx2, and Lhx9 genes are expressed in bipotential gonads, where they govern key events during the early stages of gonadogenesis [15–18]. Several homeobox factors, including Irx3, Obox, Nobox, and Lhx8, contribute to the establishment of primordial ovarian follicles and postnatal folliculogenesis [19–22]. However, most of the ovarian transcription factors of interest are oocyte-specific [23]. Few homeobox factors have been investigated in ovarian somatic cells. Recent in vitro studies support a role for HOXA7 in granulosa cell proliferation via an epidermal growth factor-dependent mechanism [24]. The expression of Lbx2 is differentially regulated in thecal and granulosa cells during folliculogenesis, but its role in these cells has not been established [25].
The reproductive homeobox X-linked (Rhox) genes are good candidates for the regulation of both male and female reproductive tissue development and physiology as they are selectively expressed in the gonads, epididymis, and placenta [12]. While many Rhox genes have been detected in whole adult ovaries [12], detailed analysis of Rhox cluster expression has been limited to embryonic ovary, where they are predominantly restricted to primordial germ cells [26]. In the postnatal ovary, only the expression and regulation of the founding member of the cluster, Rhox5, have been demonstrated. In mice, RHOX5 is detected in the granulosa cells of preovulatory follicles [27]. In rats, Rhox5 expression in primary granulosa cells is dependent on the coordinated actions of SP1 and ETS family of transcription factors [28]. However, the expression of Rhox5 is transient, peaking prior to the induction of PGR and waning to near background levels during the dominant phase of progesterone signaling. We previously reported that Rhox5-null female mice are viable and fertile, suggesting that RHOX5 is either not essential for ovulation or that one of the other RHOX factors may compensate functionally in granulosa cells [29]. In order to identify uniquely expressed, as well as potentially redundant, RHOX factors, we examined the expression patterns of all 33 Rhox genes by using an equine chorionic gonadotropin (eCG)-primed, human CG (hCG)-induced superovulation model. Our gene profiling revealed that the Rhox cluster is differentially regulated during folliculogenesis, which identified one gene selectively induced by PGRA, and provides further evidence for Rhox gene cross-regulation as we have recently reported in the epididymis [30].
MATERIALS AND METHODS
Animal Care and Breeding
All animals were handled according to National Institutes of Health (NIH) guidelines and in compliance with the Southern Illinois University Carbondale Institutional Animal Care and Use Committee. All animals were maintained under a 12L:12D schedule and fed NIH-31 mouse chow (Labdiet 5008; Purina). Rhox5-null C57BL/6 female mice were generated by breeding knockout (Rhox5−/y) males either with homozygous (Rhox5−/−) females or with heterozygous (Rhox5+/−) females to produce wild-type (WT) and knockout (KO) comparisons between littermates, as described previously [27, 29]. For the superovulation studies, female mice at Postnatal Day 21 (P21) were treated with 3.25 IU of eCG (Sigma Chemical Co.). This treatment was followed 48 h later by treatment with 2.2 IU of hCG (Sigma). Both eCG and hCG were dissolved in 0.85% saline solution and injected subcutaneously in a total volume of 0.1 ml. At least five samples were collected for each time point and each genotype spanning all segments of the ovarian cycle. The time points matched immature phase (no hormone), preovulatory phase (eCG alone and 0, 2, and 4 h after hCG administration), periovulatory phase (6, 8, and 10 h after hCG administration), and postovulatory phase (12, 16, and 24 h after hCG). All mice were humanely euthanized by carbon dioxide asphyxiation, and both ovaries were extirpated. One ovary was homogenized in 500 μl of Trizol (Invitrogen) for RNA isolation according to the manufacturer's recommendations, and the other ovary was fixed in 4% paraformaldehyde (dissolved in PBS, pH 7.4) for 12–16 h, and then processed and embedded in paraffin for immunohistochemistry analysis.
Plasmids
We cloned 3.0-kb mouse Rhox8 5′-flanking genomic DNA into pGEM (Promega). From this plasmid, we used high-accuracy PCR to generate deletion fragments that were cloned into the pGL3 luciferase reporter plasmid (Promega) that contained 2556, 2062, 1981, 1412, 1357, 1200, 989, 873, 702, 552, 402, 249, and 72 nucleotides (nt) of putative Rhox8 promoter. Deletion mutagenesis was performed to eliminate the putative progesterone response element (PRE) between nt 1412 and 1357 in the 2062-nt promoter construct as described previously [31]. To overexpress RHOX5, Rhox5 coding sequence was cloned into HaloTag pHT2 (Promega), which expresses its insert under the control of the cytomegalovirus (CMV) promoter. Plasmids encoding progesterone receptors have been described previously and were kindly provided by Daniel Carson (Rice University [32]) and Lydia Arbogast (Southern Illinois University [33]).
Quantitative Real-Time RT-PCR Analysis
The quantity and quality of total RNA were determined by spectrometry and denaturing agarose gel electrophoresis, respectively. The cDNA was synthesized from total RNA (2 μg) using iScript Select cDNA synthesis kit (Bio-Rad). Real-time quantitative RT-PCR (qPCR) analysis of mRNA expression was performed using a MyiQ single-color real-time PCR detection system (Bio-Rad) with iQ SYBR Green Supermix (Bio-Rad) as the detector according to manufacturer's recommendations. Primers (described previously [30]) were designed to amplify cDNAs of approximately 200 bp, and all cDNAs exhibited similar amplification efficiency (97% ± 3%) as assessed by amplification of cDNA dilution series. PCR cycle parameters were 95°C for 15 sec and 60°C for 1 min for 40 cycles. The threshold line was set in the linear region of the plots above the baseline noise, and threshold cycle (CT) values were determined as the cycle number at which the threshold line crossed the amplification curve. PCR without template or template substituted with total RNA were used as negative controls to verify experimental results. After amplification, the specificity of the PCR was determined by both melting curve analysis and gel electrophoresis to verify that only a single product of the correct size was present. Data were normalized against Rpl19 and are shown as the average fold-value increase ± SEM.
Immunohistochemistry
Immunolocalization of RHOX8 was performed in cross-sections (5 μm) of paraffin-embedded ovarian sections by using rabbit polyclonal antibody at 1:2000 dilution (product 2223B; Imgenex) and a Vectastain Elite ABC kit (Vector Laboratories). Negative controls were created in which the primary antibody was replaced by preimmune serum from the rabbit used by Imgenex prior to injection of the peptide QEMKEREENAGIQDC, corresponding to amino acids 30–44 of the amino domain of mouse RHOX8. This peptide was selected based on its highly divergent sequence from other mouse RHOX proteins and possessed only 53% identity to the corresponding region of rat RHOX8, suggesting that cross-reactivity in rat ovaries and granulosa cells would be unlikely. Unfortunately, a second antibody (2224A; Imgenex) produced against the middle portion of RHOX8 (residues 235–251), which was highly conserved between mouse and rat, failed to stain any testicular or ovarian cells (data not shown). Antigen retrieval using a boiling citrate buffer was performed as described previously [34].
Cell Culture
Primary granulosa cells were isolated as described previously [28]. Briefly, 21-day-old mice were given a single injection of 3.25 IU of eCG subcutaneously in a total volume of 0.1 ml. Ovaries were removed and immediately transferred to a 60-mm cell culture dish containing 5 ml of Dulbecco modified Eagle medium/F12 medium (DMEM/F12) (50:50 mixture) supplemented with 1 mg/ml BSA, 2.5 μg/ml amphotericin B (Fungizone), and 50 μg/ml gentamicin (all reagents from Invitrogen). Granulosa cells from multiple ovaries were pooled according to genotype and treatment and were treated with 20 μg/ml trypsin for 1 min, and then 300 μg/ml soybean trypsin inhibitor and 160 μg/ml DNase I were added to remove necrotic cells. Cells were cultured at 37°C in 95% air and 5% CO2 for 16 h before transfection. Spontaneously immortalized clonal granulosa cells (SIGC) were grown in DMEM/F12 medium supplemented with 5% FBS (Atlanta Biologicals) that was charcoal-stripped of hormones. Transient transfection of both primary granulosa and SIGC was performed using Attractene transfection reagent (Qiagen), which outperformed Lipofectamine 2000 (Invitrogen) and Turbofect (Fermentas), as assessed by cotransfection with green fluorescent protein (GFP) expression plasmids. Cell lysates were prepared and used to measure luciferase activity according to standard protocols (dual luciferase assay system; Promega). Relative light units were normalized to the internal control plasmid pRL-TK and expressed as fold-change greater than that of the empty pGL3-basic vector.
In Situ Hybridization
In situ hybridization analysis in mouse ovaries was conducted using methods described previously [30, 35]. Briefly, deparaffinized, rehydrated, and deproteinated cross-sections (5 μm) of one ovary from each mouse were hybridized with radiolabeled sense or antisense cRNA probes generated from linearized plasmid DNA templates, using in vitro transcription with α-35S-uridine 5′-triphosphate. For Rhox8, we used a 500-nt cDNA probe (corresponding to 681-1181 nt downstream of the transcription start site) to exclude the large GAA repeat region to preclude potential cross-reactivity with trinucleotide repeat genes [29]. After hybridization, washing, and ribonuclease A digestion, slides were dipped in NTB liquid photographic emulsion (Kodak), stored at 4°C for 4–30 days, and developed in D-19 developer (Kodak). Slides were then counterstained with Gill modified hematoxylin (Stat Lab), dehydrated through a graded series of alcohol to xylene (Fisher), and protected with a coverslip.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed using a protocol previously used to analyze PDX1 homeobox factor target promoters in pancreatic cells [36], with some modifications. Ovaries were extirpated from immature or superovulated mice (eCG, hCG, 8 h), washed with PBS, and disrupted in 500 μl of 1% formaldehyde (in PBS) using a single short pulse of the tissue homogenizer on its lowest setting. An additional 9.5 ml of 1% formaldehyde was quickly added and cells were incubated for 10 min at RT. Fixation was stopped by adding glycine to a final concentration of 0.125 M. The ovarian cells were then washed three times with cold PBS containing 1 mM PMSF and 1% protease inhibitor cocktail (Sigma), harvested, and lysed on ice for 10 min in 600 μl of ChIP sonication buffer (50 mM Tris [pH 8.0], 0.1% deoxycholate, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100) with freshly added proteinase inhibitors, and the DNA was sheared by sonication. Cellular debris was pelleted and the supernatant was transferred to a fresh tube and brought to 1.2 ml with additional ChIP sonication buffer. Chromatin preparations were precleared by incubation with a Dynabead protein G-agarose/salmon-sperm DNA slurry (Novex) according to the manufacturer's instructions (Life Technologies) and then immunoprecipitated overnight at 4°C with either 1 μg of anti-PGR (RB-9017-P0; Thermo Fisher) or preimmune rabbit serum (immunoglobulin G [IgG]). Dynabead protein G-agarose/salmon sperm DNA slurry was added to immunoprecipitate the antibody-protein complexes, and these complexes were washed following the manufacturers' instructions. Immune complexes were eluted with a buffer containing 1% SDS and 0.1 M NaHCO3. Reverse-crosslinking was performed by incubation with 200 mM NaCl at 65°C for 4 h. Samples were treated with proteinase K for 1 h, and the DNA was purified by phenol/chloroform extraction, ethanol precipitated, and resuspended in 100 μl of H2O. PCR amplification was done with 2 μl of each DNA sample, 200 μM of each dNTP, 1.5 mM MgCl2, 1 unit of ExTaq DNA polymerase (Takara), 1× buffer (supplied), and 0.2 μM of each primer. Amplification of the PRE-containing region of the Rhox8 promoter was performed using the specific forward 5′-CTTTTGTTTGGTGCAGGCCC-3′ and reverse 5′-GAGGACTTTTGTTTGGTGCAG-3′ primers. The 5-kb downstream control region was amplified using the forward 5′-TCTGGAGTGCTCTCATGGTG-3′ and reverse 5′-GCCTATGTGGTCAGAGAAAGCCCACTC-3′ primers. The PCR products were resolved by electrophoresis in a 1% agarose gel and visualized by ethidium bromide staining.
Statistical Analysis
All qPCR and transfection data were subjected to one-way ANOVA, and differences between individual means were tested by a Tukey multiple-range test using Prism version 4.0 software (GraphPad). All qPCR data were corrected for differences in sample loading by using the Rpl19 data as a covariate. Tests of significance were performed using the appropriate error terms according to the expectation of the mean squares for error. A P value of 0.05 or less was considered significant. Data are presented as least-square means ± SEM.
RESULTS
Rhox Cluster Expression Is Regulated During the Ovarian Cycle
The mouse Rhox gene cluster contains 33 genes. Ten single-copy genes, Rhox1 and Rhox5–Rhox13 [29, 37], and several almost identical paralog copies of three genes (Rhox2, Rhox3, and Rhox4) that were recently derived by tandem duplication of a trimer unit [12, 38] (Fig. 1A). To determine the expression of these Rhox genes during folliculogenesis, we used qPCR analysis with primer pairs specific for each of the single-copy genes and the universal Rhox2, Rhox3, or Rhox4 primer, as described previously [30] (Fig. 1B). Rhox1 mRNA expression is initially higher in the immature ovary. When the ovary is stimulated with eCG, Rhox1 expression apparently decreases by 50% prior to ovulation. Stimulation by hCG caused further decline in relative Rhox1 mRNA levels during the preovulatory period (2 h and 4 h post-hCG administration). After ovulation (12 h post-hCG), there is no detectable Rhox1 expression. In contrast, Rhox2 expression was low in the immature ovary. However, Rhox2 was induced by eCG and reached its peak level at 4 h post-hCG during the preovulatory phase. During and after ovulation, Rhox2 mRNA was still detected, but it was significantly lower. Rhox3 expression also peaked during the preovulatory phase at 4 h post-hCG, but its total expression was less than that of Rhox2. Thus, Rhox3 expression during ovulation and afterwards was not as diminished relative to its peak as was the case for Rhox1 or Rhox2. The final gene in the alpha cluster, Rhox4, exhibited its highest expression in postovulatory follicles, but relative expression levels were not variable enough to reach significant differences at any time point.
FIG. 1.
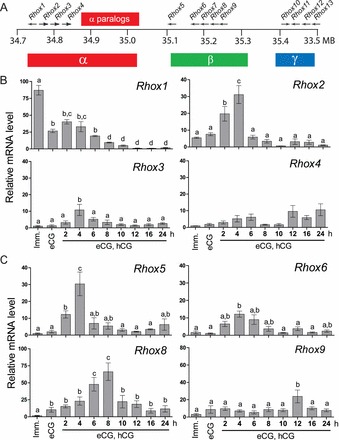
Differential expression of mouse Rhox cluster family members in superovulated mice. A) Schematic of the mouse Rhox gene cluster, which contains 13 distinct primary genes and a 7-unit tandem duplication of Rhox2, Rhox3, and Rhox4, indicated as “α-paralogs” for simplicity. B) qPCR analysis of relative α subcluster Rhox gene expression in mice induced to superovulate with eCG and hCG. Values were normalized relative to those of ribosomal L19 (Rpl19) mRNA and are expressed as fold-change above background (±SEM), which was arbitrarily given a value of 1. Equivalent results were obtained when cyclophilin mRNA was used as the internal control (data not shown). Letters denote means that were significantly different (P < 0.05). C) qPCR analysis of the Rhox β-subcluster analyzed as above. Imm, immature.
We previously reported the expression and regulation of the first gene in the beta subcluster Rhox5 in rat granulosa cells [28], and RHOX5 protein has been localized to granulosa cells of preovulatory follicles in mice [27]. In agreement with these findings, our qPCR analysis demonstrated that in superovulated mice, Rhox5 is expressed after stimulation of eCG and peaks 4 h after hCG injection (Fig. 1C). Rhox6 expression is also induced by eCG and reaches its peak 4 h post-hCG, but it is not as highly expressed as Rhox5. Interestingly, Rhox7 expression is not consistently detected in the ovary at any phase of ovulation (data not shown). Conversely, the next gene in the sequence, Rhox8, exhibited the highest level of expression in ovulatory follicles, reaching its peak during the periovulatory phase at 8 h post-hCG administration. Rhox9 mRNA levels were fairly consistent during follicular development but did display a 2.5-fold increase only at 12 h post-hCG. Threshold cycles observed for the gamma subcluster genes Rhox10–Rhox13 were near those of no RT background controls (CT ∼35) or failed entirely, suggesting they are not highly expressed in the ovary. Detection of these genes between time points, and between animals within the same time point, was too inconsistent to characterize their differential expression during folliculogenesis and to identify their peak phase (data not shown). These primers have been used previously to quantify mRNA expression in testis and epididymal developmental time courses, mid-term placenta, and embryonic stem cells [29, 30, 37, 39].
RHOX8 Is Expressed Predominantly in Granulosa Cells of the Mouse Ovary
The mRNA expression pattern we observed for Rhox8 suggested that the gene is induced by FSH in proliferating granulosa cells and remains high through the LH surge until the granulosa cells differentiate into luteal cells. To confirm this prediction, immunohistochemistry was performed using a newly derived polyclonal antibody raised against the amino terminus (residues 30–45) of RHOX8 (Fig. 2). The oocyte is devoid of RHOX8 protein staining at all stages of follicular development. RHOX8 protein was not detected in the granulosa layers of primordial and primary follicles. However, as multiple layers of granulosa cells proliferate in the growing follicles, weak staining can be observed in granulosa cells adjacent to the oocyte (Fig. 2C, arrowhead, and see Supplemental Fig. S1, available online at www.biolreprod.org). This staining becomes weaker and more diffuse in granulosa cells of large preantral follicles (Fig. 2C and Supplemental Fig. S1). However, strong nuclear RHOX8 staining appears in the thecal layers of follicles at this stage and persists through the remainder of follicular development (Fig. 2D, arrowheads). After antrum formation, strong nuclear staining of granulosa cells is reacquired (Fig. 2, C–E) and becomes murally distributed, reaching a peak at 8 h post-hCG injection, as correlated with the qPCR findings. Interestingly, RHOX8 protein is excluded from cumulus granulosa cells surrounding the oocyte in periovulatory follicles (Fig. 2, C and E, arrows). After ovulation, RHOX8 protein remains in the developing corpus luteum (Fig. 2F). However, given that Rhox8 mRNA is highly downregulated after 8 h post-hCG administration, it is unclear if new RHOX8 continues to be translated or if the observed protein is a carry-over from granulosa cells as luteinization proceeds. Our initial in situ hybridization analysis indicated that Rhox8 mRNA is downregulated in luteal cells (data not shown), but we have not formally examined this with subsequent analyses.
FIG. 2.
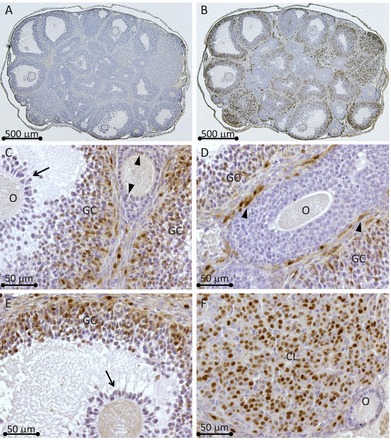
Immunolocalization of RHOX8 protein in mice. A) Serial ovarian sections from eCG primed mice, after 8 h hCG-treated mice were incubated with either preimmune serum (1:250 dilution) or RHOX8 polyclonal antiserum (1:2500 dilution) (B) from Imgenex rabbit no.2223B immunized with a RHOX8 amino domain peptide. Visualization by diaminobenzidine staining reveals that most of the RHOX8 protein is localized to granulosa cells of large antral follicles (granulosa cells are shown at higher magnification in C−F). Weak RHOX8 protein staining can be observed in some granulosa cells of preantral follicles (C, arrowheads) and is absent in cumulus granulosa cells (C and E, arrows) surrounding the oocyte (O). D) Nuclei of thecal cells (arrowheads) are also positive for RHOX8. F) RHOX8 expression remains in the corpus luteum (CL) of postovulatory follicles.
Identification of the Rhox8 Promoter
As a first step to examine the mechanism by which Rhox8 is regulated in granulosa cells, serial deletions of the Rhox8 5′-flanking sequence were cloned into the pGL3-basic luciferase reporter vector. These constructs were transiently transfected into primary mouse granulosa cells from superovulated mice, as described previously [28], and relative promoter activity was determined by luciferase assay. Strong Rhox8 promoter activity was conferred by the 2-kb 5′-flanking sequence (Fig. 3A) and did not increase when extended to 3 kb (data not shown). Truncation of the 1.4-kb sequence by 55 bp resulted in a 50% loss of promoter activity (Fig. 3A). Interestingly, the only predicted transcription factor binding site (as assessed by TFSEARCH version 1.0 and TESS software analysis) present in this region is a PRE (Fig. 3B). Further deletion of the Rhox8 promoter did not elicit any statistically significant decline in luciferase activity. However, eliminating the binding sites for globin transcription factor 1 (GATA1), estrogen response element (ERE), c-MYC binding sites, resulted in complete loss of promoter activity (data not shown), suggesting these factors contribute to the minimal essential promoter for Rhox8 expression in granulosa cells. Because the largest significant drop in promoter activity occurred upon elimination of the putative PRE element, we further examined its importance by deletion of this region within the context of the 2-kb promoter region. We found that the presence of a putative homeobox (HBOX), glucocorticoid receptor response element (GRE), and potential unidentified transcription factor binding motifs between nt −2062 and −1412 did not confer any additional Rhox8 promoter activity as luciferase activity was equivalent to the 1357-nt promoter construct.
FIG. 3.
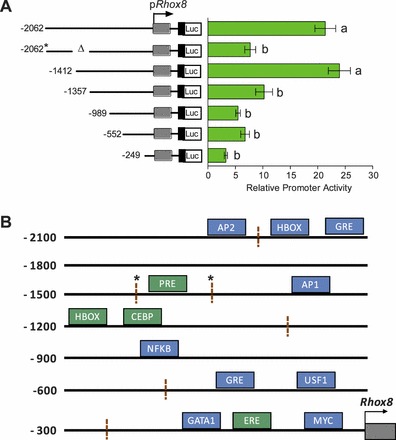
PRE and homeobox consensus binding sites contribute to Rhox8 transcription. A) Immature mice were induced to superovulate and euthanized at 8 h post-hCG treatment, their ovaries were removed, and granulosa cells were purified and cultured. A deletion series of Rhox8 genomic DNA starting 2 kb upstream of the transcription start site was cloned in the pGL3 luciferase promoter vector. These constructs were transiently transfected along with pRL-TK and analyzed by dual luciferase measurement 36 h after introduction into granulosa cells. The relevance of the putative PRE element was tested by deletion within the context of the 2-kb promoter (−2062 nt). Transfection assays were performed in triplicate, and the means ± SEM for multiple determinations are indicated. Results shown are fold-increases relative to those from the empty vector pGL3-basic, which was given an arbitrary value of 1. B) The TFSEARCH and TESS algorithms were used to identify putative transcription factor binding sites within the Rhox8 promoter. These factors are color coded according to their predicted role in Rhox8 regulation based on a survey of the literature and our transfection data: stimulatory factors are green and factors of unknown relevance are blue. Hashed lines indicate breakpoints used for the deletion series displayed in A; * indicates the region deleted from the 2-kb promoter flanking the putative PRE.
RHOX5 Control in the Regulation of Rhox8 Expression in Granulosa Cells
While not statistically significant in cultured primary granulosa cells, truncation of the Rhox8 promoter from 1357 to 989 nt resulted in a two-fold decline in relative promoter activity (Fig. 3A). This region has an activator of protein 1 (AP1) binding site, one CTAAT consensus homeodomain protein binding element (HBOX), and a CCAAT-enhancer-binding protein (CEBP) site. The identification of a relevant homeobox binding site suggested the possibility that other RHOX factors may regulate Rhox8. We have recently demonstrated that Rhox8 expression is downregulated in the epididymis following ablation of Rhox5 [30, 40]. Thus, we first examined Rhox8 expression in Rhox5-null mice and found that during the window in which Rhox5 expression is observed (Fig. 1C), Rhox8 mRNA is significantly decreased in eCG-primed and 2-h and 4-h post-hCG-superovulated ovaries (Fig. 4A). At 6 h post-hCG and beyond, no significant differences in Rhox8 mRNA levels were found. Next, primary granulosa cells were transiently cotransfected with the 1200-nt Rhox8 promoter reporter vector and vectors overexpressing each Rhox gene. Only Rhox5 elicited a three-fold increase in Rhox8 expression (Fig. 4B and data not shown). In situ hybridization analysis confirmed that Rhox8 mRNA is expressed in preantral follicles (although it was near to the detection limit) (Fig. 5A) and reduced in Rhox5-null mice (Fig. 5B). However, no change in Rhox8 expression was observed in larger Graafian follicles (Fig. 5, C and D). The Rhox8 in situ probe appeared to hybridize to some oocytes (Fig. 5A, arrows), and this hybridization could still be observed in Rhox5-null animals (Fig. 5B, arrows). However, there was no obvious RHOX8 protein localized to oocytes (Fig. 2), so at present, it is not clear if oocytes store Rhox8 mRNA that is not translated or if the in situ signal in oocytes is nonspecific. Subtle differences in RHOX8 protein levels can be observed between preantral follicles from WT and Rhox5-null mice (data not shown). However, because most of the RHOX8 protein is found in granulosa cells of antral follicles where Rhox5 is waning and Rhox8 mRNA is not diminished in these follicles from Rhox5-null animals, we chose to pursue other transcriptional regulators of Rhox8.
FIG. 4.
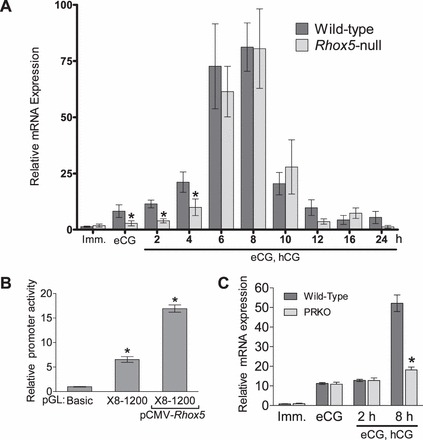
RHOX5 and progesterone signaling induce Rhox8 transcription in granulosa cells. A) Endogenous Rhox8 expression in superovulated Rhox5-null mice, determined as described in the legend to Figure 1. B) Primary granulosa cells were transfected with the 1200-bp promoter construct and RHOX5 expression plasmid as described in the legend to Figure 3. C) Endogenous Rhox8 mRNA expression in superovulated PRKO mice. Imm., immature. *P < 0.01.
FIG. 5.
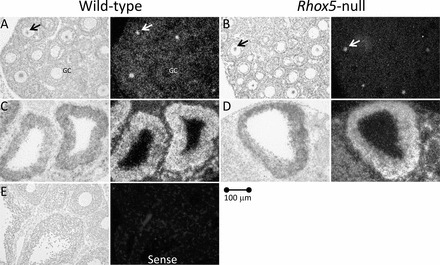
In situ hybridization of Rhox8 in mouse ovary. Representative photomicrographs show that Rhox8 mRNA is reduced in granulosa cells of preantral follicles in Rhox5-null mice (A, B) eCG primed, 2 h post-hCG but not altered in large antral follicles (C, D) eCG primed, 6 h post-hCG. Arrows show accumulation of Rhox8 antisense probe in some oocytes that is not translated (Fig. 2C) or may be nonspecific. E) No appreciable signal was observed with the Rhox8 sense strand probe in any tissue section.
Progesterone Regulation of Rhox8 Expression in Granulosa Cells
The observations that Rhox8 mRNA was not decreased in Rhox5-null animals beyond 4 h post-hCG administration (Fig. 4A and 5) and that Rhox8 promoter activity decreased after elimination of a PRE (Fig. 3A) suggested that progesterone signaling may induce Rhox8 expression in periovulatory granulosa cells. In support of this, endogenous Rhox8 expression was lower in progesterone receptor knockout mice (PRKO) that had been induced by exogenous gonadotropins (Fig. 4C). Subsequent immunohistochemical analysis of RHOX8 protein in PRKO animals revealed strong downregulation of RHOX8 expression in mural granulosa cells (Fig. 6). However, a minor subpopulation of antral granulosa cells continues to express RHOX8 in PRKO ovaries (Fig. 6, B and C, arrows). The residual Rhox8 transcription in PRKO ovaries (Fig. 4C) likely stems from a mixture of these cells, preantral follicles where putative RHOX5 stimulation is still present, and the thecal layer, which interestingly remains RHOX8-positive (Fig. 6, B and C, arrowheads).
FIG. 6.
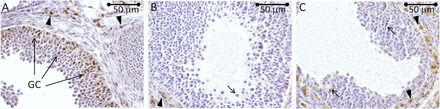
RHOX8 is downregulated in PRKO ovaries. Immunolocalization of RHOX8 in WT mice was performed as described in the legend to Figure 1. Abundant expression of RHOX8 was detected in mural granulosa cells of antral follicles from WT ovaries (A) and nearly ablated in similar follicles from PRKO animals (B and C), although a few granulosa cells retained expression (arrows). Expression of RHOX8 in the thecal layer remains prevalent (arrowheads) in PRKO ovaries.
The elimination of a PRE in Rhox8's promoter reduced its activity (Fig. 3A), suggesting that regulation by progesterone may be direct rather than through secondary activation by PGR-dependent transcription factors. To address this issue, we used ChIP to investigate PGR occupancy of the putative PRE within Rhox8's promoter (Fig. 7). The addition of antibody against PGR successfully pulled down PGR-PRE complexes from superovulated mice but not from immature animals that were not induced with gonadotropins, suggesting that peak Rhox8 expression is achieved by direct stimulation of Rhox8 by PGR. This interaction was specific, as PGR antiserum did not interact with a chromatin region downstream of the Rhox8 gene and the nonspecific control rabbit IgG failed to produce a signal with the PRE-specific PCR probe.
FIG. 7.
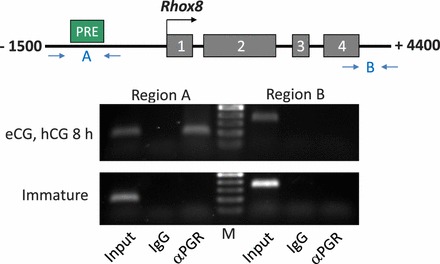
Gonadotropin-induced recruitment of PGR to the PRE region of the Rhox8 promoter. ChIP analysis was performed as described in Materials and Methods. Cross-linked sheared chromatin was immunoprecipitated with PGR antibody, and recovered PGR-chromatin complexes were subjected to PCR analysis using primers flanking the putative PRE (region A) or a 5-kb downstream, non-PRE containing genomic region (region B). Schematic shows the Rhox8 promoter (−1500 nt; the four coding exons of Rhox8 and the downstream region are not drawn to scale). The IgG lanes are ChIP assays performed using nonspecific IgG. Positive controls for the PCR represent inclusion of 10% of the starting material used for ChIP (Input). The ethidium bromide-stained gel of PCR products shows a representative result from six gonadotropin-stimulated ovaries and two immature animals. M, 100-bp ladder for PCR product size determination (top band, 500 bp).
The PGR antibody we used for immunohistochemistry and ChIP does not distinguish between PGRA and PGRB. To determine which form may be more relevant for Rhox8 regulation, we used an immortalized rat granulosa cell line (SIGC) that lacks expression of endogenous nuclear progesterone receptors [41, 42]. First we examined the endogenous levels of rat Rhox genes in the cell line by qPCR. Interestingly, we found expression similar to that which we observed in superovulated mice, namely that the gamma cluster genes were not detected and that the alpha and beta cluster genes that were induced by eCG treatment were expressed in low levels (Fig. 8A). Rhox5 was highly expressed in SIGC, which is likely due in part to normal granulosa-specific promoter induction but also to the immortalization of this cell line by TRP53 mutation [41] as Rhox5 is well known to be aberrantly expressed in tumor cells regardless of their tissue of origin [14]. SIGC were transiently transfected with the pGL3-Rhox8 promoter constructs containing (1412 nt) and lacking (1357 nt) the PRE sequence. Cotransfection of an expression construct encoding PGRA stimulated Rhox8 promoter activity upon addition of P4, whereas constructs encoding PGRB or a mutant form of PGRA lacking signaling activity failed to increase Rhox8 expression (Fig. 8B). Elimination of the PRE resulted in loss of PGRA's stimulatory capability, further indicating that induction of Rhox8 expression is direct rather than via induction of a progesterone-dependent factor that binds elsewhere in Rhox8's regulatory region.
FIG. 8.
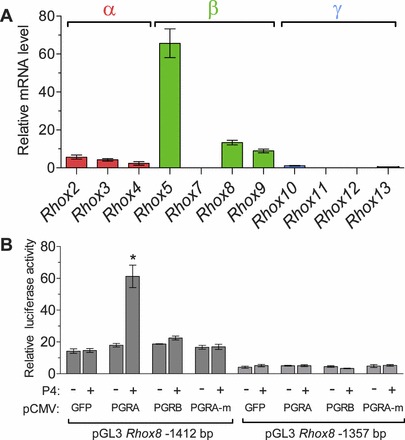
PGR regulation of Rhox8 in SIGC. A) qPCR analysis of endogenous rat Rhox gene expression in SIGC. B) SIGC were transiently cotransfected with Rhox8 promoter reporter constructs (as described in the legend to Fig. 3) and expression plasmids encoding PGRA, PGRB, or a mutant nontransducing form of PGRA (PGRA-m). Data are fold-change above empty vector with and without 10 nM progesterone. *P < 0.001.
DISCUSSION
Homeobox genes were initially characterized in Drosophila melanogaster as master regulators of embryonic development, whose ablation resulted in improper body symmetry, segmentation, and limb development [43]. However, those transcription factors have subsequently been identified in most eukaryotes, where they regulate both embryonic and postnatal developmental programs [10, 11, 13, 24]. The subject of this report is the Rhox genes, which are a cluster of X-linked homeobox genes that are selectively expressed in reproductive tissues [12, 29]. To date, most Rhox studies have focused on the regulation and function of Rhox5, the founding member of the cluster [12, 44]. Those studies have focused primarily on the male due in part to Rhox5's well-established androgen-dependent regulation in the epididymis and Sertoli cells of the testis but also because Rhox5-null males exhibit subfertility, whereas female KO mice have no obvious defects. We have previously examined the regulation of Rhox5 in granulosa cells of rats [28], but prior to this report, the expression and regulation of the other Rhox genes had not been examined during folliculogenesis.
Results of the present study indicate that the most Rhox alpha and beta subcluster genes are induced by FSH and LH, whereas Rhox7 and the gamma subcluster remained silent in our superovulation study. Eight Rhox genes exhibited slightly overlapping windows of expression, which varied in the timing and abundance of peak expression during the course of follicular development. We found that as in rats, Rhox5 was induced by eCG and reached peak expression at 4 h post-hCG administration in mouse ovary, suggesting that the regulatory mechanism we previously reported in rat granulosa cells may be conserved in rodents [28]. The temporal profiles for Rhox2, Rhox3, and Rhox5 overlap precisely, and Rhox6 also peaks at 4 h post-hCG (although its expression is lower and less transient), suggesting that these genes may govern similar events during the early stages of folliculogenesis. However, the amino acids in the third helix of the homeodomain that confer DNA binding and promoter specificity are highly variable among these four genes [29]. Thus, it is likely that these transcription factors have evolved to regulate distinct cadres of subordinate genes despite being coexpressed. Three factors, Rhox1, Rhox8, and Rhox9, have unique patterns of expression that exhibit peak levels in the immature, periovulatory, and post-ovulatory follicle, respectively. Presently no ortholog for Rhox1 has been identified in rats [29, 30, 38], and Rhox9 has already been characterized by genetic ablation [45]. Thus, we chose to examine Rhox8 regulation in further detail.
Rhox8 is the most highly expressed Rhox factor in growing follicles. The timing of peak Rhox8 expression, 8 h post-hCG injection, was not observed for any other Rhox gene and is consistent with previously described progesterone-regulated genes [32, 46, 47]. While critical downstream effectors of progesterone signaling, such as the proteinases cathepsin-L and ADAMTS1 that contribute to follicular rupture, have been identified [7, 46, 48], these factors are not directly regulated by progesterone. Because of its unique expression profile and possible function as a bridge between progesterone signaling and induction of ovulation promoting genes, we sought to further examine the regulation of Rhox8. We provide several lines of evidence to suggest that Rhox8 is regulated by progesterone. Rhox8 expression is significantly diminished in PRKO mice. PGR is recruited to the Rhox8 promoter following stimulation by gonadotropins. Ablation of a putative PRE within the Rhox8 promoter reduces luciferase reporter activity in granulosa cells. This element can be stimulated by the addition of PGRA and P4 in granulosa cells. The spatial expression pattern of PGR develops ∼4 h following LH stimulation and is highly restricted to mural granulosa cells [49], where we predominantly observed RHOX8 protein localization.
KO studies have provided definitive proof that progesterone signaling is required for ovulation [50]. Both PGRA and PGRB are induced by LH stimulation in preovulatory follicles [51]. However, administration of superovulatory gonadotropins to PGRB KO mice reveals no blockage to ovulation, whereas mice lacking PGRA exhibit severely impaired ovulation [50]. In these mice, follicle development proceeds normally, but the fertilization-competent oocyte remains trapped within the granulosa layers which undergo luteinization [5]. Because our analysis revealed that Rhox8 is specifically stimulated by PGRA, it is tempting to speculate that it may contribute to LH-dependent follicular rupture by inducing secondary progesterone-regulated genes that are essential for ovulation. However, it is currently unknown what role RHOX8 may play in granulosa cell proliferation, survival, or differentiation. Given that Rhox8 is found in other cells in addition to periovulatory mural granulosa cells, its function during ovulation is likely complex. PGR is absent in thecal cells, oocytes, and cumulus granulosa cells [49]. While we occasionally observe RHOX8 positive granulosa cells adjacent to the oocyte in small follicles, its expression is excluded from cumulus stalk cells in large antral follicles. Our in situ hybridization analysis indicated that Rhox8 mRNA may be present in the oocyte, but we have not observed RHOX8 protein staining in oocytes that was not also observed in preimmune or no primary antibody control slides. We do, however, see RHOX8-positive thecal cell nuclei around growing follicles. This expression is only slightly diminished in PRKO mice, suggesting that while RHOX8 expression is largely consistent with PGR regulation, there must be additional factors that act on the Rhox8 promoter.
We recently identified RHOX5 as the master regulator of Rhox cluster expression in the epididymis ([30] and commentary [40]). In that study, Rhox8 was downregulated in Rhox5-null caput epididymis, suggesting that it is positively regulated by RHOX5. The 5′-flanking sequence of Rhox8 contains three putative homeobox binding sites within 2.5 kb of its transcription start site that are nearly identical to the RHOX5-binding element we have characterized in Sertoli cells [52, 53]. The first two sites were indispensable in our in vitro promoter assays as activity did not drop when the promoter reporter construct was shortened from 2.5 to 1.5 kb, although a role for these two sites in vivo cannot be ruled out. We did observe a two-fold loss in Rhox8 promoter activity when the site 1200 nt upstream of the transcription start site was deleted. Cotransfection of RHOX expression constructs revealed that RHOX5 uniquely induces increased Rhox8 promoter activity in cultured granulosa cells. Ovaries from superovulated Rhox5-null mice exhibit reduced Rhox8 mRNA expression during the window in which Rhox5 is normally found. However, the peak expression of Rhox8 recovers 6 h post-hCG administration when Rhox5 is essentially turned off. Taken together, our results suggest a model in which Rhox8 regulation in granulosa cells is achieved in multiple stages. Initially, Rhox8 is induced by FSH (directly or indirectly) and is augmented by the stimulation of RHOX5, which is also induced by FSH signaling. RHOX5 maintains Rhox8 expression until LH induces PGR expression which then induces peak Rhox8 levels through ovulation. It is not clear whether other LH-dependent factors coregulate Rhox8 during the periovulatory window, but this may be the case as Rhox8 expression is not abolished at 8 h post-hCG in PRKO animals. However, the residual Rhox8 mRNA signal could be derived from thecal cells which would depend on factors other than PGR and RHOX5 which are granulosa-specific. We have not yet directly examined Rhox8 regulation in thecal cells. However, the logical assumption is that the estrogen response element with Rhox8's minimal essential promoter may be important, and of interest to pursue in the future as thecal-specific Esr1-null mice have compromised fertility [54].
The results herein and those from our prior studies have focused on the action of Rhox genes in preovulatory granulosa cells, but they may additionally function elsewhere in the ovary. Rhox9 transiently peaks at 12 h post-hCG, suggesting it may govern the luteinization of granulosa cells. However, Rhox9 KO mice have no observable phenotype and ovulate normally [45], although a role in female germ cell development in the prenatal ovary is suspected [26, 55]. One explanation for the lack of phenotype is that the functions of RHOX9 are compensated for by RHOX6. Rhox6 and Rhox9, which possesses the highest amino acid sequence identity (83%) among nonparalogous Rhox genes, are coexpressed in the embryo, placenta, epididymis, and testis [12, 29, 30, 38, 45]. If RHOX6 does compensate for the loss of Rhox9, then it must do so without being upregulated as Rhox6 mRNA is not increased in trophoblast, testis, or ovary in Rhox9-null mice [55]. Our expression analysis indicates that Rhox6 and Rhox9 may have independent functions in the ovary as their peak expression falls in different windows. To this end, recent in vitro studies using embryonic stem cell and primordial germ cell models indicate that RHOX6 and RHOX9 may not be redundant as they possess distinct abilities to promote germ cell differentiation [56]. Thus, if the primary site of action of RHOX6 and RHOX9 is in oocytes, then the differential expression patterns we observe in the total ovary may be superfluous and compensation by RHOX6 in the oocytes may still explain the lack of ovarian phenotype in Rhox9-null mice.
To date, the functions of RHOX factors in the ovary have remained elusive. Ablation of Rhox5 in Sertoli cells that nurse male germ cell development results in reduced germ cell survival and ultimately male subfertility [29]. However, loss of RHOX5 in the analogous granulosa cells does not apparently result in increased oocyte death or failure in folliculogenesis. Directed studies to identify RHOX-regulated genes in the ovary have not been performed. Candidate RHOX5-regulated genes that contribute to male germ cell survival have been characterized in Sertoli cells [52, 53]. These include secreted metabolic regulators as well as key enzymes and transcription factors associated with the maintenance of cellular energy balance. Some of these factors can be induced by RHOX8, although not as strongly as RHOX5, in Sertoli cells (J.A. MacLean, unpublished observations), but their potential relevance in the ovary remains to be assessed. However, if any putative RHOX5/RHOX8-regulated factors are essential for ovulation, then the maintenance of RHOX8 expression, under the control of progesterone signaling in Rhox5-null female mice, may explain why those mice do not display any ovulation defects. A definitive answer to this question will await the characterization of our Rhox8-null models that are underway, as well as the generation of Rhox5/Rhox8 double knockouts. In summary, the findings of the present study provide a foundation for future experiments to uncover the redundant and independent functional role of each Rhox gene during ovulation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Robert Burghardt (Texas A&M University) for supplying the rat SIGC and Lisa Stein (Imgenex) for supplying the RHOX8 antisera used in this study. We also thank Kelsey Jarrett and Mike Collard for help establishing and troubleshooting the ChIP protocol and Josh Welborn for editorial suggestions.
Footnotes
Supported by National Institutes of Health/National Institute of Child Health and Human Development grant HD065584 to J.A.M.
REFERENCES
- Richards JS. Genetics of ovulation. Semin Reprod Med 2007; 25: 235 242. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr,, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266 2278. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS. Ovulation: a multi-gene, multi-step process. Steroids 2000; 65: 559 570. [DOI] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol 2008; 28: 1770 1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A 2000; 97: 4689 4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraman V, Sinha M, Richards JS. Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures. Biol Reprod 2010; 82: 402 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KM, Russell DL, Sriraman V, Richards JS. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol 2004; 18: 2463 2478. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Richards JS. Cathepsin L gene expression and promoter activation in rodent granulosa cells. Endocrinology 2004; 145: 582 591. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS. Cyclic guanosine 5′-monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol 2006; 20: 348 361. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell 1994; 78: 191 201. [DOI] [PubMed] [Google Scholar]
- Svingen T, Koopman P. Involvement of homeobox genes in mammalian sexual development. Sex Dev 2007; 1: 12 23. [DOI] [PubMed] [Google Scholar]
- MacLean JA, II,, Wilkinson MF. The Rhox genes. Reproduction 2010; 140: 195 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MK, Wayne CM, Wilkinson MF. Pem homeobox gene regulatory sequences that direct androgen-dependent developmentally regulated gene expression in different subregions of the epididymis. J Biol Chem 2002; 277: 48771 48778. [DOI] [PubMed] [Google Scholar]
- MacLean JA., II. The role of Rhox homeobox factors in tumorigenesis. Front Biosci 2013; 18: 474 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 2000; 403: 909 913. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet 2002; 32: 359 369. [DOI] [PubMed] [Google Scholar]
- Mazaud S, Oreal E, Guigon CJ, Carre-Eusebe D, Magre S. Lhx9 expression during gonadal morphogenesis as related to the state of cell differentiation. Gene Expr Patterns 2002; 2: 373 377. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development 1997; 124: 1653 1664. [DOI] [PubMed] [Google Scholar]
- Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod 2008; 79: 442 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns 2005; 5: 756 762. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004; 305: 1157 1159. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Yan C, Yan W, Klysik M, Matzuk MM. Obox, a family of homeobox genes preferentially expressed in germ cells. Genomics 2002; 79: 711 717. [DOI] [PubMed] [Google Scholar]
- Jagarlamudi K, Rajkovic A. Oogenesis: transcriptional regulators and mouse models. Mol Cell Endocrinol 2012; 356: 31 39. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang Q, Cheng JC, Nishi Y, Yanase T, Huang HF, Leung PC. Homeobox A7 increases cell proliferation by up-regulation of epidermal growth factor receptor expression in human granulosa cells. Reprod Biol Endocrinol 2010; 8: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan V, Robert NM, Tremblay JJ. Expression of ladybird-like homeobox 2 (LBX2) during ovarian development and folliculogenesis in the mouse. J Mol Histol 2010; 41: 289 294. [DOI] [PubMed] [Google Scholar]
- Daggag H, Svingen T, Western PS, van den Bergen JA, McClive PJ, Harley VR, Koopman P, Sinclair AH. The rhox homeobox gene family shows sexually dimorphic and dynamic expression during mouse embryonic gonad development. Biol Reprod 2008; 79: 468 474. [DOI] [PubMed] [Google Scholar]
- Pitman JL, Lin TP, Kleeman JE, Erickson GF, MacLeod CL. Normal reproductive and macrophage function in Pem homeobox gene-deficient mice. Dev Biol 1998; 202: 196 214. [DOI] [PubMed] [Google Scholar]
- MacLean JA, II,, Rao MK, Doyle KM, Richards JS, Wilkinson MF. Regulation of the Rhox5 homeobox gene in primary granulosa cells: preovulatory expression and dependence on SP1/SP3 and GABP. Biol Reprod 2005; 73: 1126 1134. [DOI] [PubMed] [Google Scholar]
- Maclean JA, II,, Chen MA, Wayne CM, Bruce SR, Rao M, Meistrich ML, Macleod C, Wilkinson MF. Rhox: a new homeobox gene cluster. Cell 2005; 120: 369 382. [DOI] [PubMed] [Google Scholar]
- MacLean JA, II,, Hayashi K, Turner TT, Wilkinson MF. The Rhox5 homeobox gene regulates the region-specific expression of its paralogs in the rodent epididymis. Biol Reprod 2012; 86: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wilkinson MF. Deletion mutagenesis of large (12-kb) plasmids by a one-step PCR protocol. Biotechniques 2001; 31: 722 724. [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol 2006; 20: 2278 2291. [DOI] [PubMed] [Google Scholar]
- Jensik PJ, Arbogast LA. Differential and interactive effects of ligand-bound progesterone receptor A and B isoforms on tyrosine hydroxylase promoter activity. J Neuroendocrinol 2011; 23: 915 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon SN, King ML, MacLean JA, II,, Mann JL, Demayo FJ, Lydon JP, Hayashi K. Cdh1 Is Essential for Endometrial Differentiation, Gland Development, and Adult Function in the Mouse Uterus. Biol Reprod 2012; 86 (5): 1 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshioka S, Reardon SN, Rucker EB, 3rd,, Spencer TE, DeMayo FJ, Lydon JP, MacLean JA., II. WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 2011; 84: 308 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem 2002; 277: 13286 13293. [DOI] [PubMed] [Google Scholar]
- Geyer CB, Eddy EM. Identification and characterization of Rhox13, a novel X-linked mouse homeobox gene. Gene 2008; 423: 194 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JA, II,, Lorenzetti D, Hu Z, Salerno WJ, Miller J, Wilkinson MF. Rhox homeobox gene cluster: recent duplication of three family members. Genesis 2006; 44: 122 129. [DOI] [PubMed] [Google Scholar]
- Maclean JA, Bettegowda A, Kim BJ, Lou CH, Yang SM, Bhardwaj A, Shanker S, Hu Z, Fan Y, Eckardt S, McLaughlin KJ, Skoultchi AI, et al. The rhox homeobox gene cluster is imprinted and selectively targeted for regulation by histone h1 and DNA methylation. Mol Cell Biol 2011; 31: 1275 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GL. Rhox5 rules in an evolving saga of reproductive diversity. Biol Reprod 2012; 86: 188. [DOI] [PubMed] [Google Scholar]
- Stein LS, Boonstra J, Burghardt RC. Reduced cell-cell communication between mitotic and nonmitotic coupled cells. Exp Cell Res 1992; 198: 1 7. [DOI] [PubMed] [Google Scholar]
- Stein LS, Stoica G, Tilley R, Burghardt RC. Rat ovarian granulosa cell culture: a model system for the study of cell-cell communication during multistep transformation. Cancer Res 1991; 51: 696 706. [PubMed] [Google Scholar]
- Duboule D. (ed.) The Guidebook To the Homeobox Genes. Oxford: Oxford University Press; 1994; [Google Scholar]
- Maclean JA, II,, Wilkinson MF. Gene regulation in spermatogenesis. Curr Top Dev Biol 2005; 71: 131 197. [DOI] [PubMed] [Google Scholar]
- Takasaki N, Rankin T, Dean J. Normal gonadal development in mice lacking GPBOX, a homeobox protein expressed in germ cells at the onset of sexual dimorphism. Mol Cell Biol 2001; 21: 8197 8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 2002; 64: 69 92. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Eichenlaub-Ritter U, Bartsch JW, Rittger A, Mulders SM, Richards JS. Regulated expression of ADAM8 (a disintegrin and metalloprotease domain 8) in the mouse ovary: evidence for a regulatory role of luteinizing hormone, progesterone receptor, and epidermal growth factor-like growth factors. Biol Reprod 2008; 78: 1038 1048. [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem 2003; 278: 42330 42339. [DOI] [PubMed] [Google Scholar]
- Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 2002; 16: 2475 2489. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction 2004; 128: 139 146. [DOI] [PubMed] [Google Scholar]
- Natraj U, Richards JS. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 1993; 133: 761 769. [DOI] [PubMed] [Google Scholar]
- Hu Z, MacLean JA, Bhardwaj A, Wilkinson MF. Regulation and function of the Rhox5 homeobox gene. Ann N Y Acad Sci 2007; 1120: 72 83. [DOI] [PubMed] [Google Scholar]
- Hu Z, Shanker S, MacLean JA, 2nd,, Ackerman SL, Wilkinson MF. The RHOX5 homeodomain protein mediates transcriptional repression of the netrin-1 receptor gene Unc5c. J Biol Chem 2008; 283: 3866 3876. [DOI] [PubMed] [Google Scholar]
- Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, Cheon Y, Gye MC, Chambon P, Ko C. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology 2009; 150: 3855 3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki N, McIsaac R, Dean J. Gpbox (Psx2), a homeobox gene preferentially expressed in female germ cells at the onset of sexual dimorphism in mice. Dev Biol 2000; 223: 181 193. [DOI] [PubMed] [Google Scholar]
- Liu C, Tsai P, Garcia AM, Logeman B, Tanaka TS. A possible role of Reproductive Homeobox 6 in primordial germ cell differentiation. Int J Dev Biol 2011; 55: 909 916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


