ABSTRACT
As advanced reproductive technologies have become routine for domesticated species, they have begun to be applied in the field of endangered species conservation. For avian conservation, the most promising technology is the transfer of germ stem cells of exotic species to domestic hosts for the production of gametes. In this study, adult quail (model for exotic species) spermatogonial stem cells were xenogeneically transferred to stages 14–17 chicken host embryos. Fluorescent cellular dyes, quail-specific antibodies, and quail-specific quantitative PCR confirmed donor cell migration to and colonization of the host gonadal ridge. Donor-derived cells were observed by fluorescent microscopy in the caudal area as early as 2 h after injection, in the gonadal ridge at 4 h after injection, as well as in the gonads of stages 35–38 host embryos. Four of eight donor-derived cell flow cytometry-positive host gonads were confirmed by quantitative PCR using quail-specific primers. There was no statistically significant effect of host stage of injection, host gonad isolation stage, or host sex on the number of hosts positive for donor cells or the percent of donor-derived cells per positive gonad. Donor-derived cells isolated from stages 35–38 host gonads costained with the germ stem cell marker SSEA-1, indicating that the donor-derived cells have maintained stem cell-ness. This is the first study to suggest that it is feasible to rescue adult germ stem cells of deceased birds to prolong the reproductive lifespan of critically endangered species or genetically valuable individuals by transferring them to an embryonic chicken host.
Keywords: chicken, embryo, gonadal ridge, quail, spermatogonial stem cell, testes
Adult quail spermatogonial stem cells will colonize the embryonic gonadal ridge in host chickens.
INTRODUCTION
Advanced reproductive technologies, including cloning, in vitro fertilization, and intracytoplasmic sperm injection, may be viable methods for conservation of endangered mammalian species. However, many of these techniques are impractical, if not unachievable, for avian species, mainly due to the large size of the oocyte and the impracticability of collecting oocytes from the body cavity following super ovulation stimulation [1], and because unfertilized oocytes degrade by the time of oviposition. Alternatively, germline stem cells, either embryonic primordial germ cells (PGCs), embryonic gonadal germ cells (GGCs), or adult germ stem cells (GSCs), from exotic species could be used for xenogeneic transfer (xenotransfer) to domestic host embryos, such as the chicken (Gallus gallus). These hosts could produce donor-derived sperm for use in artificial insemination. Thus, the transfer of germline stem cells has the potential to be an important advancement in avian conservation.
The unique behavior of avian PGCs makes germ cell manipulation a feasible technique. The PGCs originate in the anterior hypoblast at stage X [2], move to the germinal crescent, and (beginning at stage 11; see Hamburger and Hamilton [3]) passively migrate throughout the vasculature, followed by active migration to the gonadal ridge by stage 15 [4–6]. It is during this time of endogenous PGC migration that the germline can be manipulated, by either adding or genetically manipulating PGCs, GGCs, or GSCs.
This possibility of using germ stem cells to generate transgenic birds or donor-derived gametes has been discussed and tested by several investigators (reviewed in Wentworth et al. [5], Naito [7], and Petitte [8]). For example, both chicken [9] and quail (Coturnix coturnix) [10] PGCs injected into the vitelline blood vessels of stages 14–17 chicken host embryos, as well as chicken PGCs transferred into quail embryos [11], will migrate to the host's gonadal ridge. In fact, donor-derived offspring have been produced following intraspecies [5, 12–16] and interspecies [17, 18] transfer of PGCs or GGCs.
As successful as the embryonic PGC/GGC transfer technique has been, collection from live embryos does not represent a practical method for avian conservation. Instead, routine collection of GSCs at the time of necropsy of genetically valuable individuals would be a more feasible method of preserving germplasm, because self-renewing spermatogonial stem cells (spermatogonia Ad; see Lin and Jones [19]) are found throughout the reproductive lifespan of a bird. Previous studies by Minematsu et al. [20] and Jung et al. [16] have demonstrated that adult chicken gonads contain cells, presumably spermatogonia Ad, that are capable of migrating and colonizing the gonadal ridge following transfer to host chicken embryos stages 14–17. Likewise, Trefil et al. [21] and Lee et al. [22] reported that dispersed testicular cells from both prepubertal and adult donors transferred directly into sterilized adult host testes recolonized the seminiferous tubules of the conspecific host, partially resumed spermatogenesis, and produced donor-derived offspring. Pereira et al. [23] recently demonstrated resumption of spermatogenesis in sterilized adult host chickens following transfer of adult quail spermatogonia.
For this technology to be practical for the opportunistic harvest of genetically valuable individuals postmortem, the recovered cells must be maintained in culture for the period necessary for the host embryos to reach stages 14–17, as well as be able to migrate to the gonadal ridge after transfer. Gonadal germ cells and GSCs, unlike PGCs, are relatively easy to maintain in both short- and long-term culture prior to successful transfer and colonization of the host gonad [16, 24, 25]. The current study investigates the migration of presumed spermatogonia A and colonization of host gonads following xenotransfer of adult quail testicular cells to host chicken embryos.
MATERIALS AND METHODS
All experiments in this study were reviewed and approved by the San Diego Zoo Global Institutional Animal Care and Use Committee (assurance A3675-01).
Donor Cell Isolation and Staining
Testes from reproductively active adult quail (Ramona Duck Farm) were removed immediately following cervical dislocation and were stored in antibiotic antimycotic solution (Sigma-Aldrich) for 45–120 min.
Testes were mechanically dissociated using a Miltenyi MACS dissociator (Miltenyi Biotec) using the internal “spleen_04.01” program. The tissue was passed through a 40-μm filter to obtain a single-cell suspension in M199 medium. The cells were stained with the cellular membrane dye PKH67 for stages 16–20 donor GSC-host gonad colonization confirmation or PKH26 for stages 35–38 donor GSC-host gonad colonization confirmation. Dyes were used according to the manufacturer's recommendations (Sigma-Aldrich).
Incubation
Unincubated fertilized chicken eggs were obtained from McIntyre Egg Ranch. Host chicken embryos were incubated at 37.5°C and 60%–70% humidity in a forced-air incubator (GQF Manufacturing Co.).
Donor Cell Injection
A 1.5-cm2 opening in the host embryo shell was made using a standard belt sander. A total of 10 000 donor quail total testis cells suspended in 2–10 μl of M199 (Mediatech Inc.) medium were injected into the vitelline vein of unsexed chicken embryos at stages 14–17 using a SteReo discovery.V12 microscope (20×–25× magnification; Zeiss) and a 40- to 60-μm beveled glass needle mounted on a microinjection/micromanipulation setup (IM-9B Narishige; Kite-R WPI Inc.). The opening was sealed using Parafilm (Pechiney Plastic Packaging Co.) melted to the shell. The host embryos were incubated, opening down, for an additional 7–9 days. Control embryos were not opened or injected.
Stages 16–20 Donor GSC-Host Gonad Colonization Confirmation
Stages 14–17 embryos were injected with PKH67-stained quail testis cells as described above, whereas control embryos were not injected. Postinjection embryos were either incubated in ovo (as described above) or cultured in vitro (as described below) for 2–16 h prior to donor cell detection. A SteReo discovery.V12 microscope with a dual 470 LED and filter set 38 intermediate tube, an AxioCam MRm camera (Zeiss), and the ZEN image software (Zeiss) were used to record images.
In vitro embryo culture.
Whole embryos were removed from the yolk following injection using filter paper rings, as previously described by Chapman et al. [26]. The filter paper made it possible to keep the vitelline membrane stretched to not interfere with vitelline blood flow while the embryo was being cultured on an agar-albumin substrate. Cultured embryos were incubated as described above.
Whole-embryo immunohistochemistry.
Stages 16–19 embryos were removed from the yolk and associated membranes using the filter paper rings as described above. Embryos were fixed in Dietrick fixative (10% formalin, 30% ETOH, and 2% glacial acetic acid) for 30 min, incubated in blocking solution (PBS, 0.1% Triton X, 0.05% Tween 20, and 0.5% bovine serum albumin [BSA]) for 30 min followed by overnight incubation in blocking solution and fluorescein isothiocyanate (FITC)-conjugated SSEA-1 (1:100; eBiosciences) at 4°C. Antibody- and PKH67-positive GGCs were visualized using a SteReo discovery.V12 microscope equipped as described above.
Stages 35–38 Donor GSC-Host Gonad Colonization Confirmation
Whole gonads from host embryos injected with PKH26 stained or unstained donor cells were removed at stages 35–38 (10–12 days), visually sexed, and prepared for flow cytometry, quantitative PCR (qPCR), and immunocytochemistry, as described below.
Flow cytometry.
Following removal, gonads (both testes or left ovary) were dispersed in collagenase:DNAse (31 mg/ml:3.9 mg/ml) at 37°C for 30–60 min. A total of 50 000 cells from each control and injected embryo were analyzed by flow cytometry to determine the percent of PKH26-positive cells. The flow cytometry data were collected using CellQuest and a FACSCalibur (BD Biosciences) and were analyzed using FlowJo (Tree Star Inc.). Front scatter and side scatter gating was used to eliminate endogenous red blood cells and cellular debris. FL-1 (green fluorescence) and FL-2 (red fluorescence) gating was used to gate positive cells while excluding the high gonadal cell autofluorescence.
Gonadal cells from nonstained GSC-injected embryos were labeled with the quail-specific antibody QH 1 by blocking for 30 min (PBS, 0.1% Triton X, 0.05% Tween 20, and 0.5% BSA), and overnight incubation in blocking solution and QH 1 (1:100; DHSB) at 4°C, followed by incubation with goat anti-mouse FITC-conjugated secondary antibodies for 1 h at room temperature. A subset of PKH26 and the QH 1-labeled cells were stained with FITC-conjugated SSEA-1 antibodies (incubated in blocking solution [PBS, 0.1% Triton X, 0.05% Tween 20, and 0.5% BSA] for 30 min, followed by 1-h incubation in blocking solution and FITC-conjugated SSEA-1 [1:100; eBiosciences] at room temperature) to demonstrate the stem cell-ness of donor-derived cells. The QH 1 antibody has previously been shown to detect quail cells in quail/chicken chimeras [27, 28]. The SSEA-1, SSEA-3, and SSEA-4 antibodies have previously been shown to detect avian germline stem cells [29, 30].
qPCR analysis.
Following flow cytometry, DNA was isolated from the injected and control embryo gonad cells using Chelex beads (Sigma-Aldrich) in sterile H2O for 30 min at 56°C followed by boiling for 8 min, centrifugation, and storage at −20°C [31, 32]. DNA from all samples was diluted to the same concentration in sterile water. The qPCR reactions were performed on an Applied Biosystems 7900HT Real-Time PCR system (Applied Biosystems): 50°C for 2 min and denaturation at 95°C for 10 min; followed by amplification for 40 cycles at 95°C for 15 sec, and annealing and extension at 62°C for 1 min; and a final round of amplification at 95°C for 15 sec, and annealing and extension at 62°C for 15 sec and 95°C for 15 sec. The qPCR primers 65F (CGTCACCCTCTTCAAAAGCTAC) and 70R (GCTTTGGAGCTTATAGCTACGC) were designed to detect quail cells in chicken hosts. Efficiency was 101.5 as derived from the slope of the standard curve. Melting curves of all samples were checked for single peak. Ct (threshold cycle) was automatically determined by software (Applied Biosystems 7900HT sequence detection system software version 2.3). The relative amount of PCR product for each control and experimental host embryo was determined by the calibrator method using a quail testis cell DNA sample.
Immunocytochemistry.
Following flow cytometry, cells from a subset of positive and control embryos were blocked for 30 min, incubated for 1 h in blocking solution and FITC-conjugated SSEA-1 antibodies at 22°C, and stained for 10 min in Hoechst 33342 nuclear stain to test for colocalization of SSEA-1 expression and PKH26 dye. Localization of SSEA-1-positive and PKH26-positive cell staining was visualized on an Eclipse 80i (Nikon) with a Digital Sight and DS-Fi1 camera (Nikon).
Statistical Analysis
An embryonic gonad was considered positive when the percent of positive cells observed by flow cytometry exceeded two standard deviations above the average for the uninjected control embryos. The percent of positive donor-derived cells in host gonads was determined by subtracting the number of cells in the positive flow cytometry gate of experimental gonads from the average of the uninjected controls. The two-tailed Fisher exact test, t-test, and one-way ANOVA were used to determine statistical significance; a P value of 0.05 or less was considered significant.
RESULTS
Stages 16–20 Donor GSC-Host Gonad Colonization Confirmation
Donor-derived cells were observed by fluorescent microscopy throughout the embryo and vitelline vasculature immediately after injection. At 2 h (stage 16) after injection, donor-derived PKH67 cells were assembling in the caudal region (Fig. 1A). At 4 h (stage 17) after injection, donor-derived PKH67 cells were localized in the limb bud and the gonadal ridge of midgut (Fig. 1, B and C), and by 16 h (stage 20) after injection, cells were localized in the gonadal ridge (Fig. 1, D and E). In uninjected control embryos, SSEA-1-positive cells were located in the limb buds and gonadal ridge of the midgut wall at stage 16 (Fig. 1, F and G), in the gonadal ridge at stage 17 (Fig. 1, H and I), and tightly clustered in the gonadal ridge at stage 19 (Fig. 1, J and K). In addition to the limb buds and gonadal ridges, PKH-positive cells were also localized in the cephalic region and the allantoic stalk.
FIG. 1.
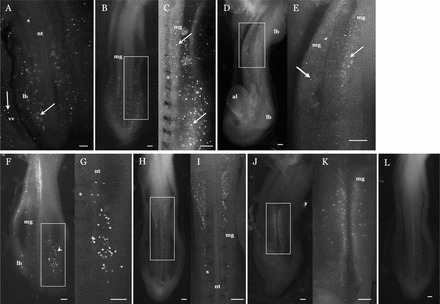
Stage 16 host embryo 2 h after injection of PKH67-stained donor quail testes cells (A). The PKH cells can be seen in the posterior and limb bud regions at different tissue depths, as well as in the vitelline vasculature. Stage 17 host embryo 4 h after injection (B), with a close-up of a PKH67-positive region (C). Note the presence of positive cells in the limb bud and gonadal ridge on the wall of the midgut. Stage 20 host embryo 16 h after injection (D), with a close-up of a PKH67-positive region (E). Note the presence of positive cells in the gonadal ridge on the wall of the midgut. In addition, positive cells are localized to the areas around the stalk of the allantois but are absent from the limb bud. Location of SSEA-1-positive cells in a stage 16 uninjected embryo (F), with a close-up of a positive region (G). Left limb bud has been dissected to show positive cells (arrowhead). Location of SSEA-1 positive cells in a stage 17 uninjected embryo (H), with a close-up of a positive region (I). Location of SSEA-1-positive cells in stage 19 uninjected embryo (J), with a close-up of a positive region (K). Note the presence of cells in the wall of the midgut. Stage 17 uninjected control embryo (L). Embryo micrographs are ventral side except for A, which is dorsal. Micrographs are cropped immediately below the heart. al, allantois; lb, limb bud; mg, wall of midgut; nt, neural tube; s, somites; vv, vitelline vasculature. Arrow, PKH-positive cells; arrowhead, positive cells exposed by dissection of limb bud. Bar = 200 μm.
Stages 35–38 Donor GSC-Host Gonad Colonization Confirmation
A total of 43 of the 83 injected embryos (51.8%) survived to gonad collection (Table 1). A total of 23 of the surviving 43 host embryos (53.5%) had detectable levels of donor-derived cells in the gonad (Table 1). The percent of donor-derived cells per gonad averaged 0.103% ± 0.02%, ranging from 0.032% to 0.188% (mean ±SEM, 0.091% ± 0.018%) in male embryos and 0.024% to 0.42% (mean ±SEM, 0.113% ± 0.032%) in female embryos (Table 2). The total number of cells in a pair of stage 36 embryo testes was 2.04 × 106 ± 2.19 × 105, and the number was 2.00 × 106 ± 1.87 × 105 for the left ovary. This yielded a range of 653-3835 and 480-8400 colonized donor-derived cells in male and female hosts, respectively (Table 2). The range of positive cells in host gonads varied considerably between individual embryos (Fig. 2).
TABLE 1.
Effect of transfer-injection on embryo survival and successful donor GSC colonization of host gonad at time of gonad retrieval at stages 35–38.
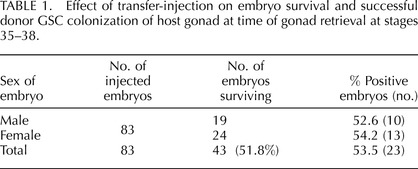
TABLE 2.
The total number of cells in host testes (both) or ovary (left), and the range of donor-derived cells detected by PKH26.

Values are mean ± SEM.
n = 3.
FIG. 2.
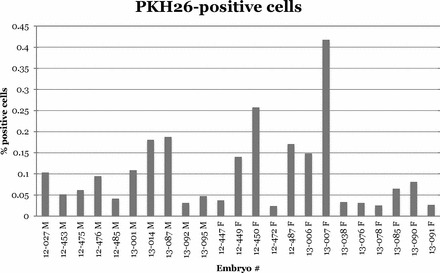
There was a considerable difference in the quantity of donor-derived cells within stages 35–38 host gonads. This graph shows the individual variation expressed as percent of positive cells per gonad (testes or left ovary), minus the average background from uninjected control embryos. Host gonads were considered to be donor cell positive if the percent of positive cells was greater than the average control plus two standard deviations for each experiment.
A total of 6 of the 8 embryos (75%) injected at stage 14 were positive, 10 of the 18 embryos (55.6%) injected at stage 15 were positive, and 7 of the 15 embryos (46.7%) injected at stages 16/17 were positive (two-tailed Fisher exact test: P = 0.47; Fig. 3A). A total of 17 of the 28 host embryo gonads (60.7%) isolated at stage 35 were positive, and 6 of the 15 host embryo gonads (40%) isolated at stages 36/37/38 were positive (two-tailed Fisher exact test: P = 0.22; Fig. 3B). A total of 10 of the 19 male host embryos (52.6%) injected were positive, and 13 of the 24 female host embryos (54.2%) injected were positive (two-tailed Fisher exact test: P = 0.99; Fig. 3C). The percentages of positive cells per gonad (testes or left ovary; mean ± SEM) were 0.128% ± 0.021%, 0.114% ± 0.041%, and 0.068% ± 0.019% for stages 14, 15, and 16/17 injected host embryos, respectively (one-way ANOVA: P = 0.49; Fig. 3A). The percentages of positive cells per gonad for isolation stages 35 and 36/37 were 0.081% ± 0.017% and 0.166% ± 0.054%, respectively (t-test: t = 2.02; df = 21; P = 0.056; Fig. 3B). The percentage of positive cells per gonad in males was 0.091% ± 0.018%, and for females it was 0.113% ± 0.032% (t-test: t = 0.53; df = 21; P = 0.60; Fig. 3C). It was not possible to determine whether there was an influence of sex on host survival, because hosts were not sexed at time of injection or death.
FIG. 3.
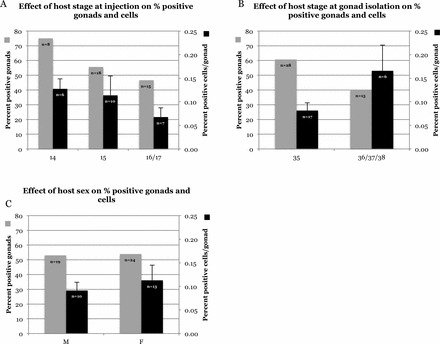
Comparison of percent of positive gonads and donor-derived cells in host gonads with: host stage at injection (A), host stage at time of gonad removal (B), and host sex (C). Hosts at injection stages 16 and 17 were pooled for statistics because of low numbers; hosts at stages 36 and 37 were pooled for statistics because of low numbers. Percent of positive gonads: A, P = 0.47; B, P = 0.22; C, P > 0.99. Percent of positive cells per gonad: A, P = 0.49; B, t = 2.02, df = 21, P = 0.056; C, t = 0.53, df = 21, P = 0.60.
A total of 1.82% ± 0.35% and 1.74% ± 0.17% (mean ± SEM) of total cells were SSEA-1, SSEA-3, SSEA-4 positive in uninjected controls (n = 5) and injected experimental (n = 10) stage 35 host embryo gonads, respectively (t = 0.24; df = 13; P = 0.82). Three of four embryos showed a decrease of 49%–92% QH 1-positive cells, and four of four embryos showed a decrease of 29%–100% PKH26-positive cells, following staining with SSEA-1, SSEA-3, and SSEA-4 (Fig. 4).
FIG. 4.
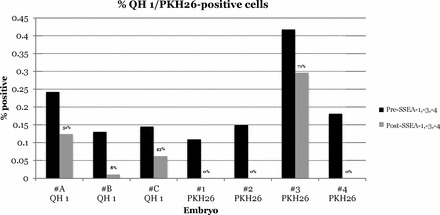
Following staining with FITC-conjugated SSEA-1, SSEA-3, and SSEA-4 antibodies, QH 1-positive and PKH26-positive cells in the FL-2 gate decreased as dual-stained cells moved into the yellow range during flow cytometry. Numbers indicate the percent of pre-SSEA-staining cells that were detectable after SSEA. Donor cells were detected by either the quail-specific QH 1 antibody or by PKH26 fluorescence. A direct measure of the increase in dual-stained yellow cells is not possible because of the high autofluorescence of embryonic gonadal cells.
Of eight experimental embryo gonads positive by flow cytometry, four were positive and four were negative when tested by qPCR (Table 3). Of six experimental embryo gonads negative by flow cytometry, one was positive and five were negative when tested by qPCR (Table 3).
TABLE 3.
Verification of flow cytometry data by qPCR.

Some donor-derived PKH26 cells exhibited dual staining with SSEA-1, whereas others were negative for expression of the gonadal stem cell marker SSEA-1 (Fig. 5).
FIG. 5.
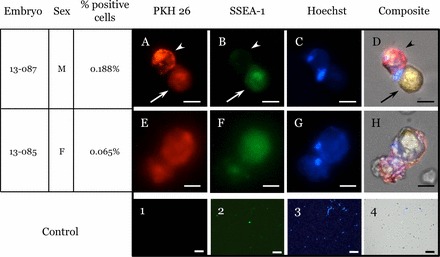
Stages 35–37 host embryo ovary and testis cells. Dissociated testes (A–D) and ovary (E–H) from positive embryos show PKH26-positive cells (A and E), SSEA-1-positive cells (B and F), Hoechst-stained nuclei (C and G), and a composite of all three markers (D and H). Donor-derived cells (PKH26) are found to be both positive (arrow), as well as negative (arrowhead) for the gonadal stem cell marker SSEA-1. Uninjected control embryos PKH 26 (1), SSEA-1 (2), Hoechst (3), and composite (4). Bars = 10 μm (A–H) or 100 μm (1–4).
DISCUSSION
This study is the first to demonstrate that xenotransferred adult quail testicular cells, specifically presumptive spermatogonial stem cells, are capable of migrating to and colonizing the gonadal ridge of embryonic chicken. A variety of cells, including somatic cells and differentiated and undifferentiated germ cells, were transferred to the host embryos during the stages of endogenous PGC migration and colonization. However, we postulate that only spermatogonial stem cells successfully migrated to the gonadal ridge, because active cell migration is required from blood vessels to the gonadal ridge. It is unlikely that Sertoli, Leydig, or meiotic cells are capable of active migration and colonization of the gonadal ridge.
Although the donor cells successfully incorporated into the host gonad, there is the possibility that PKH-dyed cell counts underestimated the actual number of donor-derived cells because of dye dilution during cell proliferation. In this case, some embryos with lower PKH-positive cell counts could represent more successful transfers. Even with these limitations, PKH represents the most convenient and cost-effective marker to track exotic donor GSC migration and colonization, as opposed to the development of species-specific antibodies, primers, or GFP-transformation protocols for multiple exotic species.
Of the surviving host embryos in this study, 53.5% had detectable donor-derived cells, with an average of 2058 per two testes or the left ovary at embryonic stages 35–37. These counts are similar to previously reported numbers of donor-derived cells following transfer, even though those were counted at younger stages than in the present study. Kim et al. [33] reported an average of 942 donor-derived (chicken) GGCs in stage 29 host embryo (chicken) gonads following transfer of 3000 GGCs. Chang et al. [34] reported an average of 754 donor-derived cells per stage 27 host embryo (chicken) following injection of 150 chicken PGCs.
The localization of donor GSCs within 2–16 h following xenotransfer was similar, although stage-wise it was slightly behind the localization of endogenous PGCs. This effect is possibly due to the donor GSCs being injected after the initiation of endogenous PGC migration, that donor GSCs may require some time in the embryonic environment before acquiring the ability to migrate to and colonize the gonadal ridge, or that they migrate at a slower pace.
By stages 35–38, detection of donor-derived quail GSC incorporation into the host chicken embryo gonads was verified by several independent methods, including flow cytometry detection of the membrane-bound dye PKH26 and the quail-specific antibody QH 1, as well as by qPCR of quail-specific sequences. However, only four of the eight flow cytometry-positive embryos were also positive by qPCR, which may be due to the very low concentration of quail DNA compared with chicken DNA. This may also be because the quail and chicken sequences in this region are too similar to completely preclude amplification of chicken DNA following high numbers of amplification rounds.
In addition, the stem cell-ness of donor-derived cells was inferred by colocalization of the germ stem cell marker antibody SSEA-1 and the PKH dye by fluorescent photomicroscopy and flow cytometry. These data suggest that the adult donor-derived cells of one species were capable of migrating to and colonizing the gonadal ridge of the embryo of another species.
That PKH-positive cells populated stages 35–38 host gonads indicates that a subpopulation of cells, presumably the GSCs, has developmental plasticity and can undergo active migration and colonization of the embryonic gonadal ridge. These findings match previous reports; Li et al. [35] established that chicken PGCs could be directionally differentiated into adipose, neuronlike cells and osteoblasts. Jung et al. [16] reported successful transfer of both chicken adult GSCs and embryonic GGCs to embryo and adult chicken hosts, whereas Trefil et al. [21, 36] restored spermatogenesis in sterilized roosters by transplantation of dispersed testicular cells from chicken adult donors. Pereira et al. [23] used dispersed adult quail testis cells to recolonize and reinitiate spermatogenesis in irradiated adult roosters. These studies, along with our study, demonstrate that both embryonic and adult premeiotic germline cells have developmental plasticity, allowing them to dedifferentiate/differentiate in response to their environment. The ability of adult GSCs to colonize host gonads following xenotransfer makes xenotransfer a potentially important technique for saving exotic species in the near future.
In summary, this study showed there was no statistically significant effect of host stage at the time of injection, host gonad isolation stage, or host sex on the number of hosts positive for donor cells or the percent of donor-derived cells per positive gonad. At 2–19 h after injection, donor-derived cells in experimental embryos localized to the same general area as cells positive for the germ stem cell marker SSEA-1 in control embryos. In addition, donor-derived cells in stages 35–38 host gonads costained with SSEA-1, indicating that a subpopulation of adult testis cells has the ability to migrate to and colonize the host gonad while maintaining stem cell-ness.
Although this study is only a first step in developing a viable method of saving genetically valuable exotic species, it demonstrates that the germplasm from recently deceased or killed adult birds can potentially be saved by xenotransfer.
ACKNOWLEDGMENT
The authors would like to acknowledge Suellen Charter for technical support during this project. The SSEA-1, SSEA-3, and SSEA-4 antibodies developed by D. Solter and B.B. Knowles, and the QH 1 antibody developed by F. Dieterlen were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and were maintained by The University of Iowa Department of Biology (Iowa City, IA).
Footnotes
Supported by a grant from the NOJ Foundation to T.J., a fellowship supported by the California Institute for Regenerative Medicine (grant TB1-01186) to M.R., and a fellowship supported by the National Institute of General Medical Sciences Research Initiative for Scientific Enhancement (grant GM064783) to N.M. Presented in part at the 45th Annual Meeting of the Society for the Study of Reproduction, August 12–15 2012, State College, Pennsylvania.
REFERENCES
- Nakanishi A, Miyake M, Utsumi K, Iritani A. Fertilizing competency of multiple ovulated eggs in the domestic fowl (Gallus domesticus). Mol Reprod Dev 1991; 28: 131 135. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H, Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol 1976; 49: 321 337. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49 92. [PubMed] [Google Scholar]
- Eyal-Giladi H, Kochav S, Menashi MK. On the origin of primordial germ cells in the chick embryo. Differentiation 1976; 6: 13 16. [DOI] [PubMed] [Google Scholar]
- Wentworth BC, Tsai H, Hallett JH, Gonzales DS, Rajcic-Spasojevic G. Manipulation of avian primordial germ cells and gonadal differentiation. Poult Sci 1989; 68: 999 1010. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci 2007; 86: 2182 2193. [DOI] [PubMed] [Google Scholar]
- Naito M. Development of avian embryo manipulation techniques and their application to germ cell manipulation. Anim Sci J 2003; 74: 157 168. [Google Scholar]
- Petitte JN. Avian germplasm preservation: embryonic stem cells or primordial germ cells? Poult Sci 2006; 85: 237 242. [DOI] [PubMed] [Google Scholar]
- Petitte JN, Clark ME, Etches RJ. Assessment of functional gametes in chickens after transfer of primordial germ cells. J Reprod Fertil 1991; 92: 225 229. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yoshinaga K, Fujimoto T. Histochemical identification and behavior of quail primordial germ cells injected into chick embryos by the intravascular route. J Exp Zool 1992; 261: 479 483. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Tajima A, Fujimoto T, Kuwana T. A method to obtain avian germ-line chimaeras using isolated primordial germ cells. J Reprod Fertil 1992; 96: 521 528. [DOI] [PubMed] [Google Scholar]
- Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ line chimera by transfer of primordial germ cells in the domestic chicken (Gallus domesticus) . Theriogenology 1993; 40: 509 519. [DOI] [PubMed] [Google Scholar]
- Naito M, Tajima A, Yasuda Y, Kuwana T. Donor primordial germ cell-derived offspring from recipient germline chimaeric chickens: Absence of long term immune rejection and effects on sex ratios. Br Poult Sci 1998; 39: 20 23. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Kawashima T, Naito M, Yamashita H, Matsuzaki M, Takano T. Conservation of a threatened indigenous fowl (Kureko dori) using the germline chimeras transplanted from primordial germ cells. J Poult Sci 2006; 43: 60 66. [Google Scholar]
- Naito M, Minematsu T, Harumi T, Kuwana T. Testicular and ovarian gonocytes from 20-day incubated chicken embryos contribute to germline lineage after transfer into bloodstream of recipient embryos. Reproduction 2007; 134: 577 584. [DOI] [PubMed] [Google Scholar]
- Jung JG, Lee YM, Kim JN, Kim TM, Shin JH, Kim TH, Lim JM, Han JY. The reversible developmental unipotency of germ cells in chicken. Reproduction 2010; 139: 113 119. [DOI] [PubMed] [Google Scholar]
- Ono T, Yokoi R, Aoyama H. Transfer of male or female primordial germ cells of quail into chick embryonic gonads. Exp Anim 1996; 45: 347 352. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Choi JW, Kim SY, Park KJ, Kim TM, Lee YM, Kim H, Lim JM, Han JY. Reproduction of wild birds via interspecies germ cell transplantation. Biol Reprod 2008; 79: 931 937. [DOI] [PubMed] [Google Scholar]
- Lin M, Jones RC. Renewal and proliferation of spermatogonia during spermatogenesis in the Japanese quail, Coturnix coturnix japonica. Cell Tissue Res 1992; 267: 591 601. [DOI] [PubMed] [Google Scholar]
- Minematsu T, Tajima A, Kanai Y. The migratory ability of gonadal germ cells in the domestic chicken. J Poult Sci 2004; 41: 178 185. [Google Scholar]
- Trefil P, Micakova A, Mucksova J, Hejnar J, Poplstein M, Bakst MR, Kalina J, Brillard JP. Restoration of spermatogenesis and male fertility by transplantation of dispersed testicular cells in the chicken. Biol Reprod 2006; 75: 575 581. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jung JG, Kim JN, Park TS, Kim TM, Shin SS, Kang DK, Lim JM, Han JY. A testis-mediated germline chimera production based on transfer of chicken testicular cells directly into heterologous testes. Biol Reprod 2006; 75: 380 386. [DOI] [PubMed] [Google Scholar]
- Pereira RJ, Napolitano A, Garcia-Pereira FL, Baldo CF, Suhr ST, King LE, Cibelli JB, Karcher DM, McNiel EA, Perez GI. Conservation of avian germplasm by xenogeneic transplantation of spermatogonia from sexually mature donors. Stem Cells Dev 2013; 22 (5): 735 749. [DOI] [PubMed] [Google Scholar]
- Park TS, Hong YH, Kwon SC, Lim JM, Han JY. Birth of germline chimeras by transfer of chicken embryonic germ (EG) cells into recipient embryos. Mol Reprod Dev 2003; 65: 389 395. [DOI] [PubMed] [Google Scholar]
- Park TS, Jeong DK, Kim JN, Song GH, Hong YH, Lim JM, Han JY. Improved germline transmission in chicken chimeras produced by transplantation of gonadal primordial germ cells into recipient embryos. Biol Reprod 2003; 68: 1657 1662. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn 2001; 220: 284 289. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, García-Martín M, Martin C, Ríos A. Haemopoietic phagocytes in the early differentiating avian retina. J Anat 1991; 177: 145 158. [PMC free article] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Ordahl CP, Christ B. The fate of the first avian somite. Anat Embryol (Berl) 1997; 195: 435 449. [DOI] [PubMed] [Google Scholar]
- Urven LE, Erickson CA, Abbott UK, McCarrey JR. Analysis of germ line development in the chick embryo using an anti-mouse EC cell antibody. Development 1988; 103: 299 304. [DOI] [PubMed] [Google Scholar]
- Jung JG, Kim DK, Park TS, Lee SD, Lim JM, Han JY. Development of novel markers for the characterization of chicken primordial germ cells. Stem Cells 2005; 23: 689 698. [DOI] [PubMed] [Google Scholar]
- Walsh PS, Metzger DA, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 1991; 10: 506 513. [PubMed] [Google Scholar]
- Jensen T, Pernasetti FM, Durrant B. Conditions for rapid sex determination in 47 avian species by PCR of genomic DNA from blood, shell-membrane bloodvessels, and feathers. Zoo Biol 2003; 22: 561 571. [Google Scholar]
- Kim JN, Park TS, Park SH, Park KJ, Kim TM, Lee SK, Lim JM, Han JY. Migration and proliferation of intact and genetically modified primordial germ cells and the generation of a transgenic chicken. Biol Reprod 2010; 82: 257 262. [DOI] [PubMed] [Google Scholar]
- Chang IK, Yoshiki A, Kusakabe M, Tajima A, Chikamune T, Naito M, Ohno T. Germ line chimera produced by transfer of cultured chick primordial germ cells. Cell Biol Int 1995; 19: 569 576. [DOI] [PubMed] [Google Scholar]
- Li BC, Tian ZQ, Sun M, Xu Q, Wang XY, Qin YR, Xu F, Gao B, Wang KH, Sun HC, Chen GH. Directional differentiation of chicken primordial germ cells into adipocytes, neuron-like cells, and osteoblasts. Mol Reprod Dev 2010; 77: 795 801. [DOI] [PubMed] [Google Scholar]
- Trefil P, Bakst MR, Yan H, Hejnar J, Kalina J, Mucksova J. Restoration of spermatogenesis after transplantation of c-Kit positive testicular cells in the fowl. Theriogenology 2010; 74: 1670 1676. [DOI] [PubMed] [Google Scholar]


