Highlights
-
•
Quantification of volatiles emitted by human skin by SPME-GCMS.
-
•
Determination of emission rates of 64 skin-borne species.
-
•
Selection of potential skin-borne markers of human presence for rescue applications.
Keywords: Volatile organic compounds, Human skin emanation, Human odor, Entrapped victims, Emission rate, SPME-GCMS
Abstract
Gas chromatography with mass spectrometric detection (GC–MS) coupled with solid phase micro-extraction as pre-concentration method (SPME) was applied to identify and quantify volatile organic compounds (VOCs) emitted by human skin. A total of 64 C4-C10 compounds were quantified in skin emanation of 31 healthy volunteers. Amongst them aldehydes and hydrocarbons were the predominant chemical families with eighteen and seventeen species, respectively. Apart from these, there were eight ketones, six heterocyclic compounds, six terpenes, four esters, two alcohols, two volatile sulphur compounds, and one nitrile. The observed median emission rates ranged from 0.55 to 4790 fmol cm−2 min−1. Within this set of analytes three volatiles; acetone, 6-methyl-5-hepten-2-one, and acetaldehyde exhibited especially high emission rates exceeding 100 fmol cm−2 min−1. Thirty-three volatiles were highly present in skin emanation with incidence rates over 80%. These species can be considered as potential markers of human presence, which could be used for early location of entrapped victims during Urban Search and Rescue Operations (USaR).
1. Introduction
The human body emits hundreds of volatile organic compounds (VOCs) offering unique insight into biochemical processes ongoing in healthy and diseased human organism [1–4]. In the medical context, they can be considered as a non-invasive biochemical probe capable of diagnosing disease processes and monitoring therapy. For instance, over the last years a robust correlation has been established between profiles of breath volatiles and lung cancer [5–8], oxidative stress [9,10], or organ rejection after transplantation [11]. Thus, the VOC pattern directly relates to the physiological status of an individual. However, the main obstacle limiting the application of this chemical fingerprint within a diagnostic context is the insufficient understanding of the origin and metabolic fate of its constituents.
A human-specific VOC signature also opens up new prospects for Urban Search and Rescue operations (USaR) organized after natural, or man-made disasters (e.g., earthquakes, tropical storms, explosions, or terrorist attacks). In urban areas such events may entail building collapses and, thereby, entrapment of people under ruins. Although USaR teams increasingly rely on specialized technical equipment supporting the rapid detection of trapped humans, canines are still the preferred choice [12]. Sniffer dogs can rapidly scan ruins and track human scent. However, their abilities are limited. They become tired after approximately 30 min of intensive search and may easily get stressed or frustrated once they are unsuccessful [13]. Consequently, there is a need for sensitive human scent detectors, which could support or even replace searching dogs. A number of recent studies suggest that chemical analysis of debris air aiming at constituents of human scent could meet this demand and considerably improve the effectiveness of USaR operations [14–20].
Skin, next to breath, is a principal source of human scent constituents. Contrary to some incidental and temporal sources like blood or urine, it offers a long-lasting emission of VOCs. The composition of skin emanation in humans has received a broad attention [21]. Numerous studies employing a number of analytical techniques such as Gas Chromatography–Mass Spectrometry (GC–MS) [22–31], High Performance Liquid Chromatography (HPLC) [32], Ion Mobility Spectrometry (IMS) [33], Proton Transfer Reaction Mass Spectrometry (PTR-MS) [34–36], or Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) [37] aimed at the identification of constituents forming the human scent. However, GC–MS with solid phase microextracxtion (SPME) as the pre-concentration method seems to be a gold standard in this context [20,25,26,30,31,38–40]. This is due to the simplicity of SPME, its ease of operation, its potential for automation and the small amounts of sample required to perform extraction. Up to now more than 400 volatiles belonging to numerous chemical classes have been reported in the literature to be emitted by human skin [21]. However, the overwhelming majority of studies present only qualitative data (e.g. occurrence) based frequently on tentative identification [20,22–31,39]. The emission rate/flux (quantitative data) has been determined only for a limited number of species [32,41]. Moreover, most of the studies were focused on indirect analyses of skin emanation (e.g., species adsorbed on different materials rubbed on human skin) [20,23,24,26,27,31], thereby inducing high losses of very volatile compounds during sampling.
The primary goal of this work was the quantification of a wide range of reliably identified VOCs emitted by skin of healthy volunteers in order to fill the literature gap with respect to emission rates (flux) of skin-borne volatiles. A particular focus has been on highly volatile analytes, which cannot properly be detected using indirect sampling. Another main objective was the selection of species showing high potential as markers of human presence to be used during USaR operations. Within this study all volatiles of interest were quantified on the basis of SPME-GC–MS as the pre-concentration method.
2. Materials and methods
2.1. Calibration mixtures
Since the preparation of gaseous calibration mixtures was described in detail in our recent article [18] only a brief outline of the procedure will be presented here. Gaseous multi-compound calibration mixtures were prepared from pure liquid or gaseous substances. The reference substances with purities ranging from 90 to 99% were purchased from Sigma–Aldrich (Austria), Fluka (Switzerland), ChemSampCo (USA), Acros Organic (Belgium), and SAFC (USA).
Gaseous mixtures of less volatile species were produced by means of a GasLab calibration mixtures generator (Breitfuss Messtechnik, Germany). The generator supports the preparation of gas mixtures at pre-defined humidity levels from pure liquid substances containing 10 ppb to 100 ppm of each solute. For the aim of this study, pure substances were additionally diluted at ratios of 1:2000 to 1:3000 to reduce the resulting concentration levels. Gas mixtures exhibiting 90% relative humidity at 25 °C with analyte volume fractions ranging from approximately 0.05 to 1000 ppb were used during calibration and validation. Alternatively, in case of highly volatile compounds (e.g., hydrocarbons) multi-compound gaseous standards were prepared by injecting and evaporating a few microliters of liquid or gaseous analyte into evacuated 1-L glass bulbs (Supelco, Canada). The desired calibration levels were achieved by transferring appropriate volumes of the bulb standard into Tedlar bags (SKC Inc., USA) filled with predefined amounts of humidified zero air (90% RH at 25 °C as above), the latter being produced by the GasLab generator. Calibration curves were obtained on the basis of 3-fold analyses of 6 distinct and independent concentration levels.
2.2. Human subjects and sampling.
A cohort of 31 healthy volunteers (16 males, 15 females, age range 23–55 years, median 35 years, 4 smokers) was recruited. All subjects gave written informed consent to participate and completed a questionnaire describing their health and smoking status, as well as recent food intake. The sample collection was approved by the Ethics Commission of the Innsbruck Medical University. No special dietary regimes were applied.
Skin emission measurements followed a modified version of the procedure applied by Turner et al. [37]. Hand and forearm were selected for skin emission analyses as this part of the body can most easily be accessed during the applied sampling protocol and thus is the most convenient option for volunteers. Volatiles emitted by human skin were collected into in-house made disposable Nalophan bags (Kalle Nalo GmbH, Germany). The Nalophan tube (film thickness 20 μm, diameter 165 mm) was cut into 45–50 cm long pieces. Next, a polypropylene valve (SKC Inc.) equipped with a rubber septum was installed on each Nalophan piece and the open ends of the tube were sealed with Polyamide (PA) closures (WeLoc, Sweden) to form a bag. In order to desorb potential contaminants all bags were pre-conditioned. For this purpose, they were flushed five times with high-purity nitrogen (99.9999%), filled with nitrogen and heated for approximately 12 h in an oven at 50 °C and finally flushed again five times with nitrogen [42].
Prior to each experiment involving a human subject, a blank experiment was performed. The pre-conditioned Nalophan bag was filled with 2.5 L of high-purity nitrogen and stored at room temperature for 30 min (exposed to daylight). Next, the bag content was analyzed to identify possible bag contaminants, which could affect the underlying skin emission profiles.
Directly before the main experiment the volunteers were asked to wash their arm thoroughly exclusively with tap water and dry it with a paper towel. Next, one of the bag closures was removed and the volunteer was asked to place his/her arm into the bag. The bag was then sealed around the forearm using an elastic strap and tape. The residual room air filling the bag was afterwards evacuated using a membrane pump and 2.5 L of purified air (GasLab calibration mixtures generator, Breitfuss Messtechnik, Germany) were introduced into the bag via the polypropylene valve using an EL-FLOW F201CV digital mass flow controller (Bronkhorst high-tech B.V., Netherlands). The introduction of high purity air considerably reduced the VOCs background levels and improved the detection of species under study. After 30 min of skin VOCs accumulation a volume of 50 mL was drawn from the bag using a heated (40 °C) 50 mL glass syringe (Roth, Germany). During each experiment additional room air samples were taken to monitor VOCs levels in ambient air and measurements of bag and room air temperatures were performed. Finally, the basic dimensions of the part of the volunteer's forearm located in the bag were measured to approximately calculate the skin surface involved.
2.3. SPME extraction and chromatographic analysis
The skin emission samples for the SPME-GC–MS analysis were prepared using a 50 mL gas-tight glass syringe (Roth, Germany) equipped with a replaceable needle. Using the latter, a volume of 50 mL was drawn from the sampling bag and injected into an evacuated SPME vial (55 mL nominal volume, Macherey-Nagel, Germany) sealed with a 1.3 mm butyl/PTFE septum (Macherey-Nagel, Germany). The SPME procedure was carried out automatically using a multipurpose sampler MPS (Gerstel, Germany). SPME was achieved by inserting a 75 μm carboxen-polydimethylsiloxane (CAR-PDMS) fiber (Supelco, Canada) into the vial and exposing it to its content for 25 min at 20 °C. Immediately after extraction the fiber was introduced into the inlet of the gas chromatograph where the pre-concentrated VOCs were thermally desorbed at 290 °C. The fiber was conditioned at 290 °C for 5 min prior to each extraction.
GC–MS analyses were performed using an Agilent 7890A/5975C system (Agilent, USA). During desorption the split/splitless inlet operated in the splitless mode (1 min), followed by a split mode at the ratio of 1–35. The volatiles of interest were separated using a PoraBond Q column (25 m × 0.32 mm, film thickness 5 μm, styrene-divinylbenzene copolymer phase, Varian, USA) working in a constant flow of helium at 1.4 mL/min. The column temperature program was as follows: 40 °C for 2 min, increase to 260 °C at a rate of 7 °C/min, constant temperature of 260 °C for 7 min. The mass spectrometer worked in a SCAN mode with an associated m/z range set from 20 to 200. The quadrupole, ion source, and transfer line temperatures were kept at 150 °C, 230 °C, and 280 °C, respectively.
The identification of compounds was performed in two steps. First, the peak spectrum was checked against the NIST mass spectral library. Next, the NIST identification was confirmed by comparing the retention times of peaks of interest with retention times obtained for standard mixtures prepared from pure compounds. Peak integration was based on extracted ion chromatograms. The applied quantifier ions are presented in Table 1.
Table 1.
Retention times Rt [min], quantifier ions, LODs [ppb], RSDs (%), coefficients of variation (R2), linear ranges [ppb] and average flux LODs [fmol cm−2 min−1] of compounds under study for skin emission measurements. Compounds are ordered with respect to increasing retention time.
| VOC | CAS | Rt [min] | Quantifier ion | LOD [ppb] | RSD [%] | R2 | linear range [ppb] | Average flux LOD [fmol × cm−2 × min−1] |
|---|---|---|---|---|---|---|---|---|
| Propene | 115-07-1 | 5.84 | 41 | 0.59 | 3.5 | 0.998 | 1.76–15.5 | 3.15 |
| Acetaldehyde | 75-07-0 | 8.08 | 44 | 6 | 8 | 0.997 | 18–760 | 32.1 |
| Ethanol | 64-17-5 | 11.01 | 45 | 29 | 5 | 0.999 | 86–1520 | 155 |
| Acetonitrile | 1975-05-08 | 11.46 | 41 | 0.14 | 5.5 | 0.999 | 0.42–100 | 0.75 |
| 2-Butene, (E)- | 624-64-6 | 11.69 | 56 | 0.017 | 2 | 0.998 | 0.05–4.6 | 0.09 |
| 2-Butene, (Z)- | 590-18-1 | 11.94 | 56 | 0.014 | 4.2 | 0.998 | 0.04–1.8 | 0.07 |
| 2-Propenal | 107-02-8 | 12.90 | 56 | 0.11 | 4.5 | 0.999 | 0.33–40 | 0.59 |
| n-Propanal | 123-38-6 | 13.56 | 58 | 0.124 | 2.4 | 0.999 | 0.37–26 | 0.66 |
| Acetone | 67-64-1 | 13.76 | 58 | 0.125 | 3.5 | 0.999 | 0.38–766 | 0.67 |
| 2-Propanol | 67-63-0 | 14.16 | 45 | 14.7 | 8 | 0.988 | 44–150 | 78.6 |
| Dimethyl sulfide | 75-18-3 | 14.37 | 62 | 0.045 | 6 | 0.999 | 0.13–11 | 0.24 |
| 1-Pentene | 109-67-1 | 16.03 | 55 | 0.035 | 2 | 0.999 | 0.1–7.2 | 0.19 |
| Isoprene | 78-79-5 | 16.15 | 67 | 0.009 | 2.7 | 0.999 | 0.03–5.3 | 0.05 |
| n-Pentane | 109-66-0 | 16.62 | 43 | 0.05 | 1.7 | 0.998 | 0.15–5 | 0.27 |
| 1,3-Dioxolane | 646-06-0 | 16.80 | 74 | 0.69 | 6.5 | 0.997 | 2.1–21 | 3.69 |
| 2-Propenal, 2-methyl- | 78-85-3 | 17.04 | 70 | 0.025 | 3.4 | 0.993 | 0.08–11 | 0.13 |
| Propanal, 2-methyl- | 78-84-2 | 17.34 | 72 | 0.127 | 5 | 0.999 | 0.38–20.5 | 0.68 |
| 3-Buten-2-one | 78-94-4 | 17.64 | 55 | 0.086 | 1.8 | 0.998 | 0.26–19 | 0.46 |
| Furan, 2-methyl- | 534-22-5 | 18.13 | 82 | 0.015 | 1.6 | 0.999 | 0.04–9 | 0.08 |
| n-Butanal | 123-72-8 | 18.16 | 72 | 0.2 | 3.3 | 0.999 | 0.6–45 | 1.07 |
| 2-Butanone | 78-93-3 | 18.25 | 72 | 0.08 | 7 | 0.998 | 0.17–14 | 0.43 |
| Furan, 3-methyl- | 930-27-8 | 18.42 | 82 | 0.015 | 2 | 0.997 | 0.04–8 | 0.08 |
| Ethyl Acetate | 141-78-6 | 19.01 | 43 | 0.016 | 5 | 0.992 | 0.05–75 | 0.09 |
| 2-Butenal, (E)- | 123-73-9 | 19.37 | 70 | 0.057 | 3.5 | 0.996 | 0.17–11.5 | 0.30 |
| 1,3-Dioxolane, 2-methyl- | 497-26-7 | 19.69 | 73 | 0.022 | 5 | 0.997 | 0.07–14 | 0.12 |
| 2-Pentene, 2-methyl- | 625-27-4 | 20.31 | 69 | 0.011 | 1.7 | 0.999 | 0.03–12.6 | 0.06 |
| 2-Butene, 2,3-dimethyl- | 563-79-1 | 20.63 | 69 | 0.012 | 5 | 0.996 | 0.04–8.3 | 0.06 |
| 1,3-Pentadiene, 2-methyl-, (E)- | 926-54-5 | 20.90 | 67 | 0.014 | 3 | 0.998 | 0.04–7.3 | 0.07 |
| 1,3-Pentadiene, 2-methyl-, (Z)- | 1118-58-7 | 21.02 | 67 | 0.014 | 3.5 | 0.996 | 0.04–10 | 0.07 |
| Butanal, 3-methyl- | 590-86-3 | 21.46 | 44 | 0.13 | 5 | 0.956 | 0.38–13 | 0.69 |
| Butanal, 2-methyl- | 96-17-3 | 21.51 | 57 | 0.065 | 4.3 | 0.999 | 0.2–18 | 0.35 |
| Isopropyl acetate | 108-21-4 | 21.69 | 43 | 0.025 | 3 | 0.995 | 0.08–50 | 0.13 |
| 2-Pentanone | 107-87-9 | 22.03 | 43 | 0.024 | 7 | 0.994 | 0.07–9 | 0.13 |
| Furan, 2,5-dimethyl- | 625-86-5 | 22.07 | 96 | 0.018 | 0.5 | 0.999 | 0.05–6.5 | 0.10 |
| Sulfide, allyl methyl | 10152-76-8 | 22.14 | 88 | 0.014 | 0.9 | 0.996 | 0.04–5 | 0.07 |
| n-Pentanal | 110-62-3 | 22.23 | 44 | 0.103 | 2.2 | 0.998 | 0.31–8.5 | 0.55 |
| 2-Butenal, 3-methyl- | 107-86-8 | 23.82 | 84 | 0.188 | 12 | 0.942 | 0.6–44 | 1.00 |
| 1-Heptene | 592-76-7 | 23.96 | 56 | 0.015 | 1 | 0.998 | 0.05–4 | 0.08 |
| 2-Heptene | 592-77-8 | 24.20 | 55 | 0.034 | 1.5 | 0.996 | 0.1–5 | 0.18 |
| n-Heptane | 142-82-5 | 24.38 | 71 | 0.022 | 1.8 | 0.996 | 0.06–6 | 0.12 |
| Butanal, 2-ethyl- | 97-96-1 | 25.02 | 72 | 0.23 | 11 | 0.995 | 0.68–30 | 1.23 |
| 3-Penten-2-one, 4-methyl- | 141-79-7 | 25.39 | 83 | 0.23 | 9 | 0.983 | 0.68–41 | 1.23 |
| Isobutyl acetate | 110-19-0 | 25.55 | 43 | 0.07 | 7 | 0.988 | 0.21–14 | 0.37 |
| 2-Hexanone | 591-78-6 | 25.64 | 58 | 0.08 | 5 | 0.992 | 0.24–6 | 0.43 |
| n-Hexanal | 66-25-1 | 25.80 | 56 | 0.063 | 12 | 0.994 | 0.19–25 | 0.34 |
| γ-Butyrolactone | 96-48-0 | 26.01 | 42 | 0.37 | 8 | 0.989 | 1.1–100 | 1.98 |
| n-Butyl acetate | 123-86-4 | 26.27 | 56 | 0.033 | 11 | 0.997 | 0.1–1000 | 0.18 |
| 2-Hexenal, (E)- | 6728-26-3 | 26.68 | 55 | 0.25 | 7 | 0.985 | 0.75–5.5 | 1.34 |
| 1-Octene | 111-66-0 | 27.26 | 55 | 0.055 | 1.7 | 0.994 | 0.17–7.3 | 0.29 |
| n-Octane | 111-65-9 | 27.64 | 43 | 0.04 | 3.4 | 0.991 | 0.12–6 | 0.21 |
| 2-Heptanone | 110-43-0 | 28.84 | 58 | 0.16 | 10 | 0.968 | 0.46–13 | 0.86 |
| n-Heptanal | 111-71-7 | 29.00 | 70 | 0.19 | 12 | 0.963 | 0.57–14 | 1.02 |
| Benzaldehyde | 100-52-7 | 29.00 | 106 | 0.53 | 10 | 0.969 | 1.59–41 | 2.83 |
| 1-Nonene | 124-11-8 | 30.26 | 56 | 0.055 | 2.7 | 0.985 | 0.17–6 | 0.29 |
| n-Nonane | 111-84-2 | 30.58 | 57 | 0.1 | 1.4 | 0.986 | 0.29–8 | 0.53 |
| 5-Hepten-2-one, 6-methyl- | 110-93-0 | 31.20 | 108 | 0.15 | 9 | 0.958 | 0.43–210 | 0.80 |
| Furan, 2-pentyl- | 3777-69-3 | 31.62 | 81 | 0.026 | 1.3 | 0.995 | 0.08–8 | 0.14 |
| β-Pinene | 127-91-3 | 31.90 | 93 | 0.085 | 4 | 0.999 | 0.25–10.5 | 0.45 |
| n-Octanal | 124-13-0 | 31.92 | 43 | 0.3 | 12 | 0.981 | 0.9–30 | 1.60 |
| p-Cymene | 99-87-6 | 32.72 | 119 | 0.1 | 1.4 | 0.995 | 0.3–12.5 | 0.53 |
| DL-Limonene | 5989-27-5 | 32.94 | 68 | 0.125 | 1.6 | 0.993 | 0.38–54 | 0.67 |
| Styrene, p,α-dimethyl- | 1195-32-0 | 33.49 | 117 | 0.46 | 3 | 0.992 | 1.38–32 | 2.46 |
| Eucalyptol | 470-82-6 | 33.50 | 81 | 0.16 | 6 | 0.990 | 0.47–100 | 0.86 |
| n-Nonanal | 124-19-6 | 34.73 | 57 | 0.6 | 8 | 0.977 | 1.8–50 | 3.21 |
3. Results and discussion
3.1. Validation parameters
The calculated validation parameters are presented in Table 1. Limits of detection (LODs) were calculated using the algorithm presented by Huber [43]. More specifically, standard deviation of 9 consecutive blank signals and 1% probability (1 − α) for the type 1 error resulting in the coverage factor of 3.05 were used for these purposes. The LOD values ranged from 0.009 ppb for isoprene to 29 ppb for ethanol. The limit of quantification (LOQ) was defined as 3 × LOD. Relative standard deviations (RSDs) were calculated on the basis of consecutive analyses of five independent standard mixtures exhibiting concentrations close to the medians of the observed levels in real samples. The RSDs fall within the range of 0.5–12% and were recognized as satisfactory for the goals of this study. The instrument response was found to be linear within the investigated concentration ranges, with coefficients of variation ranging from 0.942 to 0.999. The LODs of emission rates were estimated using the average skin surface involved. It must be stressed here that these values should be treated as approximate, as in the applied experimental setup forearm skin area was not fixed and differed amongst volunteers.
3.2. Human skin profile of VOCs
Overall 64 compounds emitted by human skin were reliably identified and quantified. Their associated detection and quantification incidences as well as the observed concentration ranges in Nalophan bags are given in Table 2. The presented concentration levels are diminished (if applicable) by the respective values obtained for the associated blank samples. Several compounds appearing in very high and variable levels in room air before and during experiments (e.g., methanol, methyl acetate, 2-methyl-butane, 2-methyl-1-propene, hexane, toluene, benzene) were excluded from the following discussion as it was assumed that their possible skin emissions would be too distorted by contamination to allow for a sound analysis. The obtained bag concentration levels of VOCs together with the dimensions of a volunteer's forearm skin involved into experiments were used to calculate the emission rates of VOCs under study. Since the origin and emission type of species stemming from human skin is in many cases ambiguous a constant emission was assumed for these calculations. The obtained values expressed in femtomols emitted by one square cm of skin within 1 min of experiment (fmol cm−2 min−1) are presented in Table 2.
Table 2.
Detection (nd) and quantification (nq) incidences of the compounds under study, together with their concentration ranges in Nalophan bags and calculated emission rates. Compounds are divided into chemical classes and ordered with respect to decreasing incidence.
| Class |
VOC |
Incidence nd(nq) |
Concentration range (median) [ppb] |
Emission rate range (median) [fmol × cm−2 × min−1] |
Reported also by: |
Tentative origin |
|
|---|---|---|---|---|---|---|---|
| [%] | [–] | ||||||
| Aldehydes | Acetaldehyde | 100(100) | 31(31) | 27.8–688 (43) | 164–3989 (244) | A, B, C, D, O | (a) Ethanol metabolism [59] (b) Oxidative degradation of linolenic acid [45,46] |
| 2-Propenal | 100(100) | 31(31) | 1.36–7.45 (3.44) | 6.37–45 (19.5) | (a) Smoking [47] | ||
| Butanal, 3-methyl- | 100(100) | 31(31) | 1.19–6.45 (2.45) | 6.09–26.9 (13.4) | |||
| Butanal, 2-methyl- | 100(100) | 31(31) | 0.92–5.43 (2.47) | 5.3–24.8 (14) | E | ||
| n-Propanal | 97(97) | 30(30) | 0.56–20.8 (2.3) | 3.44–112 (12.4) | B, C, D, E, F | (a) Oxidative degradation of linolenic acid and oleic acid [45,46] | |
| 2-Propenal, 2-methyl- | 97(97) | 30(30) | 1.08–12.3 (3.35) | 6.42–55.9 (17.4) | (a) OH-initiated degradation of isoprene [60] | ||
| Propanal, 2-methyl- | 97(97) | 30(30) | 0.90–4 (2.23) | 5.48–17.7 (11.7) | E | ||
| n-Hexanal | 97(97) | 30(30) | 3.43–32.5 (7.56) | 16.8–168 (41.9) | F, G, H, N | (a) Oxidative degradation of linoleic acid, palmitoleic acid and vaccenic acid, [24,45,46] | |
| n-Nonanal | 94(94) | 29(29) | 3.26–19.2 (11.4) | 18.1–119 (58.9) | E, F, G, H, I, J, K, L, M, N | (a) Oxidative degradation of oleic acid [24,45,46] | |
| n-Octanal | 87(87) | 27(27) | 4.29–25.9 (6.17) | 22.5–150 (33.1) | E, F, G, H, I, J, K, M | (a) Oxidative degradation of oleic acid [45,46] | |
| n-Heptanal | 81(77) | 25(24) | 2.34–11.2 (4.81) | 12–60 (27.6) | D, E, F, H, M | (a) Oxidative degradation of palmitoleic acid, vaccenic acid [24] | |
| 2-Butenal, (E)- | 71(71) | 22(22) | 0.12–5.48 (0.6) | 0.62–30.2 (3.06) | D | (a) Oxidative degradation of linolenic acid [45] | |
| n-Pentanal | 71(71) | 22(22) | 0.79–3.13 (1.62) | 3.74–14.9 (8.59) | D, F | (a) Oxidative degradation of linoleic acid [46] | |
| Benzaldehyde | 71(71) | 22(22) | 12.7–42.5 (29.1) | 62–238 (147) | E, I, J, K, M, N, O | ||
| n-Butanal | 48(48) | 15(15) | 0.98–53.8 (2.1) | 4.6–311 (12) | F | (a) Oxidative degradation of linolenic acid [45] | |
| 2-Hexenal, (E)- | 48(35) | 15(11) | 0.7–1.72 (1.06) | 3.29–9.51 (6.29) | |||
| 2-Butenal, 3-methyl- | 39(39) | 12(12) | 2.36–15.1 (5.28) | 13.5–68.7 (28.3) | K | ||
| Butanal, 2-ethyl- | 6(6) | 2(2) | 1.37–3.99 (2.68) | 6.33–26.6 (16.5) | |||
| HCs | 1-Heptene | 100(100) | 31(31) | 0.04–0.72 (0.29) | 0.17–3.28 (1.79) | ||
| n-Heptane | 100(100) | 31(31) | 0.30–1.73 (0.6) | 1.41–7.89 (2.94) | D, E | (a) Oxidative degradation of oleic acid [45] | |
| n-Octane | 100(100) | 31(31) | 0.28–3.66 (1.37) | 1.75–16.9 (7.14) | E | (a) Oxidative degradation of oleic acid [45] | |
| Isoprene | 97(97) | 30(30) | 0.16–3.89 (0.82) | 0.99–17.7 (4.6) | (a) Endogenous cholesterol synthesis [61,62] (b) Peroxidation of squalene [54] (c) Cutaneous synthesis of squalene [63] |
||
| n-Nonane | 97(97) | 30(30) | 0.80–8.84 (2.42) | 4.84–40.3 (12.1) | E, M, N | ||
| 1-Octene | 94(94) | 29(29) | 0.18–1.16 (0.51) | 0.74–7.76 (2.9) | D, H | ||
| 1-Nonene | 90(90) | 28(28) | 0.07–1.72 (0.67) | 0.35–8.1 (3.75) | |||
| 2-Pentene, 2-methyl- | 87(87) | 27(27) | 0.17–11.8 (1.96) | 1.05–54 (9.34) | (a) Peroxidation of squalene [54] | ||
| 1,3-Pentadiene, 2-methyl-, (Z)- | 81(81) | 25(25) | 0.12–1.66 (0.31) | 0.62–8.19 (1.7) | |||
| 1,3-Pentadiene, 2-methyl-, (E)- | 81(77) | 25(24) | 0.10–0.93 (0.23) | 0.51–4.44 (1.24) | |||
| Propene | 65(61) | 20(19) | 1.21–4.96 (2.56) | 4.97–29.3 (13.13) | (a) Peroxidation of squalene [54] | ||
| 1-Pentene | 48(48) | 15(15) | 0.26–0.68 (0.35) | 1.24–3.81 (2.1) | |||
| n-Pentane | 39(39) | 12(12) | 0.49–2.86 (0.99) | 2.69–13.1 (5.19) | D | (a) Oxidative degradation of linoleic acid [45] | |
| 2-Butene, (E)- | 26(26) | 8(8) | 0.05–0.9 (0.12) | 0.30–4.17 (0.57) | |||
| 2-Heptene | 23(23) | 7(7) | 0.15–0.35 (0.21) | 0.75–2.01 (1.29) | |||
| 2-Butene, (Z)- | 10(10) | 3(3) | 0.11–0.76 (0.36) | 0.52–4.42 (1.93) | |||
| 2-Butene, 2,3-dimethyl- | 6(6) | 2(2) | 2.98–9.12 (6.05) | 13.7–43.1 (28.4) | |||
| Ketones | Acetone | 100(100) | 31(31) | 86–808 (206) | 493–3680 (1100) | A, D, E, G, J, L, O | (a) Endogenous decarboxylation of Acetyl–CoA [50] (b) Oxidative degradation of squalene [28,55] |
| 2-Butanone | 100(100) | 31(31) | 0.59–3.64 (1.17) | 3.7–16.6 (6.4) | D, E | ||
| 2-Pentanone | 100(100) | 31(31) | 0.18–1.66 (0.36) | 0.85–7.56 (1.94) | E | (a) Diet [64] (b) 2-Pentanol metabolism [65] |
|
| 5-Hepten-2-one, 6-methyl- | 97(97) | 30(30) | 2.63–167 (24.8) | 14–918 (133) | E, G, H, J, K, L, M, N | (a) Oxidative degradation of squalene [28,55] | |
| 3-Buten-2-one | 87(87) | 27(27) | 0.75–3.23 (1.54) | 4.12–19.5 (8.31) | (a) OH-initiated degradation of isoprene [60] | ||
| 3-Penten-2-one, 4-methyl- | 10(6) | 3(2) | 3.81–42.63 (23.3) | 20.6–247 (133) | |||
| 2-Hexanone | 6(6) | 2(2) | 0.34–0.53 (0.43) | 1.74–3.55 (2.65) | E | ||
| 2-Heptanone | 6(6) | 2(2) | 1.59–1.66 (1.62) | 9.02–10.3 (9.66) | |||
| Heterocycles | Furan, 3-methyl- | 97(97) | 30(30) | 0.08–0.75 (0.17) | 0.44–4.15 (0.9) | E | (a) OH-initiated degradation of isoprene [60] (b) Produced by skin microbiota (Penicillium sp., Aspergillus flavus) [66] |
| Furan, 2-pentyl- | 94(94) | 29(29) | 0.23–1.03 (0.36) | 1.17–5.42 (1.94) | (a) Oxidative degradation of linolenic acid [67] (b) Produced by skin microbiota (Fusarium sp. and Aspergillus flavus) [68] |
||
| Furan, 2-methyl- | 87(87) | 27(27) | 0.13–1.01 (0.33) | 0.6–4.6 (1.8) | E | (a) Smoking [47] | |
| 1,3-Dioxolane, 2-methyl- | 77(77) | 24(24) | 0.11–3.15 (0.57) | 0.63–16 (3.07) | |||
| Furan, 2,5-dimethyl- | 39(39) | 12(12) | 0.07–1.38 (0.09) | 0.37–8.28 (0.55) | (a) Smoking [47] | ||
| 1,3-Dioxolane | 32(32) | 10(10) | 2.81–21.85 (18.1) | 16.4–124 (99) | |||
| Terpenes | DL-Limonene | 97(90) | 30(28) | 0.18–60.64 (1.64) | 0.88–377 (8.76) | I, N | (a) Diet (flavoring) [64] (b) Cosmetics, solvents |
| p-Cymene | 94(52) | 29(16) | 0.31–2.45 (0.46) | 1.43–15.3 (2.68) | J | (a) Diet [64] | |
| γ-Butyrolactone | 74(74) | 23(23) | 0.97–18.90 (4.84) | 5.65–104.5 (26.6) | (a) Produced by skin microbiota (Malassezia)[69] | ||
| β-Pinene | 13(13) | 4(4) | 0.28–2.81 (0.7) | 1.59–18.8 (4.25) | M | (a) Perfumes, cosmetics | |
| Styrene, p,α-dimethyl- | 10(10) | 3(3) | 0.76–10.17 (1.38) | 4.63–63.2 (8) | |||
| Eucalyptol | 10(10) | 3(3) | 1.85–14.24 (2.21) | 9.07–86 (10.1) | (a) Cosmetics (b) Diet (beverages, meat) [64] (c) Insecticide component [70] |
||
| Esters | Ethyl Acetate | 10(10) | 3(3) | 3.74–81.05 (33.6) | 21.7–469 (182) | N | (a) Cosmetics |
| Isopropyl acetate | 6(6) | 2(2) | 23.87–43.78 (33.8) | 138–237 (187) | (a) Cosmetics | ||
| Isobutyl acetate | 6(6) | 2(2) | 11.63–15.52 (13.6) | 63–90 (76) | (a) Cosmetics | ||
| n-Butyl acetate | 10(10) | 3(3) | 130.20–1409 (885) | 659–8140 (4790) | (a) Cosmetics | ||
| Alcohols | Ethanol | 19(10) | 6(3) | 109–7377(329.2) | 683–42773 (2005) | A, D | (a) Oxidative degradation of linoleic acid [45] |
| (b) Diet | |||||||
| 2-Propanol | 6(3) | 2(1) | 87.6 | 506 | D | (a) Disinfectants, cosmetics | |
| Sulphurs | Dimethyl sulfide | 81(77) | 25(24) | 0.13–1.12 (0.48) | 0.60–6.06 (2.52) | E | (a) Endogenous metabolism of sulfur-containing amino acids [71] (b) Bacterial decomposition of sulfur-containing amino acids acids [71] |
| Sulfide, allyl methyl | 19(19) | 6(6) | 0.06–0.5 (0.36) | 0.28–3.13 (1.73) | (b) Diet, garlic consumption [72] | ||
| Other | Acetonitrile | 90(90) | 28(28) | 0.47–89 (1.14) | 2.39–407 (6.31) | D | (a) Smoking [47] |
Legend: A – Turner, C., et al., Rapid Commun Mass Spectrom, 2008, 22(4), 536; B – Moeskops, B.W., et al., Physiol Meas, 2006, 27(11), 1187; C – Steeghs, M.M.L., et al., Int. J. Mass Spectrom., 2006, 253, 58; D – Ellin, R.I., et al.,. J. Chromatogr, 1974, 100(1), 137; E – Bernier, U.R., et al., Anal Chem, 2000, 72(4), 747; F – Luo, X.P., et al., Anal Biochem, 1995, 228(2), 294; G – Wisthaler, A. and C.J. Weschler, Proc Natl Acad Sci U S A, 2010, 107(15), 6568; H – Haze, S., et al., J Invest Dermatol, 2001, 116(4), 520; I – Zhang, Z.M., et al., J Chromatogr B, 2005, 822(1–2), 244; J – Gallagher, M., et al., Br J Dermatol, 2008, 159(4), 780; K – Ruzsanyi, V., et al., J Chromatogr B, 2012, 911, 84; L – Fruekilde, P., et al., Atmospheric Environment, 1998, 32(11), 1893., M – Curran, A.M., et al., J Chromatogr, 2007, 846, 86, N – Dormont, L., et al., J. Exp. Bol., 2013, 216, 2783, O – Sekine Y., et al., J Chromatogr B, 2007, 859, 201.
Ten compounds were omnipresent and further nine were found in all samples but one. The predominant chemical classes were aldehydes and hydrocarbons with eighteen and seventeen species, respectively. Apart from these, there were eight ketones, six heterocyclic compounds, six terpenes, four esters, two alcohols, two volatile sulphur compounds (VSCs), and one nitrile. 33 species were found in more than 80% of samples. This group can be considered as particularly interesting for safety applications.
Aldehydes comprised 28% of all quantified species. Eight of them were n-alkanals (C2–C9), four branched-alkanals, and five alkenals. Aromatic aldehydes were represented only by benzaldehyde. Decanal was also found in all skin samples; however, it was not quantified due to dissatisfactory validation parameters. Aldehydes exhibited relatively high incidence rates. Only 4 species from this class were present in less than 70% of samples. The highest concentrations and thereby emission rates were noted for acetaldehyde, benzaldehyde, and n-nonanal (see Table 2). Within this group acetaldehyde showed particularly high emission rates spread around the value of 244 fmol cm−2 min−1. This acetaldehyde flux is three-fold smaller than the one provided by Sekine et al. [32] for human forearm skin (0–6400, median 756 fmol cm−2 min−1). This discrepancy can stem from the differences in diet regimes between populations, or different intensities of reactive oxygen species inducing the acetaldehyde production in the sebum [44–46].
A total number of seventeen hydrocarbons (HC) were found to be emitted by human-skin. They were predominantly (75%) unsaturated (alkenes or dienes). The remaining ones were n-alkanes. Three HCs (1-heptene, n-heptane and n-octane) were omnipresent and further seven were detected in more than 80% of the samples. The concentration levels of the observed hydrocarbons were lower than the ones noted for aldehydes and did not exceed values of several ppb. A number of hydrocarbons reported within this study have not yet been reported in the literature (see Table 2). This may result from insufficient identification mechanisms (e.g., based solely on the NIST mass spectral library check), high volatility of HCs inducing losses during sampling and indirect analysis, or both.
A number of ketones were detected in the skin head-space. Acetone was definitely the most abundant compound amongst the species quantified within this study. Its levels in bags fell within the range of 86–808 ppb (median 206 ppb), which agrees reasonably well with values observed by Turner et al. [37] for a similar experimental setup. This compound was reported to be released from human skin by numerous investigators [23,26,28,32,37]. The emission rates of acetone obtained within this study (493–3680 fmol cm−2 min−1, median 1100) agree reasonably well with the ones reported by Sekine et al. [32] (<140–1800 fmol cm−2 min−1, mean 490). However, it should be mentioned here that Sekine et al. were able to detect acetone in skin emission of only 37% of volunteers (n = 60), which presumably stems from the fact that acetone LOD was about 200 times higher in Sekine's study (137 fmol cm−2 min−1). Again, some differences in acetone emission can derive from fasting condition, or different exposure to factors inducing oxidative stress on the skin surface and thereby acetone production (UV-radiation, or O3) [28,36]. The emission rates and incidence of the next frequently observed skin-borne ketone, 6-methyl-5-hepten-2-one, were also high and spread around the median value of 133 fmol cm−2 min−1. With the exception of 2-butanone the remaining species from this family were characterized by much lower abundances; however, two of them (2-pentanone and 3-buten-2-one) exhibited high occurrence.
Amongst heterocyclic compounds only three (2-methyl furan, 3-methyl furan, and 2-pentyl furan) occurred in more than 80% of the cases. The highest levels were noted for 1,3 dioxolane, however, this compound was detected only in 30% of samples. Interestingly, 2,5-dimethyl furan – a volatile usually associated with smoking [47]was found in 40% of the volunteers. Bearing in mind that only four smokers were recruited this finding could provide evidence of environmental exposure to cigarette smoke.
Six terpenes were quantified in the human skin headspace. The highest incidence rates were observed for DL-limonene (96%), p-cymene (93%), and γ-butyrolactone (74%). Within this group, γ-butyrolactone exhibited the highest emission rates ranging from 6 to 105 fmol cm−2 min−1. Other terpenes generally occurred in less than 15% of all samples.
The great majority of species from the remaining classes (e.g., esters, alcohols) showed very poor detection incidence, which did not exceed 20%. Only two compounds dimethyl sulfide and acetonitrile were characterized by high incidences of 80% and 90%, respectively. Nevertheless, their head-space concentrations and thereby emission rates were rather low (with the exception of acetonitrile levels in smokers).
3.3. Skin-borne VOCs as markers of human presence
An ideal marker of human presence should be omnipresent, volatile, relatively non-reactive and continuously emitted at relatively high concentrations in the proximity of an entrapped victim. Analytical possibilities with respect to the field identification of such a biomarker are also of utmost importance. Thus, its physicochemical characteristics should allow for its detection by miniature, easy-to-use, low-power, fast, however, sensitive instruments (e.g., chemical sensor arrays, ion mobility spectrometers).
Following this definition systemic skin volatiles seem to be the best candidates as their presence mirrors the physiological processes ongoing in the living organism. Amongst species from this study acetone shows the highest potential in this context. It is formed in the liver during fatty acids oxidation from acetoacetate undergoing spontaneous decarboxylation [48–50] and released in considerable amounts not only through skin, but also via breath [18,50,51], and urine [52,53]. Another source of acetone emitted from human skin is ROS-induced degradation of squalene, an abundant component of human sebum [28,44,54,55]. Finally, acetone can already be detected at low ppb levels by some portable sensor-based instruments [17,56,57].
The entrapped victim is inherently cut off from the predominant factors inducing oxidative stress on the skin surface such as UV radiation, or O3. Thus, it can be expected that the production of UV-induced species will be stopped shortly after entrapment. Consequently, the applicability of compounds from this group may be limited to the initial period of rescue operations. On the other hand, some of these species stem from constituents unique for human sebum (e.g., squalene, sapienic acid, sebaleic acids) and may therefore be regarded as unique and specific markers of human beings. For example, the squalene-related volatile 6-methyl-5-hepten-2-one is very abundant in human odor [24,26,30,33,38]. Although it was also found to be emitted by some plants [28] it could be a biomarker of trapped humans in urban environments. Nevertheless, the UV-, or bacterial-induced decomposition of these unique lipids is poorly known and demands additional studies.
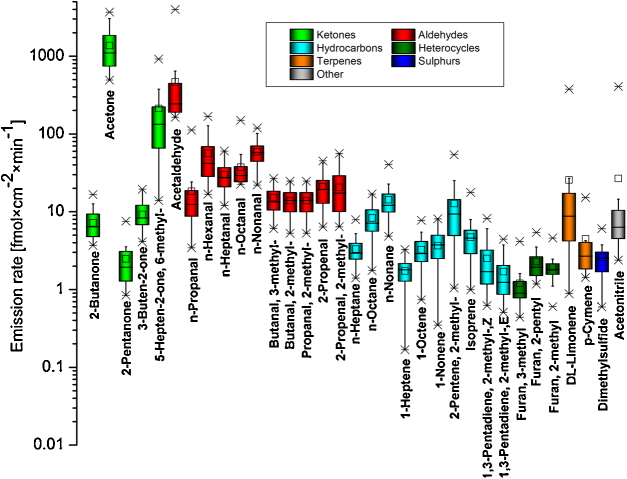
Bearing in mind the considerable shortage of information on the origin of numerous VOCs reported here the only reasonable criteria for the selection of tentative markers of human presence are their omnipresence and concentration levels. An emission pattern of skin-borne volatiles with occurrence greater than 80% is presented in Fig. 1. Three compounds (acetone, 6-methyl-5-hepten-2-one, and acetaldehyde) were emitted with particularly high emission rates exceeding 100 fmol cm−2 min−1. In terms of chemical class, aldehydes are the most represented and abundant species in the above subset. The 33 selected species in Fig. 1 create an initial library of potential skin-borne markers of human presence to be verified during field studies. Interestingly, five compounds from this list (acetone, n-hexanal, DL-limonene, n-octanal and n-nonanal) have recently been proposed as preliminary human markers during simulation experiments with entrapped volunteers involving ion mobility spectrometry [58].
Fig. 1.
Emission pattern of skin-borne volatiles with occurrence rates greater than 80%. Various chemical classes of compounds are indicated by different colors.
4. Conclusions
The present study aimed at the reliable identification and quantification of a wide range of VOCs released by human skin. 64 volatiles were quantified in skin emanations of 31 healthy volunteers. The observed median emission rates ranged over several orders of magnitude from 0.55 to 4790 fmol cm−2 min−1. The quantified compounds belong to several chemical classes; however, aldehydes and hydrocarbons are the most numerous ones. The observed emission rates agree reasonably well with the available literature data. It should be stressed that some species may originate from several distinct sources and their production is still far from being completely understood. In particular, the term endogenous as used in this manuscript embraces metabolic species as well as diet-, and drug-related ones. Moreover, the contribution of gut bacteria to the generation of endogenous VOCs cannot be ignored. 33 volatiles occurred in more than 80% of the samples and these species can be regarded as potential markers of human presence. Amongst them acetone, 6-methyl-5-hepten-2-one, and acetaldehyde exhibited the highest emission rates. These compounds can also be detected by some miniature, easy-to-use, low-power analytical instruments such as ion mobility spectrometry (multicapillary column-ion mobility spectrometry (MCC-IMS), aspiration ion mobility spectrometry (AIMS), field asymmetric waveform ion mobility spectrometry (FAIMS)), or sensor boards, which appear to be natural candidates for human scent detectors to be used during USaR operations. Nevertheless, further field studies are necessary to confirm their applicability for the fast detection of entrapped victims.
Several limitation of the study should be indicated. Firstly, highly volatile species C3-C10 were targeted. This resulted from the fact that the compounds characterized by high vapor pressure have a higher potential for being markers of human presence. Next, only reliably identified and quantified volatiles were reported within this study. A number of VOCs could not be identified and/or quantified properly, either due to the unavailability of pure substances from commercial vendors, or due to problems related to the preparation of reliable standard mixtures. Consequently, some interesting volatile compounds emitted from skin may not be included in the context of the present study. Finally, only forehand skin was involved in the experiments. Due to the differences in the distribution of sebaceous glands, the composition and thickness of the sebum vary between different parts of the body [44]. Consequently, the emission of sebum-related VOCs may be affected by these variations [26].
Acknowledgments
P.M. and K.U. gratefully acknowledge support from the Austrian Science Fund (FWF) under Grant No. P24736-B23. We appreciate funding from the Austrian Federal Ministry for Transport, Innovation and Technology (BMVIT/BMWA, project 836308, KIRAS) and from the Austrian Agency for International Cooperation in Education and Research (OeAD-GmbH, project SPA 04/158 – FEM_PERS). We thank the government of Vorarlberg (Austria) for its generous support.
Contributor Information
Paweł Mochalski, Email: pawel.mochalski@uibk.ac.at.
Anton Amann, Email: anton.amann@i-med.ac.at.
References
- 1.Horvath I., de Jongste J.E. European Respiratory Society; 2010. European Respiratory Monograph, Number 49: Exhaled Biomarkers. [Google Scholar]
- 2.Amann A., Smith D. World Scientific; New Jersey: 2005. Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring. [Google Scholar]
- 3.Amann A., Smith D.e. Elsevier; Amsterdam: 2013. Volatile Biomarkers Non-Invasive Diagnosis in Physiology and Medicine. [Google Scholar]
- 4.de Lacy Costello B., Amann A., Al-Kateb H., Flynn C., Filipiak W., Khalid T., Osborne D., Ratcliffe N.M. J. Breath Res. 2014;8:014001. doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- 5.Amann A., Corradi M., Mazzone P., Mutti A. Expert Rev. Mol. Diagn. 2011;11:207–217. doi: 10.1586/erm.10.112. [DOI] [PubMed] [Google Scholar]
- 6.Bajtarevic A., Ager C., Pienz M., Klieber M., Schwarz K., Ligor M., Ligor T., Filipiak W., Denz H., Fiegl M., Hilbe W., Weiss W., Lukas P., Jamnig H., Hackl M., Haidenberger A., Buszewski B., Miekisch W., Schubert J., Amann A. BMC Cancer. 2009;9:348. doi: 10.1186/1471-2407-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poli D., Carbognani P., Corradi M., Goldoni M., Acampa O., Balbi B., Bianchi L., Rusca M., Mutti A. Respir. Res. 2005;6:71. doi: 10.1186/1465-9921-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips M., Altorki N., Austin J.H., Cameron R.B., Cataneo R.N., Kloss R., Maxfield R.A., Munawar M.I., Pass H.I., Rashid A., Rom W.N., Schmitt P., Wai J. Clin. Chim. Acta. 2008;393:76–84. doi: 10.1016/j.cca.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholpp J., Schubert J.K., Miekisch W., Geiger K. Clin. Chem. Lab. Med. 2002;40:587–594. doi: 10.1515/CCLM.2002.101. [DOI] [PubMed] [Google Scholar]
- 10.Kanoh S., Kobayashi H., Motoyoshi K. Chest. 2005;128:2387–2392. doi: 10.1378/chest.128.4.2387. [DOI] [PubMed] [Google Scholar]
- 11.Phillips M., Boehmer J.P., Cataneo R.N., Cheema T., Eisen H.J., Fallon J.T., Fisher P.E., Gass A., Greenberg J., Kobashigawa J., Mancini D., Rayburn B., Zucker M.J. J. Heart Lung Transplant. 2004;23:701–708. doi: 10.1016/j.healun.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Ferworn A. In: Canine Ergonomics: The Science of Working Dogs. Helton W.S., editor. CRC Press; Boca Raton: 2009. pp. 205–244. [Google Scholar]
- 13.Wong J., Robinson C. 2004. Federal Emergency Management Agency (FEMA) and the National Institute of Justice (NIJ). Document number 207771. [Google Scholar]
- 14.Mochalski P., Buszewska M., Agapiou A., Statheropoulos M., Buszewski B., Amann A. Chromatographia. 2011;74 [Google Scholar]
- 15.Mochalski P., Krapf K., Ager C., Wiesenhofer H., Agapiou A., Statheropoulos M., Fuchs D., Ellmerer E., Buszewski B., Amann A. Toxicol. Mech. Methods. 2012;22:502–511. doi: 10.3109/15376516.2012.682664. [DOI] [PubMed] [Google Scholar]
- 16.Rudnicka J., Mochalski P., Agapiou A., Statheropoulos M., Amann A., Buszewski B. Anal. Bioanal. Chem. 2010;398:2031–2038. doi: 10.1007/s00216-010-4147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mochalski P., Rudnicka J., Agapiou A., Statheropoulos M., Amann A., Buszewski B. J. Breath Res. 2013;7:026002. doi: 10.1088/1752-7155/7/2/026002. [DOI] [PubMed] [Google Scholar]
- 18.Mochalski P., King J., Klieber M., Unterkofler K., Hinterhuber H., Baumann M., Amann A. Analyst. 2013;138:2134–2145. doi: 10.1039/c3an36756h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huo R., Agapiou A., Bocos-Bintintan V., Brown L.J., Burns C., Creaser C.S., Devenport N.A., Gao-Lau B., Guallar-Hoyas C., Hildebrand L., Malkar A., Martin H.J., Moll V.H., Patel P., Ratiu A., Reynolds J.C., Sielemann S., Slodzynski R., Statheropoulos M., Turner M.A., Vautz W., Wright V.E., Thomas C.L. J. Breath Res. 2011;5:046006. doi: 10.1088/1752-7155/5/4/046006. [DOI] [PubMed] [Google Scholar]
- 20.Curran A.M., Prada P.A., Furton K.G. J. Forensic Sci. 2010;55:50–57. doi: 10.1111/j.1556-4029.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 21.Dormont L., Bessiere J.M., Cohuet A. J. Chem. Ecol. 2013;39:569–578. doi: 10.1007/s10886-013-0286-z. [DOI] [PubMed] [Google Scholar]
- 22.Ellin R.I., Farrand R.L., Oberst F.W., Crouse C.L., Billups N.B., Koon W.S., Musselman N.P., Sidell F.R. J. Chromatogr. 1974;100:137–152. doi: 10.1016/s0021-9673(00)86048-3. [DOI] [PubMed] [Google Scholar]
- 23.Bernier U.R., Kline D.L., Barnard D.R., Schreck C.E., Yost R.A. Anal. Chem. 2000;72:747–756. doi: 10.1021/ac990963k. [DOI] [PubMed] [Google Scholar]
- 24.Haze S., Gozu Y., Nakamura S., Kohno Y., Sawano K., Ohta H., Yamazaki K. J. Invest. Dermatol. 2001;116:520–524. doi: 10.1046/j.0022-202x.2001.01287.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z.M., Cai J.J., Ruan G.H., Li G.K. J. Chromatogr. B. 2005;822:244–252. doi: 10.1016/j.jchromb.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher M., Wysocki C.J., Leyden J.J., Spielman A.I., Sun X., Preti G. Brit. J. Dermatol. 2008;159:780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X.P., Yazdanpanah M., Bhooi N., Lehotay D.C. Anal. Biochem. 1995;228:294–298. doi: 10.1006/abio.1995.1353. [DOI] [PubMed] [Google Scholar]
- 28.Fruekilde P., Hjorth J., Jensen N.R., Kotzias D., Larsen B. Atmos. Environ. 1998;32:1893–1902. [Google Scholar]
- 29.Penn D.J., Oberzaucher E., Grammer K., Fischer G., Soini H.A., Wiesler D., Novotny M.V., Dixon S.J., Xu Y., Brereton R.G. J. Roy. Soc. Interface/the Roy. Soc. 2007;4:331–340. doi: 10.1098/rsif.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curran A.M., Ramirez C.F., Schoon A.A., Furton K.G. J. Chromatogr. B. 2007;846:86–97. doi: 10.1016/j.jchromb.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Brown J.S., Prada P.A., Curran A.M., Furton K.G. Forensic Sci. Int. 2013;226:173–182. doi: 10.1016/j.forsciint.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Sekine Y., Toyooka S., Watts S.F. J. Chromatogr. B. 2007;859:201–207. doi: 10.1016/j.jchromb.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Ruzsanyi V., Mochalski P., Schmid A., Wiesenhofer H., Klieber M., Hinterhuber H., Amann A. J. Chromatogr. B. 2012;911:84–92. doi: 10.1016/j.jchromb.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeskops B.W., Steeghs M.M., van Swam K., Cristescu S.M., Scheepers P.T., Harren F.J. Physiol. Meas. 2006;27:1187–1196. doi: 10.1088/0967-3334/27/11/011. [DOI] [PubMed] [Google Scholar]
- 35.Steeghs M.M.L., Moeskops B.W.M., van Swam K., Cristescu S.M., Scheepers P.T.J., Harren F.J.M. Int. J. Mass Spectrom. 2006;253:58–64. doi: 10.1088/0967-3334/27/11/011. [DOI] [PubMed] [Google Scholar]
- 36.Wisthaler A., Weschler C.J. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6568–6575. doi: 10.1073/pnas.0904498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner C., Parekh B., Walton C., Spanel P., Smith D., Evans M. Rapid Commun. Mass Spectrom: RCM. 2008;22:526–532. doi: 10.1002/rcm.3402. [DOI] [PubMed] [Google Scholar]
- 38.Dormont L., Bessiere J.M., McKey D., Cohuet A. J. Exp. Biol. 2013;216:2783–2788. doi: 10.1242/jeb.085936. [DOI] [PubMed] [Google Scholar]
- 39.Kusano M., Mendez E., Furton K.G. J. Forensic Sci. 2013;58:29–39. doi: 10.1111/j.1556-4029.2012.02215.x. [DOI] [PubMed] [Google Scholar]
- 40.Prada P.A., Curran A.M., Furton K.G. J. Forensic Sci. 2011;56:866–881. doi: 10.1111/j.1556-4029.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt F.M., Vaittinen O., Metsala M., Lehto M., Forsblom C., Groop P.H., Halonen L. J. Breath Res. 2013;7:017109. doi: 10.1088/1752-7155/7/1/017109. [DOI] [PubMed] [Google Scholar]
- 42.Mochalski P., King J., Unterkofler K., Amann A. Analyst. 2013;138:1405–1418. doi: 10.1039/c2an36193k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber W. Accred Qual. Assur. 2003;8:213–217. [Google Scholar]
- 44.De Luca C., Valacchi G. Mediators Inflamm. 2010 doi: 10.1155/2010/321494. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frankel E.N. Prog. Lipid Res. 1980;19:1–22. doi: 10.1016/0163-7827(80)90006-5. [DOI] [PubMed] [Google Scholar]
- 46.Fujisaki M., Endo Y., Fujimoto K. JAOCS. 2002;79:909–914. [Google Scholar]
- 47.Filipiak W., Ruzsanyi V., Mochalski P., Filipiak A., Bajtarevic A., Ager C., Denz H., Hilbe W., Jamnig H., Hackl M., Dzien A., Amann A. J. Breath Res. 2012;6:036008. doi: 10.1088/1752-7155/6/3/036008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musa-Veloso K., Likhodii S.S., Rarama E., Benoit S., Liu Y.M., Chartrand D., Curtis R., Carmant L., Lortie A., Comeau F.J., Cunnane S.C. Nutrition. 2006;22:1–8. doi: 10.1016/j.nut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Musa-Veloso K., Likhodii S.S., Cunnane S.C. Am. J. Clin. Nutr. 2002;76:65–70. doi: 10.1093/ajcn/76.1.65. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz K., Pizzini A., Arendacka B., Zerlauth K., Filipiak W., Schmid A., Dzien A., Neuner S., Lechleitner M., Scholl-Burgi S., Miekisch W., Schubert J., Unterkofler K., Witkovsky V., Gastl G., Amann A. J. Breath Res. 2009;3:027003. doi: 10.1088/1752-7155/3/2/027003. [DOI] [PubMed] [Google Scholar]
- 51.King J., Unterkofler K., Teschl G., Teschl S., Mochalski P., Koc H., Hinterhuber H., Amann A. J. Breath Res. 2012;6:016005. doi: 10.1088/1752-7155/6/1/016005. [DOI] [PubMed] [Google Scholar]
- 52.O’Hara M.E., Clutton-Brock T.H., Green S., Mayhew C.A. J. Breath Res. 2009;3:027005. doi: 10.1088/1752-7155/3/2/027005. [DOI] [PubMed] [Google Scholar]
- 53.Mills G.A., Walker V. J. Chromatogr. B. 2001;753:259–268. doi: 10.1016/s0378-4347(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 54.Stein R.A., Mead J.F. Chem. Phys. Lipids. 1988;46:117–120. doi: 10.1016/0009-3084(88)90121-1. [DOI] [PubMed] [Google Scholar]
- 55.Petrick L., Dubowski Y. Indoor Air. 2009;19:381–391. doi: 10.1111/j.1600-0668.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- 56.Righettoni M., Tricoli A., Gass S., Schmid A., Amann A., Pratsinis S.E. Anal. Chim. Acta. 2012;738:69–75. doi: 10.1016/j.aca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyooka T., Hiyama S., Yamada Y. J. Breath Res. 2013;7:036005. doi: 10.1088/1752-7155/7/3/036005. [DOI] [PubMed] [Google Scholar]
- 58.Vautz W., Slodzynski R., Hariharan C., Seifert L., Nolte J., Fobbe R., Sielemann S., Lao B.C., Huo R., Thomas C.L., Hildebrand L. Anal. Chem. 2013;85:2135–2142. doi: 10.1021/ac302752f. [DOI] [PubMed] [Google Scholar]
- 59.Crabb D.W., Matsumoto M., Chang D., You M. Proc. Nutr. Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 60.Dibble T.S. J. Phys. Chem. A. 1999;103:8559–8565. [Google Scholar]
- 61.Kushch I., Arendacka B., Stolc S., Mochalski P., Filipiak W., Schwarz K., Schwentner L., Schmid A., Dzien A., Lechleitner M., Witkovsky V., Miekisch W., Schubert J., Unterkofler K., Amann A. Clin. Chem. Lab. Med. 2008;46:1011–1018. doi: 10.1515/CCLM.2008.181. [DOI] [PubMed] [Google Scholar]
- 62.King J., Mochalski P., Unterkofler K., Teschl G., Klieber M., Stein M., Amann A., Baumann M. Biochem. Biophys. Res. Commun. 2012;423:526–530. doi: 10.1016/j.bbrc.2012.05.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turunen M., Olsson J., Dallner G. Biochim. Biophys. Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Yannai S. Chapman & Hall/CRC; Boca Raton: 2004. Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients; p. 1764. [Google Scholar]
- 65.Burnell J.C., Li T.K., Bosron W.F. Biochemistry. 1989;28:6810–6815. doi: 10.1021/bi00443a005. [DOI] [PubMed] [Google Scholar]
- 66.Borjesson T., Stollman U., Schnurer J. Appl. Environ. Microbiol. 1992;58:2599–2605. doi: 10.1128/aem.58.8.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krishnamurthy R.G., Smouse T.H., Mookherjee B.D., Reddy B.R., Chang S.S. J. Food Sci. 1967;32:372–374. [Google Scholar]
- 68.Syhre M., Scotter J.M., Chambers S.T. Med. Mycol. 2008;46:209–215. doi: 10.1080/13693780701753800. [DOI] [PubMed] [Google Scholar]
- 69.Labows J.N., McGinley K.J., Leyden J.J., Webster G.F. Appl. Environ. Microbiol. 1979;38:412–415. doi: 10.1128/aem.38.3.412-415.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sfara V., Zerba E.N., Alzogaray R.A. J. Med. Entomol. 2009;46:511–515. doi: 10.1603/033.046.0315. [DOI] [PubMed] [Google Scholar]
- 71.Tangerman A. J. Chromatogr. B. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 72.Gupta N., Porter T.D. J. Nutr. 2001;131:1662–1667. doi: 10.1093/jn/131.6.1662. [DOI] [PubMed] [Google Scholar]