Abstract
Submerged macrophyte communities are frequently subjected to disturbance of various frequency and strength. However, there is still little experimental evidence on how mechanical disturbance affects the performance and species composition of such plant communities. In a greenhouse experiment, we constructed wetland communities consisting of five co-occurring clonal submerged macrophyte species (Hydrilla verticillata, Elodea canadensis, Ceratophyllum demersum, Chara fragilis, and Myriophyllum spicatum) and subjected these communities to three mechanical disturbance regimes (no, moderate and strong disturbance). Strong mechanical disturbance greatly decreased overall biomass, number of shoot nodes and total shoot length, and increased species diversity (evenness) of the total community. It also substantially decreased the growth of the most abundant species (H. verticillata), but did not affect growth of the other four species. Our data reveal that strong disturbance can have different effects on different submerged macrophyte species and thus alters the performance and species composition of submerged macrophyte communities.
Disturbance is a widespread phenomenon in both terrestrial and aquatic ecosystems1,2,3,4. It is defined as environmental fluctuations (e.g., temperature fluctuation and precipitation variability)5, destructive events (e.g., fire, flooding, or storm)6 or any other events causing biomass removal, thus creating open space7,8. Mechanical disturbance, caused by abiotic factors such as tidal scouring, waves9,10, and severe storms and also by human and/or animal activities, has profound effects on both individual plants and plant communities11,12.
Aboveground mechanical disturbance can have significant impact on clonal plants because it may break clonal networks formed by stolons or rhizomes and thus cut off resource translocation between previously connected ramets13. It can also affect the regeneration and colonization of plants by producing fragments differing in size and viability14,15. Furthermore, mechanical disturbance can impact the distribution of clonal plants since shoot fragments produced by mechanical disturbance can be dispersed over long distance by, e.g., water16, wind and animals, which is likely to facilitate a quick spread of the population16.
Different plant species may vary in their responses to mechanical disturbance, because they differ in relevant traits such as stem mechanical properties, vegetative regeneration ability and rooting ability14. Some species that can adjust to mechanical disturbance survive in heavily disturbed environments but others cannot17,18,19. Because of such species-specific responses, mechanical disturbance may affect the performance of a community, here defined as the sum of the performance of all component species, expressed as growth parameters such as biomass, shoot length and node number. Moreover, it may affect the species composition, hence diversity, of plant communities. Numerous studies have tested the effects of mechanical disturbance on performance and species composition of terrestrial plant communities2,20,21,22, but experimental work is scarce on aquatic plant communities, especially submerged macrophyte communities.
Submerged macrophyte communities are one of the most important components of aquatic ecosystems and play an important role in, among others, water purification, inhibition of algae growth and buffering the stability of the ecosystem23,24,25. However, submerged macrophyte communities are frequently subjected to mechanical disturbance caused by strong current14, waterfowl trampling, fish and benthonic fauna perturbation, and transportation26 and fishing vessels27,28. Since most submerged macrophytes are clonal plants29,30, mechanical disturbance may affect their performance and such effects might also differ among different submerged macrophytes. Consequently, mechanical disturbance is very likely to have an impact on the performance and species composition of submerged macrophyte communities.
Effects of disturbance will depend on its frequency and/or strength22,31,32,33. Evidence shows that both high frequency and intensity of disturbance decreased community diversity34. In terrestrial ecosystem, species composition of plant communities is also influenced by the frequency of mechanical disturbance35. Collins et al. found that increasing clipping frequency led to an increase of species richness but a decrease of vegetation abundance22. In aquatic ecosystem, if physical damage caused, e.g., by waterfowl trampling happens less frequently or is not severe, submerged macrophytes may hardly be fragmented and thus the performance and species composition of submerged macrophyte communities may not change36. In contrast, if physical damage happens frequently or strongly, then macrophytes will be broken into small fragments and the performance and species composition of communities may change greatly36.
In a greenhouse experiment, we constructed wetland communities consisting of five co-occurring submerged macrophytes and subjected the communities to three mechanical disturbance regimes (no disturbance, moderate disturbance and strong disturbance). Specifically, we addressed the following questions: 1) Do mechanical disturbance and its frequency affect the performance, measured as biomass, node number and shoot length, and species diversity of submerged macrophyte communities? 2) Can the changes at the community level be explained by the responses of the individual species?
Results
Effects of mechanical disturbance on community performance and species composition
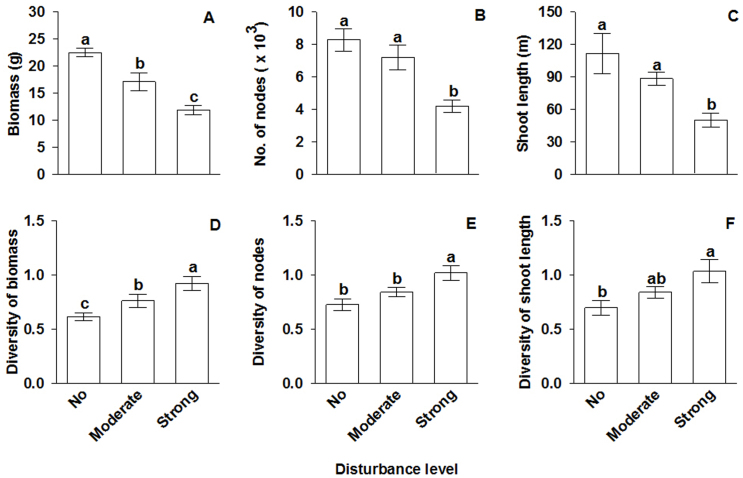
Plant performance here is expressed as three growth parameters: biomass, node number and shoot length. Biomass, node number and shoot length of the submerged macrophyte communities were all significantly greater in the control (no disturbance) than in the strong disturbance treatment, but number of nodes or shoot length did not differ between the control and the moderate disturbance treatment (Table 1, Figure 1 A–C). Shannon-Wiener diversity indices of biomass, number of nodes and shoot length were all significantly smaller in the control than in the strong disturbance treatment (Table 1, Figure 1 D–F). The diversity index of biomass was also significantly smaller in the control than in the moderate disturbance treatment, but the diversity indices of neither number of nodes nor shoot length differed (Table 1, Figure 1 D–F).
Table 1. ANOVA results for the effects of block and mechanical disturbance on the growth and diversity indices of the submerged macrophyte communities.
| Variable | Block | Disturbance | ||
|---|---|---|---|---|
| F5,15 | P | F2,15 | P | |
| Biomass | 4.10 | 0.028 | 42.49 | <0.001 |
| No. of nodes | 3.35 | 0.049 | 20.09 | <0.001 |
| Shoot length | 2.62 | 0.091 | 10.77 | 0.003 |
| Diversity based on biomass | 2.51 | 0.101 | 11.15 | 0.003 |
| Diversity based on node number | 1.84 | 0.192 | 8.76 | 0.006 |
| Diversity based on shoot length | 2.09 | 0.150 | 6.16 | 0.018 |
Figure 1. Growth (A–C) and Shannon-Wiener diversity indices (D–E) of the submerged macrophyte communities under the three mechanical disturbance treatments.
Bars and vertical lines are means (±1 SE). Bars sharing the same letters are not significantly different at P = 0.05.
Effects of mechanical disturbance on growth of individual species
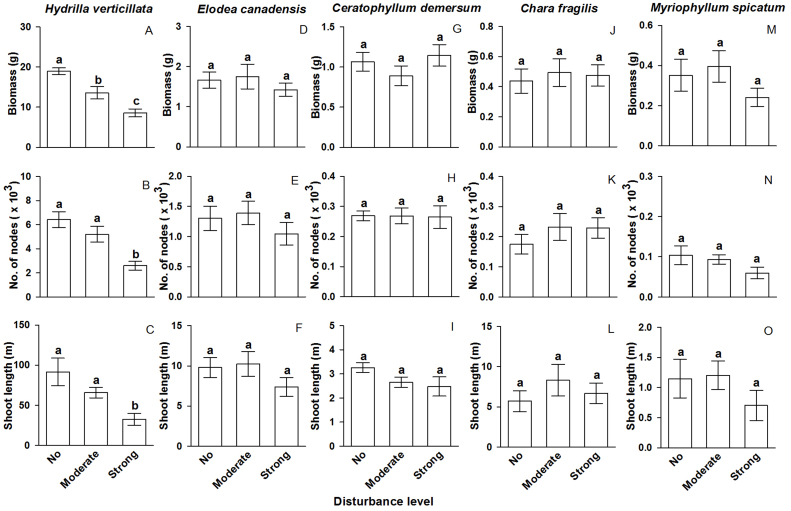
The five submerged macrophyte species in the communities responded differently to the disturbance treatments (Table 2, Figure 2). Responses of H. verticillata were similar to those of the total community: all three growth parameters of H. verticillata had significantly greater values in the control than in the strong disturbance treatment, but only biomass differed significantly between the control and the moderate disturbance treatment (Table 2, Figure 2 A–C). In contrast, increasing disturbance strength did not significantly affect biomass, number of nodes or shoot length of the other four macrophytes (Table 2, Figure 2 D–O).
Table 2. ANOVA results for the effects of block and mechanical disturbance on the growth of each of the five submerged macrophytes.
| Species | Variable | Block | Disturbance | ||
|---|---|---|---|---|---|
| F5,15 | P | F2, 15 | P | ||
| (A) H. verticillata | Biomass | 4.07 | 0.028 | 40.21 | <0.001 |
| No. of nodes | 4.28 | 0.024 | 24.55 | <0.001 | |
| Shoot length | 3.46 | 0.045 | 12.14 | 0.002 | |
| (B) E. canadensis | Biomass | 0.41 | 0.831 | 0.43 | 0.660 |
| No. of nodes | 0.17 | 0.969 | 0.61 | 0.565 | |
| Shoot length | 0.11 | 0.989 | 0.94 | 0.423 | |
| (C) C. demersum | Biomass | 3.20 | 0.055 | 1.87 | 0.204 |
| No. of nodes | 0.59 | 0.709 | 0.01 | 0.994 | |
| Shoot length | 3.14 | 0.058 | 3.50 | 0.071 | |
| (D) C. fragilis | Biomass | 3.68 | 0.038 | 0.24 | 0.794 |
| No. of nodes | 3.37 | 0.048 | 1.32 | 0.309 | |
| Shoot length | 2.62 | 0.091 | 1.13 | 0.362 | |
| (E) M. spicatum | Biomass | 1.31 | 0.334 | 1.45 | 0.280 |
| No. of nodes | 0.35 | 0.873 | 1.42 | 0.286 | |
| Shoot length | 1.02 | 0.454 | 1.03 | 0.393 | |
Figure 2. Growth of each of the five submerged macrophytes under the three mechanical disturbance treatments.
Bars and vertical lines are means and SE. Bars sharing the same letters are not significantly different at P = 0.05.
Discussion
Our results suggest that strong mechanical disturbance could dramatically alter the performance and species composition of submerged macrophyte communities, whereas moderate mechanical disturbance may not. The change in performance and species composition of the communities by strong mechanical disturbance can be explained by the fact that the five submerged macrophyte species responded differently to strong disturbance. Strong disturbance reduced the abundance of the most abundant species H. verticillata in the present experiment, but did not affect the abundance of the second most abundant species E. canadensis or the other three, less abundant species (C. demersum, C. fragilis and M. spicatum). Furthermore, strong mechanical disturbance may have caused great physical injury to the submerged macrophytes, which decreased the performance of the whole community37.
Changes in the performance of abundant species usually affect the performance and species composition of plant communities19. Smith and Knapp19 found that the total aboveground net primary productivity of grassland communities decreased due to the reduction in the abundance of the three dominant species Andropogon gerardii, A. scoparius and Sorghastrum nutans. At harvest in the present experiment, the most abundant species H. verticillata produced four to ten times more biomass, nodes and shoot length than the second most abundant species E. canadensis and about two to four times more than the sum of E. canadensis and the other three less abundant species (C. demersum, C. fragilis and M. spicatum) under undisturbed conditions. Consequently, H. verticillata was the superior competitor in the submerged macrophyte communities. The rapid growth and dominance of H. verticillata is likely related to its low light requirement for photosynthesis38,39,40 and its high adaptation to both low and high nutrients in water41. However, superior competitor species are in general more susceptible to disturbance, and thus subjected to stronger negative effects by disturbance than inferior competitors42. In the present study, strong disturbance decreased biomass, node number and shoot length of H. verticillata by 55–60%, which in turn had strong effects on the species composition of the community.
Previous studies showed that fragmentation into small pieces caused by mechanical disturbance can affect the growth of wetland plants36,43,44,45. In the present study, strong mechanical disturbance greatly decreased the growth of the submerged macrophyte communities. Strong mechanical disturbance repeatedly broke integrated clonal fragments of the submerged macrophytes into smaller fragments. As small fragments usually have a lower regeneration and growth capacity compared with larger fragments43,46, strong mechanical disturbance may have impeded the regeneration and subsequent growth of the plants47. Moreover, the submerged macrophyte communities that were subjected to repeated damage caused by strong mechanical disturbance had much less time to recover compared to those under moderate, less frequent mechanical disturbance. In addition, the interval of the two physical damages in strong mechanical disturbance was 20 days, which might be too short for the fragments caused by the first mechanical disturbance to regenerate and regrow. The lack of response of the subordinate species also contributed to the alteration of the species composition of the submerged macrophyte communities48. Compared to H. verticillata, the other four species were weaker competitors, and thus they may grow slowly as a result of insufficient light availability under the shade of H. verticillata, even after its dominance was significantly decreased under strong mechanical disturbance. Repeated fragmentation and physical injury caused by strong mechanical disturbance may also have negatively affected the regeneration and growth of the less abundant species50. However, the fast-growing species H. verticillata had produced larger integrated clonal units that were more likely to be hit by the disturbance action in the present experiment, and thus suffered more from mechanical disturbance. As a result, the growth of none of the four less abundant species differed significantly among the three mechanical disturbance treatments. Because strong mechanical disturbance can reduce the dominance of the most abundant species and may not have equal negative effects on the subordinate species42, it inevitably increases the evenness of species abundance of the submerged macrophyte communities.
The moderate mechanical disturbance treatment used in this study had little effect on the performance and species composition of the submerged macrophyte communities, because it hardly affected the growth parameters of the five submerged macrophytes (except for biomass of H. verticillata). One possible reason for this is that the fragmentation disturbance in the moderate mechanical disturbance treatment occurred at the early establishing stage of the communities, at which time plants were relatively small and thus less affected. The results suggest that moderate mechanical disturbance such as one single fragmentation event may not have significant effects on submerged macrophyte communities, and that only stronger disturbance events matter31,51. They also suggest that the effects may strongly depend on the timing of disturbance, i.e., early disturbance when the shoot fragments are still small may have a smaller effect than late disturbance when the shoot fragments are large. As a result, much more biomass loss could be caused by late mechanical damage.
In order to know to what extent the effects were caused by changed growth of individual species or changed competitive interactions, one would need to study monocultures consisting of a single macrophyte species. However, such single-species communities were not included in our study and we thus cannot fully separate the effects of mechanical disturbance on individual species growth from those on the interactions among species. If mechanical disturbance can change the interactions among species, then even without affecting the overall performance, disturbance still can affect species composition and diversity of submerged macrophyte communities.
We conclude that strong mechanical disturbance can have different effects on submerged clonal macrophyte species, thereby altering the performance and species composition of submerged macrophyte communities. Further studies should also include communities of individual species so that the contribution of alteration of species interaction by mechanical disturbance to the effects on the performance and species composition of submerged macrophyte communities can be quantified.
Methods
Experimental communities and plant sampling
The experimental communities were constructed with five co-occurring submerged macrophytes widely distributed in China, i.e., Hydrilla verticillata (L.f.) Royle (Hydrocharitaceae), Elodea canadensis Michx. (Hydrocharitaceae), Ceratophyllum demersum L. (Ceratophyllaceae), Chara fragilis Desv (Characeae) and Myriophyllum spicatum L. (Haloragidaceae)51,52. All five species are perennial and capable of clonal growth53. The shoots of these species are easily broken into fragments of different sizes when they are subjected to disturbance such as grazing, strong current, floods and transportation vessels16,54,55. The species are common and easily collected in lakes in Beijing, which also contributed to their selection for this study.
In June 2011, plants of E. canadensis, C. fragilis and M. spicatum were collected in the lakes of the Winter Palace in Beijing, and those of H. verticillata and C. demersum in the lakes of Beijing Olympic Forestry Park. Plants of each species were collected in at least two lakes. We did not know the genetic make-up of these plants, but they might represent different genotypes. All five species were vegetatively propagated in a greenhouse at Forestry Science Co, Ltd., of Beijing Forestry University for a month to minimize the potential effect of local environments. H. verticillata grew much faster than the other four species when they were cultured in the greenhouse (Q. Zhang, personal observation).
On 15 July 2011, 92 shoots of each species were selected, and each shoot was cut to 13 cm long and had an apical bud but no lateral branches to minimize variation of the size of initial shoot materials. Of the 92 shoot fragments (cuttings) of each species, 20 were randomly selected and used for initial biomass measurements, and the other 72 were used for the experiment described below.
Experimental design
The experiment was set up in a randomized-block design with six blocks (six independent basins each containing one replicate for each of the three treatments) × three disturbance treatments (no disturbance as a control, moderate disturbance and strong disturbance). We assembled 18 submerged plant communities in the six basins made of non-transparent PVC panels. Each basin had ten compartments arranged in a line, and each compartment was 40 cm long × 40 cm wide × 80 cm deep. Within each basin, any two adjacent compartments shared a wall in which there were two narrow openings (each 30 cm long and 2 cm wide) covered by plastic filter screen (1 mm mesh). Two water faucets were installed 25 cm above the bottom of each compartment so that water in the compartments could drain and be replenished. The movement of water between the compartments ensured similar water quality in the ten compartments of each basin. At the same time, the plastic mesh prevented the submerged macrophytes in a compartment from growing into the adjacent compartments. Each compartment was first filled with 18-cm-deep yellow loam as rooting substrate covered by a 2-cm-deep layer of river sand to reduce turbidity.
In each basin, three consecutive compartments were used in this experiment, and in total 18 compartments were used. Before planting, the compartments were filled with water to a depth of 20 cm above the soil surface. On 16 July 2011, four shoot fragments of each of the five species were randomly planted in 20 fixed positions arranged in four rows and five columns in each compartment. The shoot fragments were planted following a Latin square approach, i.e. the four shoot fragments of each species were distributed in the four rows, with one shoot in each row and never 2 fragments of the same species in the same column. In this study, all shoot fragments were planted into the sediment; even though C. demersum does typically not root in nature, they do anchor into the sediment56. Immediately after planting, the compartments were filled with tap water to a depth of 55 cm above the soil surface.
After 15 days of recovery, all shoot fragments grew well and developed new leaves and elongating stems, and most of them produced new side branches. The disturbance treatments were then conducted for the first time. Three mechanical disturbance treatments were applied to mimic the physical damage caused by, e.g., water flow, waterfowl trampling, transportation and fishing vessels. The three treatments were no disturbance (as a control), moderate disturbance and strong disturbance, and only aboveground disturbance was applied. In the moderate and strong disturbance treatments, we used a plastic rake (25 cm long and 37.5 cm wide) to slash the experimental communities four times from each side of the square compartment. The mechanical disturbance treatment created approximately 16 new fragments in total in each community subjected to disturbance. As all species had very high growth rates in the greenhouse during summer, in the strong disturbance treatment, equally strong disturbance was applied again after 20 days when the shoots covered the water surface of the entire container. Therefore, the moderate and strong disturbance treatments differed in the frequency of slashing, with the strong disturbance mimicking repeated disturbance. We did not remove any plant fragments from the compartment, and thus the final biomass was the total biomass in the whole compartment.
The experiment lasted for two months, until 17 September 2011. During the experiment, tap water (4.84 ± 0.20 [mean ± SE] mg L−1 total N, 0.01 ± 0.01 mg L−1 total P, 9.70 ± 0.15 mg L−1 total organic C, and 63.54 ± 0.56 mg L−1 total inorganic C) was added every day to the compartments so that the water level was kept constant at 55 cm above the sediment. Water in each compartment was also partly replaced every two weeks to maintain its quality. To reduce damage to plants, only 50%–60% of the water in each compartment was replenished at each time. The temperature in the greenhouse was 25.5 ± 0.3°C (mean ± SE; measured hourly by two Hygrochron temperature/humidity loggers, iButton DS1923; Maxim Integrated Products, USA). Photosynthetic photon flux density at water surface at noon was 247.5–519.9 μmol m−2 s−1 (LI-250A quantum sensor; LI-COR Biosciences, USA), and the photoperiod was 11–13 h.
Harvest and measurements
On 17 September 2011, all the surviving submerged macrophytes (including roots) were carefully harvested and sorted into species. Number of shoot nodes is a measure of potential clonal growth, because every single node can potentially develop into a new plant, and total shoot length is a measure of local vegetative spread. These two measures were determined during the harvest. As the plants were easily broken into numerous shoot fragments during harvest, it was impossible to count node number and measure shoot length for all shoot fragments. We randomly selected five shoot fragments of each species from each experimental community to count the number of nodes and measure the shoot length. Biomass of these samples was measured separately. We then measured biomass of the remaining parts of each species in each community. For biomass measurements, the plants were oven-dried at 70°C for 72 h and weighed.
Data analyses
Based on the data of the five fragments, we calculated the number of shoot nodes per unit biomass and shoot length per unit biomass for each species in each community. Then we derived total number of shoot nodes for each species in each community (i.e., in each compartment) by multiplying biomass with number of nodes per unit biomass. Similarly, we derived total shoot length for each species in each community/compartment. Biomass, number of nodes and total shoot length of the five species in a compartment were pooled as measures of the whole community in that compartment. We also calculated the Shannon-Wiener diversity index based on biomass, total number of nodes and total shoot length of the five macrophytes for each community to determine the structure alteration of the communities57,58. The Shannon-Wiener diversity index (H) was calculated as: H = −ΣPi ln (Pi) (i = 1, 2… S), where S is number of the macrophyte species and Pi is the growth parameter (biomass, number of nodes or shoot length) of species i divided by the sum of the growth measure of all the five species in the community49. Because species number did not change, an increase in diversity indices suggests that the five species are distributed more evenly in the community and a decrease indicates that they are distributed less evenly.
We performed one-way ANOVAs to examine the effects of disturbance on overall growth parameters (total biomass, number of nodes and shoot length) and Shannon-Wiener diversity indices of the macrophyte communities, as well as on the three growth parameters for each of the five species individually. Basin was added as a block effect to the model to take account for potential differences between basins. Post hoc comparisons were done using Student-Newman-Keuls tests. All analyses were conducted with SPSS 16.0 software (SPSS, Chicago, IL, USA).
Author Contributions
Q.Z., L.H., M.-X.Z. and F.-H.Y. designed the experiment, Q.Z., W.X., G.-Q.S. and Y.-S.X. performed the experiment, Q.Z. wrote the first draft of the manuscript, L.H. and W.X. did the statistical analysis, M.-X.Z. and F.-H.Y. contributed substantially to the revisions.
Acknowledgments
This research is supported by the Fundamental Research Funds for the Central Universities (TD-JC-2013-1), the Program for New Century Excellent Talents in University (NECT-10-0234). We thank Jian Zhou and Rui-Hua Liu for help with sampling and harvest, Dr. Heidrun Huber for valuable comments and Dr. Eric J.W. Visser for careful language editing.
References
- Fraterrigo J. M. & Rusak J. A. Disturbance-driven changes in the variability of ecological patterns and processes. Ecol. Lett. 11, 756–770 (2008). [DOI] [PubMed] [Google Scholar]
- Cornaglia P. S., Schrauf G. E. & Deregibus V. A. Flooding and grazing promote germination and seedling establishment in the perennial grass Paspalum dilatatum. Austral Ecol. 34, 343–350 (2009). [Google Scholar]
- Lake P. Disturbance, patchiness, and diversity in streams. J. N. Am. Benthol. Soc. 19, 573–592 (2000). [Google Scholar]
- Romme W. H. et al. Twenty years after the 1988 Yellowstone fires: lessons about disturbance and ecosystems. Ecosystems 14, 1196–1215 (2011). [Google Scholar]
- White P. S. Pattern, process, and natural disturbance in vegetation. Bot. Rev. 45, 229–299 (1979). [Google Scholar]
- White P. & Pickett S. The ecology of natural disturbance and patch dynamics. 3–13 (Academic press, 1985). [Google Scholar]
- Sousa W. P. The role of disturbance in natural communities. Ann. Rev. Ecol. Syst. 15, 353–391 (1984). [Google Scholar]
- Grime J. P. Plant strategies, vegetation processes, and ecosystem properties (2nd edition) (John Wiley & Sons, 2006). [Google Scholar]
- Alongi D. & Christoffersen P. Benthic infauna and organism-sediment relations in a shallow, tropical coastal area: Influence of outwelled mangrove detritus and physical disturbance. Mar. Ecol. Prog. Ser. 81, 229–245 (1992). [Google Scholar]
- Gaylord B. Detailing agents of physical disturbance: wave-induced velocities and accelerations on a rocky shore. J. Exp. Mar. Biol. Ecol. 239, 85–124 (1999). [Google Scholar]
- Kennelly S. Physical disturbances in an Australian kelp. Mar. Ecol. Prog. Ser. 40, 145–153 (1987). [Google Scholar]
- Dong B. C. et al. Effects of fragmentation on the survival and growth of the invasive, clonal plant Alternanthera philoxeroides. Biol. Invasions, 14, 1101–1110 (2012). [Google Scholar]
- Cline M. G. Apical dominance. Bot. Rev. 57, 318–358 (1991). [Google Scholar]
- Barrat-Segretain M. H., Bornette G. & Hering-Vilas-Bôas A. Comparative abilities of vegetative regeneration among aquatic plants growing in disturbed habitats. Aquat. Bot. 60, 201–211 (1998). [Google Scholar]
- Barrat-Segretain M. H., Henry C. P. & Bornette G. Regeneration and colonization of aquatic plant fragments in relation to the disturbance frequency of their habitats. Arch. Hydrobiol. 145, 111–127 (1999). [Google Scholar]
- Barrat-Segretain M. H. & Bornette G. Regeneration and colonization abilities of aquatic plant fragments: effect of disturbance seasonality. Hydrobiologia 421, 31–39 (2000). [Google Scholar]
- Wilson S. D. & Keddy P. A. Species competitive ability and position along a natural stress/disturbance gradient. Ecology 67, 1236–1242 (1986). [Google Scholar]
- Lavorel S., McIntyre S. & Grigulis K. Plant response to disturbance in a Mediterranean grassland: How many functional groups? J. Veg. Sci. 10, 661–672 (1999). [Google Scholar]
- Smith M. D. & Knapp A. K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 6, 509–517 (2003). [Google Scholar]
- Moore J. E. & Franklin S. Understanding the relative roles of disturbance and species interactions in shaping Mississippi River island plant communities. Community Ecol. 12, 108–116 (2011). [Google Scholar]
- Bickford W. A. et al. Canopy disturbance alters competitive outcomes between two brackish marsh plant species. Aquat. Bot. 103, 23–29 (2012). [Google Scholar]
- Collins B., Wein G. & Philippi T. Effects of disturbance intensity and frequency on early old-field succession. J. Veg. Sci. 12, 721–728 (2001). [Google Scholar]
- Biswas S. R. & Mallik A. U. Disturbance effects on species diversity and functional diversity in riparian and upland plant communities. Ecology 91, 28–35 (2010). [DOI] [PubMed] [Google Scholar]
- Gasith A. & Hoyer M. V. Structuring role of macrophytes in lakes: changing influence along lake size and depth gradients. In: Jeppesen E., Sondergaard M., Sondergaard M. and Christoffersen K. (eds),. The structuring role of submerged macrophytes in lakes 131, 381–392 (Springer New York, 1998). [Google Scholar]
- van Donk E. & van de Bund W. J. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquat. Bot. 72, 261–274 (2002). [Google Scholar]
- Murphy K. & Eaton J. W. Effects of pleasure-boat traffic on macrophyte growth in canals. J. Appl. Ecol. 20, 713–729 (1983). [Google Scholar]
- Barrat-Segretain M. & Amoros C. Influence of flood timing on the recovery of macrophytes in a former river channel. Hydrobiologia 316, 91–101 (1995). [Google Scholar]
- Smith C. S. & Barko J. Ecology of Eurasian watermilfoil. J. Aquat. Plant Manage. 28, 55–64 (1990). [Google Scholar]
- Howard R. J. & Rafferty P. S. Clonal variation in response to salinity and flooding stress in four marsh macrophytes of the northern gulf of Mexico, USA. Environ. Exp. Bot. 56, 301–313 (2006). [Google Scholar]
- Riis T. & Biggs B. J. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol. Oceanogr. 48, 1488–1497 (2003). [Google Scholar]
- Miller A. D., Roxburgh S. H. & Shea K. How frequency and intensity shape diversity-disturbance relationships. PNAS 108, 5643–5648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe D. J. & Gotelli N. J. Effects of disturbance frequency, intensity, and area on assemblages of stream macroinvertebrates. Oecologia 124, 270–279 (2000). [DOI] [PubMed] [Google Scholar]
- Hobbs R. J. & Huenneke L. F. Disturbance, diversity, and invasion: implications for conservation. Conserv. Biol. 6, 324–337 (1992). [Google Scholar]
- Haddad N. M. et al. Species' traits predict the effects of disturbance and productivity on diversity. Ecol. Lett. 11, 348–356 (2008). [DOI] [PubMed] [Google Scholar]
- Armesto J. & Pickett S. Experiments on disturbance in old-field plant communities: impact on species richness and abundance. Ecology 66, 230–240 (1985). [Google Scholar]
- Kim G.-Y. et al. Impact of over-wintering waterfowl on tuberous bulrush (Bolboschoenus planiculmis) in tidal flats. Aquat. Bot. 107, 17–22 (2013). [Google Scholar]
- Belsky A. J. et al. Overcompensation by plants: herbivore optimization or red herring? Evol. Ecol. 7, 109–121 (1993). [Google Scholar]
- Haller W. T. & Sutton D. L. Community structure and competition between Hydrilla and Vallisneria. Hyacinth Contr. J. 13, 48–50 (1975). [Google Scholar]
- Bowes G. et al. Adaptation to low light levels by Hydrilla. J. Aquat. Plant Manage. 15, 32–35 (1977). [Google Scholar]
- Van T. K., Haller W. T. & Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 58, 761–768 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C. D. K. & Luond R. A revision of the genus Hydrocharis (Hydrocharitaceae). Aquat. Bot. 14, 177–204 (1982). [Google Scholar]
- Collins S. L., Glenn S. M. & Gibson D. J. Experimental analysis of intermediate disturbance and initial floristic composition: decoupling cause and effect. Ecology 76, 486–492 (1995). [Google Scholar]
- Combroux I. & Bornette G. Propagule banks and regenerative strategies of aquatic plants. J. Veg. Sci. 15, 13–20 (2004). [Google Scholar]
- Xie D. & Yu D. Size-related auto-fragment production and carbohydrate storage in auto-fragment of Myriophyllum spicatum L. in response to sediment nutrient and plant density. Hydrobiologia 658, 221–231 (2011). [Google Scholar]
- Dong B. C. et al. How internode length, position and presence of leaves affect survival and growth of Alternanthera philoxeroides after fragmentation? Evol. Ecol. 24, 1447–1461 (2010). [Google Scholar]
- Lin H.-F., Alpert P. & Yu F.-H. Effects of fragment size and water depth on performance of stem fragments of the invasive, amphibious, clonal plant Ipomoea aquatica. Aquat. Bot. 99, 34–40 (2012). [Google Scholar]
- Riis T., Madsen T. V. & Sennels R. S. H. Regeneration, colonisation and growth rates of allofragments in four common stream plants. Aquat. Bot. 90, 209–212 (2009). [Google Scholar]
- Cao Y., Williams D. D. & Williams N. E. How important are rare species in aquatic community ecology and bioassessment? Limnol. Oceanogr. 43, 1403–1409 (1998). [Google Scholar]
- Kent M. & Coker P. Vegetation description and analysis: a practical approach (John Wiley & Sons, 1992). [Google Scholar]
- Huston M. A. Biological diversity: the coexistence of species on changing landscapes (Cambridge University Press, 1994). [Google Scholar]
- Wu Z. Y., Peter H. R. & Hong D. Y. E. Flora of China (English edition) (Science Press, 1994). [Google Scholar]
- Cronk J. K. & Fennessy M. S. Wetland plants: biology and ecology (CRC, 2001). [Google Scholar]
- Nichols S. A. & Shaw B. H. Ecological life histories of the three aquatic nuisance plants, Myriophyllum spicatum, Potamogeton crispus and Elodea canadensis. Hydrobiologia 131, 3–21 (1986). [Google Scholar]
- Madsen J. D. & Smith D. H. Vegetative spread of Eurasian watermilfoil colonies. J. Aquat. Plant. Manage. 35, 63–68 (1997). [Google Scholar]
- Madsen J. D., Eichler L. & Boylen C. Vegetative spread of Eurasian watermilfoil in Lake George, New York. J. Aquat. Plant. Manage. 26, 47–50 (1988). [Google Scholar]
- Keskinkan O. et al. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresource Technol. 92, 197–200 (2004). [DOI] [PubMed] [Google Scholar]
- Yu F.-H. et al. Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. Am. J. Bot. 96, 1983–1989 (2009). [DOI] [PubMed] [Google Scholar]
- Hill M. O. Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427–432 (1973). [Google Scholar]