Abstract
A novel transcriptional proofreading mechanism associated with the beta-subunit of wild-type RNA polymerase from Escherichia coli is suggested from the following data. The purified holoenzyme contains an NTPase activity which specifically converts noncognate NTPs to their corresponding NDP in a template-dependent manner during in vitro transcription of synthetic single- and double-stranded templates. In contrast, purified enzyme from an rpoB mutant which shows increased transcriptional error lacked template-dependent NTP hydrolytic activity. The NTP hydrolytic activity of wild-type enzyme was critically dependent on the integrity of the initiation complex, and required continued transcriptional elongation. Transcription and translation of the lacZ gene proceeded 17% faster in the mutant than in its wild-type parent. These results are discussed in terms of a proofreading model in which the rate of transcription is limited by proofreading events that involve recognition and hydrolysis of noncognate NTPs before they can be misincorporated into RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank A., Gallant J. A., Burgess R. R., Loeb L. A. An RNA polymerase mutant with reduced accuracy of chain elongation. Biochemistry. 1986 Oct 7;25(20):5920–5928. doi: 10.1021/bi00368a013. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
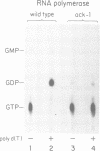
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Erlich H., Gallant J. Synthesis and turnover of ribosomal ribonucleic acid in guanine-starved cells of Escherichia coli. J Biol Chem. 1975 Apr 25;250(8):3057–3061. [PubMed] [Google Scholar]
- Gallant J., Erlich H., Weiss R., Palmer L., Nyari L. Nonsense suppression in aminoacyl-t-RNA limited cells. Mol Gen Genet. 1982;186(2):221–227. doi: 10.1007/BF00331853. [DOI] [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Greenblatt J., McLimont M., Hanly S. Termination of transcription by nusA gene protein of Escherichia coli. Nature. 1981 Jul 16;292(5820):215–220. doi: 10.1038/292215a0. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kent R. B., Guterman S. K. Pyrophosphate inhibition of rho ATPase: a mechanism of coupling to RNA polymerase activity. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3992–3996. doi: 10.1073/pnas.79.13.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- McClure W. R. On the mechanism of streptolydigin inhibition of Escherichia coli RNA polymerase. J Biol Chem. 1980 Feb 25;255(4):1610–1616. [PubMed] [Google Scholar]
- Ninio J., Bernardi F., Brun G., Assairi L., Lauber M., Chapeville F. On the mechanism of nucleotide incorporation into DNA and RNA. FEBS Lett. 1975 Sep 15;57(2):139–144. doi: 10.1016/0014-5793(75)80702-2. [DOI] [PubMed] [Google Scholar]
- Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57(5):587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Rosenberger R. F., Hilton J. The frequency of transcriptional and translational errors at nonsense codons in the lacZ gene of Escherichia coli. Mol Gen Genet. 1983;191(2):207–212. doi: 10.1007/BF00334815. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Kröger M. Participation of modified nucleosides in translation and transcription. Prog Nucleic Acid Res Mol Biol. 1979;23:151–194. doi: 10.1016/s0079-6603(08)60133-6. [DOI] [PubMed] [Google Scholar]
- Sisk W. P., Chirikjian J. G., Lautenberger J., Jorcyk C., Papas T. S., Berman M. L., Zagursky R., Court D. L. A plasmid vector for cloning and expression of gene segments: expression of an HTLV-I envelope gene segment. Gene. 1986;48(2-3):183–193. doi: 10.1016/0378-1119(86)90076-4. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Loeb L. A. On the fidelity of transcription by Escherichia coli ribonucleic acid polymerase. J Mol Biol. 1975 Oct 5;97(4):577–591. doi: 10.1016/s0022-2836(75)80060-x. [DOI] [PubMed] [Google Scholar]
- Strniste G. F., Smith D. A., Hayes F. N. X-ray inactivation of the Escherichia coli deoxyribonucleic acid dependent ribonucleic acid polymerase in aqueous solution. II. Studies on initiation and fidelity of transcription. Biochemistry. 1973 Feb;12(4):603–608. doi: 10.1021/bi00728a006. [DOI] [PubMed] [Google Scholar]
- Volloch V. Z., Rits S., Tumerman L. A possible mechanism responsible for the correction of transcription errors. Nucleic Acids Res. 1979 Apr;6(4):1535–1546. doi: 10.1093/nar/6.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]