Abstract
Changes in follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin, immunoreactive(ir)-inhibin, testosterone, estradiol-17β, and insulin-like growth factor (IGF)-I in Thoroughbred stallions along with changes in prolactin secretion in geldings were studied. The correlations of day-length with changes in the concentrations of these hormones were also studied. Five stallions and thirteen geldings were employed to draw blood samples in monthly basis and radioimmunoassay was performed to measure these hormones. All hormones showed a seasonal pattern, the levels being highest during the breeding season and lowest during the winter months. Most of the hormones were at their highest concentration during the month of April, the mid of spring in northern hemisphere. The concentration of circulating IGF-I also demonstrated seasonality, the peak lying on the month of April. The plasma concentration of prolactin also increased during the breeding season. This phenomenon was similar both in stallions and geldings although geldings had lower concentration than that of stallions. The changes in concentration of prolactin in stallions and geldings correlated more towards the day-length than towards the temperature. These results clearly indicate the seasonality of pituitary and gonadal hormones of Thoroughbred stallions, the activity being highest during the month of April and May of the breeding season.
Keywords: IGF-I, prolactin, seasonality, Thoroughbred stallion, gonadotropins
A functional reproductive status of stallion requires a complex co-ordination of endocrine, paracrine and autocrine signals. Hypothalamo-pituitary-testicular (HPT) axis and pineal gland is responsible for maintaining the long day seasonal breeding activities in stallions [11]. Although several hormones have been investigated in this mechanism, some of them have not been fully understood. The contrasting pattern of prolactin secretion in short day and long day breeders have not been fully clarified [15]. The action of growth hormones on tissues is mediated partly by insulin-like growth factor-I (IGF-I) which is secreted primarily from the liver and other non-hepatic tissues [5] to act in an endocrine-autocrine-paracrine fashion [14]. The measurement of IGF-I gives the clue of the status of the somatotropic axis as it has longer half-life with no obvious diurnal rhythm [3]. IGF-I has been demonstrated in different tissues of equine male fetus and adult [8] and placenta [1] as well. Localization of IGF-I and its receptor on equine testes at different stages of maturity has recently been demonstrated [19]. From reproductive point of view, IGF-I appears to be more important [2]. But, the plasma levels of IGF-I in stallions have not been studied for long term. The concentrations of prolactin in pituitary and serum have been studied in stallions at summer and winter [18] which showed higher values during summer. But literatures regarding long term studies on concentration of prolactin in stallions is lacking. The primary objective of this study is to characterize the pattern of plasma concentration of reproductive hormones along with IGF-I over two years in stallion and study the effect of day-length and temperature on hormone secretion. The secondary objective of this study was to compare between stallion and gelding from the perspectives of prolactin as geldings differ from the stallions in that they don’t have testicles to exert any kind of endocrine effect.
Materials and Methods
Animals
Five Thoroughbred stallions (6–8 years old) in Hayakita (42°45' N, 141°49' E), Hokkaido, Japan kept under natural condition were used for the analysis of changes in circulating follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin, immunoreactive (ir)-inhibin, testosterone, estradiol-17β, and insulin-like growth factor (IGF)-I. These stallions were engaged in breeding during March to June. Thirteen thoroughbred geldings kept in the horse farm of Tokyo University of Agriculture and Technology, Fuchu, Tokyo (35°41'N and 139°28'E) were used to measure the plasma concentration of prolactin. Monthly blood samples were taken from stallions and geldings for two and one year respectively. Blood samples were collected from Jugular vein into heparinized vacutainer. Plasma were harvested and stored at –20°C until assayed.
Radioimmunoassay of FSH, LH, ir-inhibin, testosterone, estradiol-17β, prolactin and IGF-I
Plasma concentration of FSH and LH were determined by homologous double-antibody equine RIA methods as described previously [10]. Intra- and inter-assay coefficients of variance were 4.9% and 12.2% for FSH and 12.6% and 15.1% for LH, respectively. Concentrations of testosterone and estradiol-17β were determined by double-antibody RIA systems using 125I-labeled radioligands as previously described [17]. Anti-sera against testosterone (GDN 250) and estradiol-17β (GDN 244) were used. The intra- and inter-assay coefficients of variance were 6.3% and 7.2% for testosterone and 6.7% and 17.8% for estradiol-17β, respectively. Plasma ir-inhibin concentrations were measured using a rabbit antiserum against purified bovine inhibin (TNDH 1) and 125I-labeled 32-kDa bovine inhibin, as previously described [6]. The results were expressed in terms of 32-kDa bovine inhibin. The intra- and inter-assay coefficients of variance were 8.0% and 16.2%, respectively. Plasma concentration of prolactin was measured by RIA using rat anti-sera against equine prolactin (AFP-261987) and purified 125I-labeled prolactin and reference standard (AFP-8794B) (provided by Dr. A.F. Parlow). The intra- and inter-assay coefficients of variance were 7.1% and 9.8% respectively. IGF-I was measured by RIA as previously described [5] using anti-sera against human IGF-I raised in rabbit (AFP 4892898). The intra- and inter-assay coefficients of variance were 2.7% and 14.8% respectively.
Annual temperature and day-length changes
Secondary data on daily temperature (°C), and daylight length (hr) at Hayakita, Japan was obtained from Japanese Meteorological Agency (Tokyo 100-8122, Japan). Day-lengths and temperatures were averaged over months and were compared with respect to the pattern of individual hormones.
Statistical analysis
Two years’ data was merged into one year for the ease of interpretation. All values were expressed as mean ± SEM. Duncan’s multiple range test was used to detect the significant difference in amounts of hormones in different day points using SPSS software. Bonferroni’s Multiple Comparison Test and Pearson correlation was performed using Graphpad Prism software. Correlation coefficient (r) was calculated at p=0.05. Values differing at p<0.05 were considered significant.
Results
LH
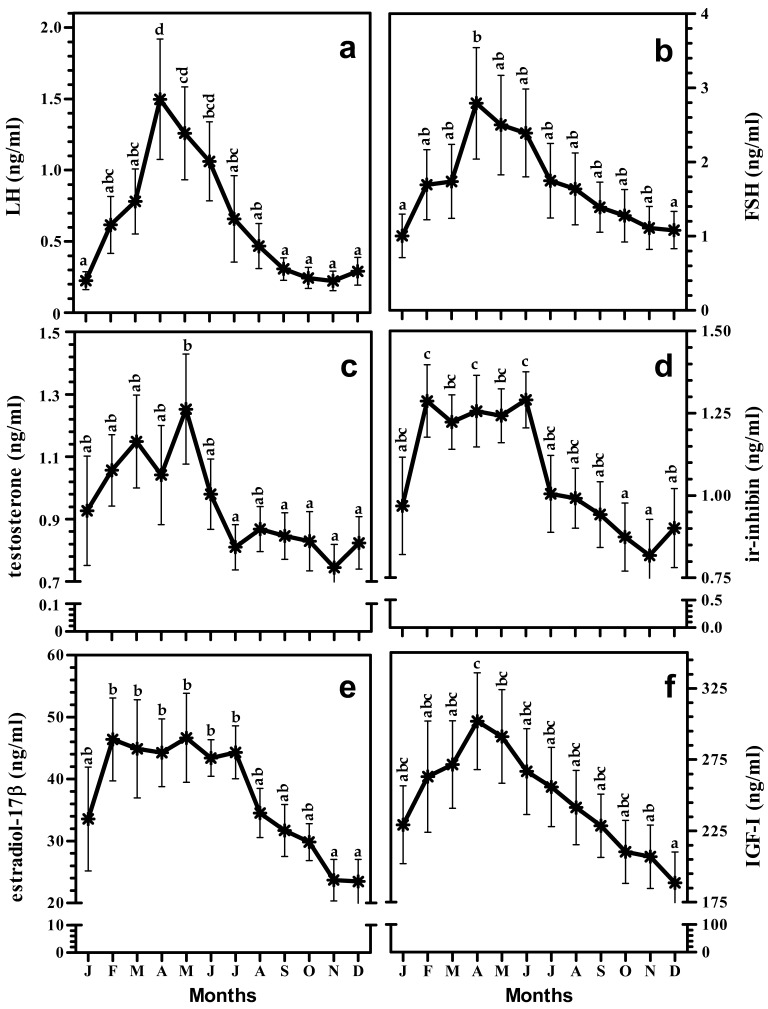
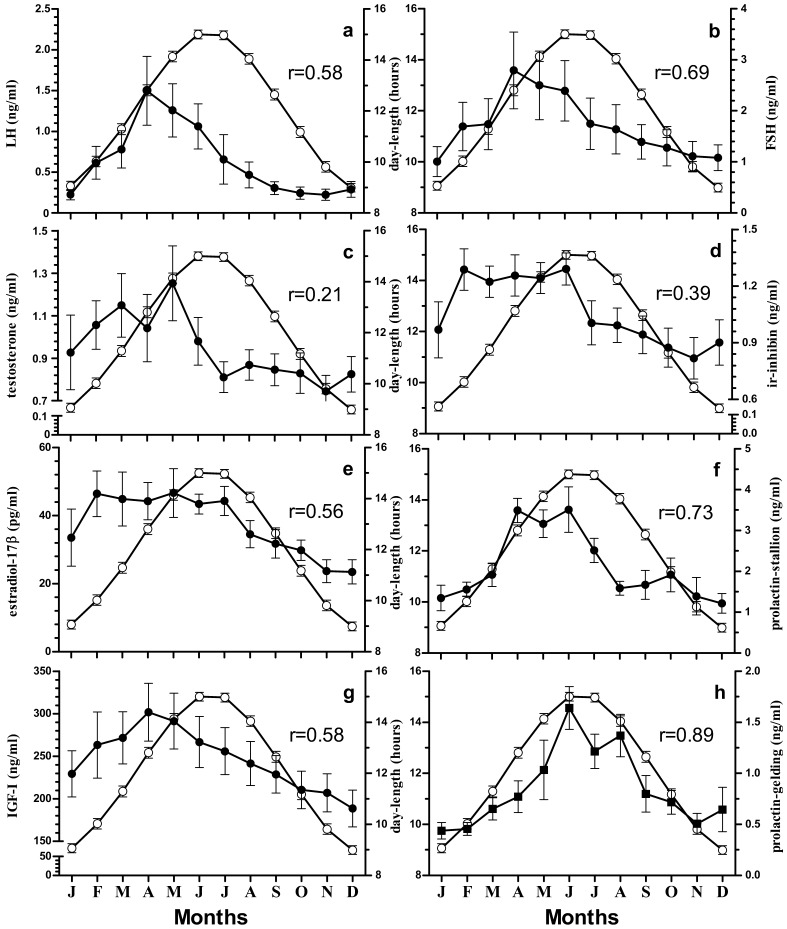
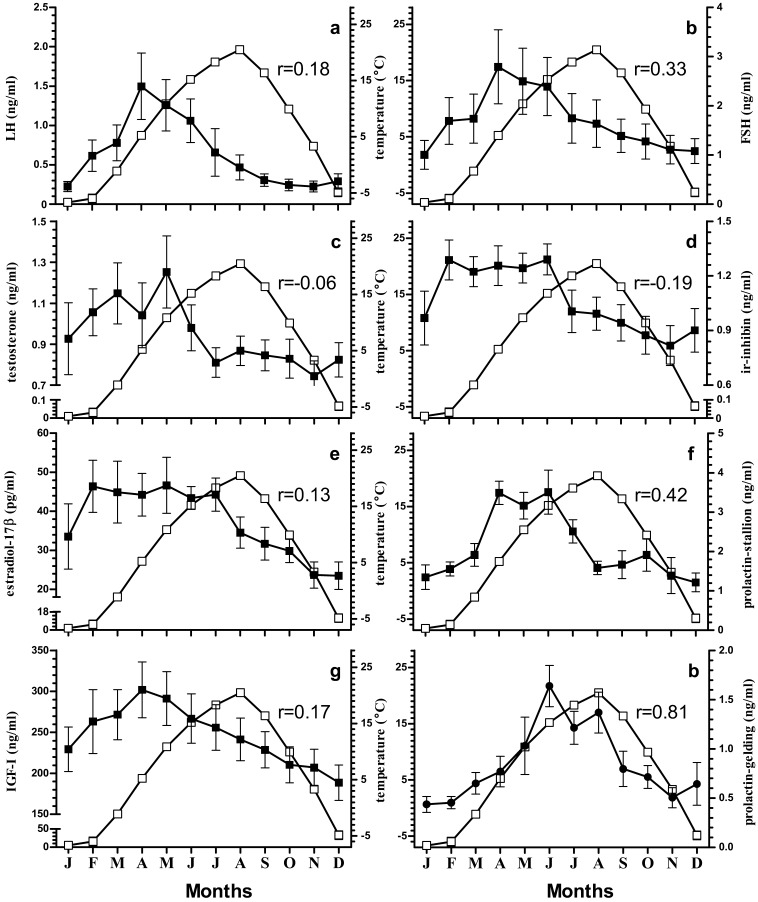
The plasma concentration of LH after January started to increase towards the maximal level at April (Fig. 1a). LH was significantly highest at April that decreased non-significantly until June and then dropped significantly reaching minimum by the month of November. LH positively correlated with day-length (r=0.58, Fig. 3a), and temperature (r=0.18, Fig. 4a).
Fig. 1.
Monthly changes in concentration of circulating LH (a), FSH (b), testosterone (c), ir-inhibin (d), estradiol-17β (e), and IGF-I (f) in stallions. Each point represents the mean ± SEM value of two years (n=5). Different alphabets reflect significant differences at p<0.05.
Fig. 3.
Correlation of day-length (○) with LH (a), FSH (b), testosterone (c), ir-inhibin (d), estradiol-17β (e), prolactin in stallion (f) (●), IGF-I (g), and prolactin in gelding (h) (■). Correlation coefficient at p<0.05 in each case is represented by r-values in respective graphs.
Fig. 4.
Correlation of temperature (□) with LH (a), FSH (b), testosterone (c), ir-inhibin (d), estradiol-17β (e), prolactin in stallion (f) (■), IGF-I (g), and prolactin in gelding (h) (●). Correlation coefficient at p<0.05 in each case is represented by r-values in respective graphs.
FSH
The plasma concentration of FSH was highest during April and was lowest in January. The values were intermediate during other months of year (Fig. 1b). The plasma concentration was higher during breeding months (March–July). FSH positively correlated with day-length (r=0.69, Fig. 3b), and temperature (r=0.33, Fig. 4b).
Testosterone
The highest concentration of testosterone was observed during the month of May and it was lowest from September to December. Among the intermediate values in rest of the months, February to June was comparatively higher (Fig. 1c). Testosterone had positive correlation with day-length (r=0.21, Fig. 3c), and had negative correlation with temperature (r=–0.19, Fig. 4c).
Ir-Inhibin
Ir-inhibin was lowest during the months of October and November while it was highest during February and June. During the remaining months, the values were intermediate except for December where the value was significantly lower than that during the highest months (Fig. 1d). In stallions, ir-inhibin correlated positively with day-length (r=0.39, Fig. 3d), and negatively with temperature (r=–0.06, Fig. 4d).
Estradiol-17β
Estradiol-17β concentration was significantly maximal from February to July while it was lowest during November and December. The values were intermediate in rest of the months (Fig. 1e). Estradiol-17β was positively correlated with day-length (r=0.56, Fig. 3e), and temperature (r=0.13, Fig. 4e).
IGF-1
IGF-I was in highest concentration on the month of April which then after decreased steadily and came to the significantly lowest value in December. The value during month of May was also significantly higher than that in December. Intermediate values were observed in rest of the months (Fig. 1f). IGF-I correlated positively with day-length (r=0.58, Fig. 3g), and temperature (r=0.17, Fig. 4g).
Prolactin, temperature, and day-length
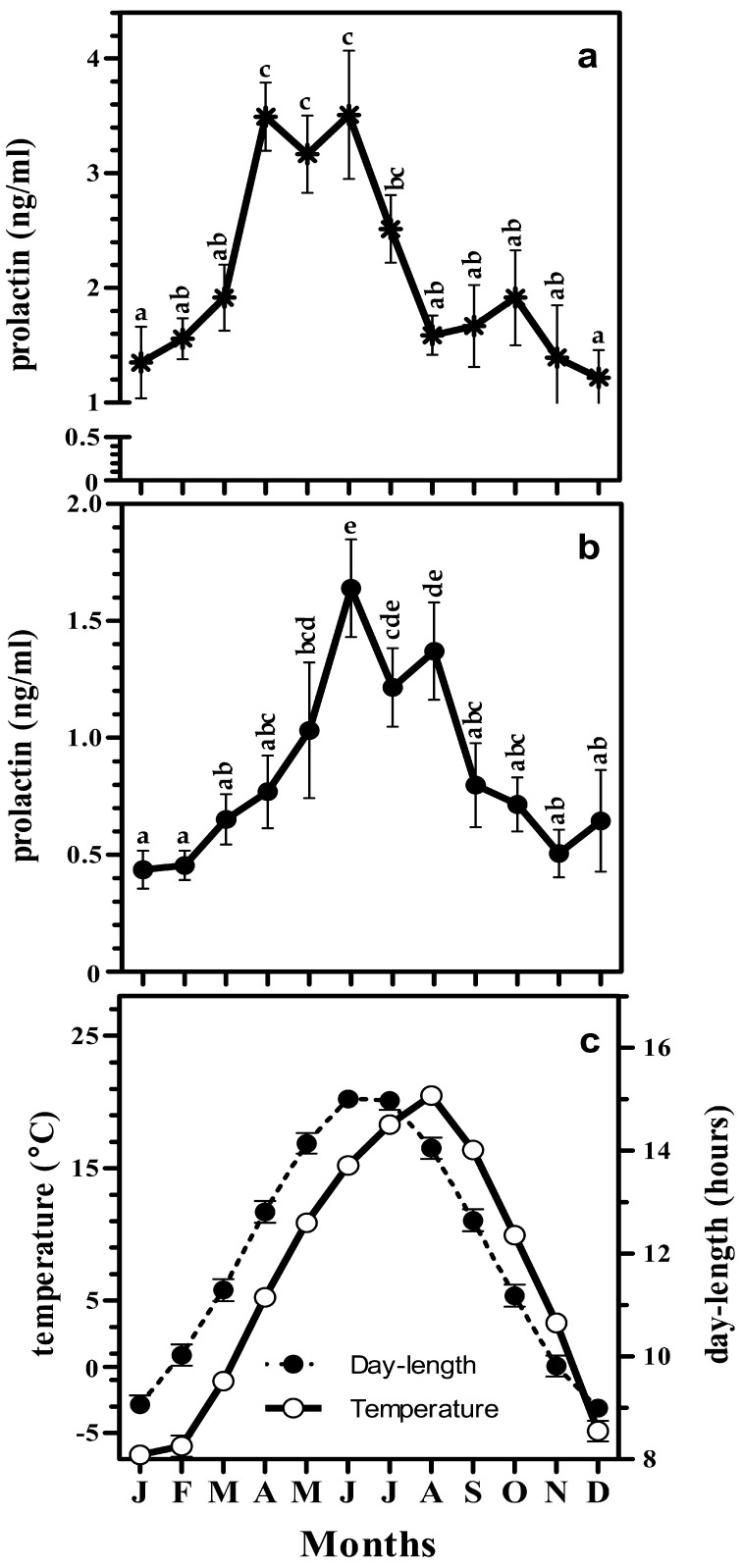
In stallions, prolactin was in highest concentration during the months of April, May, and June (Fig. 2a) whereas significantly high concentration was observed during the month of June in Geldings (Fig. 2b). But the values during July and August were significantly higher than in December in Gelding (Fig. 2b). Similarly, prolactin was at its lowest concentration during December and January in stallions. The increment of prolactin over a year both in stallions and geldings followed similar pattern like the increment in day-length (Fig. 2a, 2b, and 2c). Prolactin concentrations between stallion and gelding were positively correlated (r=0.59). Moreover, the prolactin concentration in geldings was significantly lower than that in stallions among all the months except for the month of August. Prolactin concentration in the stallions and geldings were correlated to temperature with lesser magnitude (r=0.42 for stallion, and r=0.81 geldings, Fig. 4f, and 4h) than that with the day-length (r=0.73 at for stallions and r=0.89 for geldings, Fig. 3f, and 3h).
Fig. 2.
Monthly changes in circulating levels of prolactin in stallion (a; n=5), gelding (b; n=13) and the pattern of day-length (●), and temperature (○) (c) in Japan. Values are represented as mean ± SEM and different alphabets represent the significant differences at p<0.05.
Discussion
In the present study, all the hormones exhibited a clear seasonal change. The concentration increased in spring (March–June), the industrial breeding season for Thoroughbred horses in Japan. Positive correlation was observed among ir-inhibin, IGF-I, steroid hormones, and gonadotropins of stallions. The correlation of prolactin was greater with estradiol-17β than with the testosterone. Ir-inhibin increased significantly from the month of February and maintained until June. The level decreased in transitional and non-breeding seasons. Inhibin is secreted from the testis of stallion [12, 16] and it can be the marker of the testicular function [13]. This clear seasonality in ir-inhibin is supported with previous findings in which increase in number of Sertoli cells and Leydig cell and smooth endoplasmic reticulum per testis has been shown during the breeding season of stallions [7]. Strong immunoreactions with inhibin-α in cytoplasm of Leydig cells, weak reaction in the Sertoli cells and no staining in germ cells were observed in the testis of stallions [11, 12]. The increase in number of Leydig and Sertoli cells and their increased secretion of inhibin resulted in high peripheral levels of ir-inhibin during the breeding season of stallions.
In a previous report [18], it had been concluded that season had little or no influence on FSH. The present result contradicts this as FSH showed clear seasonal rise, the peak in the month of April (spring) being significantly highest than the winter values. A positive correlation was noted between FSH and ir-inhibin of stallions.
Positive correlation of day-length and temperature remained positive in levels of prolactin in both the stallions and geldings. Lay-length seems to influence the prolactin secretion more than the temperature as there was stronger correlation between prolactin with day-length than that with temperature. Prolactin had been demonstrated to influence diverse metabolic and reproductive function [4]. The results indicate that castration doesn’t modify the responsiveness to the day length and functional mechanism of prolactin secretion by pituitary. But the concentration of this hormone between stallion and gelding was significantly different at different months of the year. Although the seasonal pattern was similar, geldings had half of the concentrations than that in stallions. Estradiol has stimulatory effect on pituitary mammotroph cells that regulate the prolactin synthesis by stimulation of gene expression pituitary cells [9]. Plasma levels of estradiol-17β decreased abruptly after bilateral gonadectomy of stallions [11]. Thus in geldings lacking testicular estradiol stimulus might had the reduced levels of prolactin concentration as compared with intact stallions.
Stallions had higher concentration of IGF-I during breeding season and the overall levels throughout the year was lower in the second year of the studied period when the age of animals increased by one year. The intensity of IGF-I and its receptor labeling in stallion Leydig cell was shown to be age dependent [19], the intensity being greater in post-pubertal stage. The values of IGF-I in stallions in our experiment are similar to those in a previous study on standardbreds [3].
In conclusion, stallions had high concentration of FSH, LH, ir-inhibin, testosterone, estradiol-17β, prolactin and IGF-I concentrations during the spring and summer months which is the natural breeding season for equines. The pattern of prolactin concentration was similar between stallions and geldings throughout the year and it correlated more with the day-length than with the temperature in a year. All the hormones were well correlated with the changes in day-length indicating that it plays important role in tuning the hypothalamo-pituitary-testicular axis for seasonal reproduction in stallions.
Acknowledgements
We are grateful to the National Hormone and Pituitary Program, NIDDK, NIH, (Torrance, CA, USA) and Dr. A.F. Parlow for the equine LH, FSH, prolactin, and IGF-I and to Dr. G.D. Niswender, Animal Reproduction and Biotechnology Laboratory, Colorado State University (Fort Collins, CO, USA) for providing antisera to progesterone (GDN 337), testosterone (GDN 250) and estradiol-17β (GDN 244). This study was supported in part by Japan Racing Horse Association.
References
- 1.Arai K.Y., Tanaka Y., Taniyama H., Tsunoda N., Nambo Y., Nagamine N., Watanabe G., Taya K.2006. Expression of inhibins, activins, insulin-like growth factor-I and steroidogenic enzymes in the equine placenta. Domest. Anim. Endocrinol. 31: 19–34 [DOI] [PubMed] [Google Scholar]
- 2.Bridges T.S., Davidson T.R., Chamberlain C.S., Geisert R.D., Spicer L.J.2002. Changes in follicular fluid steroids, insulin-like growth factors (IGF) and IGF-binding protein concentration, and proteolytic activity during equine follicular development. J. Anim. Aci. 80: 179–190 [DOI] [PubMed] [Google Scholar]
- 3.Champion Z.J., Breier B.H., Ewen W.E., Tobin T.T., Casey P.J.2002. Blood plasma concentrations of insulin-like growth factor-I (IGF-I) in resting standardbred horses. Vet. J. 163: 45–50 [DOI] [PubMed] [Google Scholar]
- 4.Curlewis J.D.1991. Seasonal prolactin secretion and its role in seasonal reproduction: a review. Reprod. Fertil. Dev. 4: 1–23 [DOI] [PubMed] [Google Scholar]
- 5.Derar R.I., Haramaki S., Hoque S.M.D., Hasizume T., Osawa T., Taya K., Watanabe G., Miyake Y.2006. Immunoreactive insulin-like growth factor in plasma during pre- and post-partum periods of thoroughbred mares from which newborn were removed: its pattern, physiological function and relation to other hormones. J. Equine Sci. 17: 75–79 [Google Scholar]
- 6.Hamada T., Watanabe G., Kokubo T., Taya K., Sasamoto S., Hasegawa Y., Miyamoto K., Igarashi M.1989. Radioimmunoassay of inhibin in various mammals. J. Endocrinol. 122: 697–704 [DOI] [PubMed] [Google Scholar]
- 7.Johnson L., Thompson D.L.1987. Effect of seasonal changes in Leydig cell number on the volume of smooth endoplasmic reticulum in Leydig cells and intratesticular testosterone content in stallions. J. Reprod. Dev. 81: 227–232 [DOI] [PubMed] [Google Scholar]
- 8.Kerstin O., Bjorn R., Asa G., Wilhelm E.1996. Cloning and sequencing of an equine insulin-like growth factor I cDNA and its expression in fetal and adult tissues. Gen. Comp. Endocrinol. 102: 11–15 [DOI] [PubMed] [Google Scholar]
- 9.Lieberman M.E., Maurer R.A., Claude P., Gorski J.1982. Prolactin synthesis in primary cultures of pituitary cells: regulation by estradiol. Mol. Cell. Endocrinol. 25: 277–294 [DOI] [PubMed] [Google Scholar]
- 10.Medan M.S., Nambo Y., Nagamine N., Shinbo H., Watanabe G., Groome N., Taya K.2004. Plasma concentrations of ir-inhibin, inhibin A, inhibin pro-alphaC, FSH, and estradiol-17β during estrous cycle in mares and their relationship with follicular growth. Endocrine 25: 7–14 [DOI] [PubMed] [Google Scholar]
- 11.Nagata S., Miyake Y., Nambo Y., Nagamine N., Watanabe G., Tsunoda N., Taniyama H., Hondo E., Yamada J., Taya K.1998. Inhibin secretion in the stallion. Equine Vet. J. 30: 98–103 [DOI] [PubMed] [Google Scholar]
- 12.Nagata S., Tsunoda N., Nagamine N., Tanaka Y., Taniyama H., Y., Nambo, Y., Watanabe, G., and Taya, K. 1998. Testicular inhibin in the stallion: cellular and seasonal changes in its secretion. Biol. Reprod. 59: 62–68 [DOI] [PubMed] [Google Scholar]
- 13.Nagata S., Nagaoka K., Shinbo H., Nagamine N., Tsunoda N., Taniyama H., Nambo Y., Groome N.P., Watanabe G., Taya K.2000. Inhibin pro-αc as the marker of testicular function in the stallion. J. Reprod. Dev. 46: 201–206 [Google Scholar]
- 14.Roser J.F.2008. Regulation of testicular function in the stallion: an intricate network of endocrine, paracrine and autocrine systems. Anim. Reprod. Sci. 107: 179–196 [DOI] [PubMed] [Google Scholar]
- 15.Tanja G.A., Jorg E.A.2000. Regulation of seasonal reproductive activity in the stallion, ram and hamster. Anim. Reprod. Sci. 58: 197–213 [DOI] [PubMed] [Google Scholar]
- 16.Taya K., Nagata S., Tsunoda N., Nagamine N., Tanaka Y., Nagaoka K., Taniyama H., Nambo Y., Watanabe G.2000. Testicular source of inhibin in stallion. J. Reprod. Fertil. 56 (Suppl): 43–50 [PubMed] [Google Scholar]
- 17.Taya K., Watanabe G., Sasamoto S.1985. Radioimmunoassay for progesterone, testosterone and estradiol-17β 125I-iodohistamine radioligands. J. Anim. Reprod. 31: 186–197 [Google Scholar]
- 18.Thompson D.L., Johnson L., George R.L., Garza F.1986. Concentrations of prolactin, luteinizing hormone and follicle stimulating hormone in pituitary and serum of horses: effect of sex, season and reproductive state. J. Anim. Sci. 63: 854–860 [DOI] [PubMed] [Google Scholar]
- 19.Yoon M., Berger T., Roser J.F.2010. Localization of insulin-like growth factor-I (IGF-I) and IGF-I Receptor (IGF-IR) in equine testes. Reprod. Domest. Anim. no. doi: 10.1111/j.1439-0531.2010.01643 [DOI] [PubMed]