Abstract
Although high oxygen consumption in skeletal muscle may result in severe oxidative stress, there are no direct studies that have documented free radical production in horse muscles after intensive exercise. To find a new parameter indicating the muscle adaptation state for the training of Thoroughbred horses, we examined free radical formation in the muscle by using electron paramagnetic resonance (EPR). Ten male Thoroughbred horses received conventional training for 18 weeks. Before and after the training period, all horses performed an exhaustive incremental load exercise on a 6% incline treadmill. Muscle samples of the middle gluteal muscle were taken pre-exercise and 1 min, 1 hr, and 1 day after exercise. Muscle fiber type composition was also determined in the pre-exercise samples by immunohistochemical staining with monoclonal antibody to myosin heavy chain. We measured the free radical in the muscle homogenate using EPR at room temperature, and the amount was expressed as relative EPR signal intensity. There was a significant increase in Type IIA muscle fiber composition and a decrease in Type IIX fiber composition after the training period. Before the training period, the mean value of the relative EPR signal intensity showed a significant increase over the pre-exercise value at 1 min after the exercise and an incomplete recovery at 24 hr after the exercise. While no significant changes were found in the relative EPR signal intensity after the training period. There was a significant relationship between percentages of Type IIA fiber and change rates in EPR signal intensity at 1 min after exercise. The measurement of free radicals may be useful for determining the muscle adaptation state in the training of Thoroughbred horses.
Keywords: free radical, EPR, Thoroughbred, training
Electron leakage via the mitochondrial electron transport chain is considered to be the mechanism predominantly responsible for the activity-independent increase in free radicals. Oxygen flux through the active muscle can increase to several times the resting values in response to intensive exercise. The electrons derived from mitochondria reduce the molecular oxygen to superoxide anion (O2–•); O2–• is dismutated to hydrogen peroxide (H2O2) by superoxide dismutase; then, the highly reactive hydroxyl radical (•OH) is formed from H2O2 in the Fenton reaction with transition metals [23]. Rapid, increased generation of •OH after exercise overwhelms the antioxidant capacity, and oxidative stress is induced via radical chain reactions [25]. The maximal oxygen uptake (VO2max) of horses can reach up to 200 ml O2/kg/min, which is more than three times the VO2 max of humans [14]. Such high oxygen consumption may result in severe oxidative stress. Therefore, the horse might be an excellent model for studying exercise-induced oxidative stress.
There are some indirect techniques used to evaluate free radicals that can detect end products of by-products of radical chain reactions. Most of the studies of oxidative stress have used these indirect markers, and they report that a bout of exercise induces lipid peroxidation [6, 7, 13, 18, 27], glutathione oxidation [24], and protein oxidation [13]. Although these indirect techniques are useful, they have been criticized for their lack of specificity and reproducibility [12]. The most specific and direct method for determining free radicals is electron paramagnetic resonance (EPR). However, there are no studies that have documented EPR measurement of free radical production in horse skeletal muscle after muscle activity, and there are only four studies of skeletal muscle in other species. In 1982, Davies et al. [7] were the first to establish an exercise-induced increase in EPR signal intensity (i.e. level of free radicals) in skeletal muscle of rodent hindlimbs. Following their report, Jackson et al. [11] reported an increase in EPR signal intensity in an electrically stimulated rodent gastrocnemius muscle. In 2007, Bailey et al. [3] reported an exercise-induced increase in EPR signal intensity in human skeletal muscle. Contradicting these results, McArdle et al. [17] reported no differences in EPR signal intensity were observed at 3 hr or 3 days after lengthening contraction in rat skeletal muscle.
In horses, some training adaptations are reported: for example, muscle hypertrophy [20], improvement of oxidative and glycolytic enzymes in muscle [29], muscle fiber type transformation toward oxidative muscle fiber [22], and improvement in antioxidant capacity [2]. In particular, muscle fiber type transformation may induce an increase in mitochondrial content and enhance the generation of free radicals after exercise. In contrast, improvement in antioxidant capacity can scavenge free radicals and reduce the generation of free radicals after exercise. Therefore, it is very interesting to consider the effects of training adaptation on the formation of free radicals after exercise. In this study, using EPR, we examined whether (a) a bout of intensive exercise increases the intramuscular concentration of free radicals, (b) the formation of free radicals is affected by the muscle adaptation state induced by training, or (c) muscle fiber transformation toward oxidative fiber has a significant impact on free radical formation after intensive exercise.
Materials and Methods
Animals, exercise test and muscle sampling
Ten Thoroughbred horses were used in this study. Before the exercise test, all horses stayed on the pasture in the daytime and were accustomed to running on a treadmill for a short time.
A bout of incremental load exercise was used for the exercise test. It consisted of a warm-up (2 min of walking 1.8 m/sec on a 6% slope) and incremental load running (2 min of running at 4, 6, 8, 10 m/sec on a 6% slope and then 2 min of running on a 6% slope at 11, 12, 13, 14,… m/sec). Incremental load running was continued until horses could not maintain the running speed. Muscle biopsy samples were obtained from the middle gluteal muscle [4] at the same depth (5 cm) before the exercise test and at 1 min, 1 hr, and 1 day after the exercise test. Each muscle sample was immediately frozen in liquid N2 and stored at –80°C for histochemical and EPR analyses.
All procedures were approved by the Animal Experiment Committee of the Equine Research Institute.
Training protocol
The training protocol for improving physical fitness, which consisted of four phases, was conducted for 18 weeks. Three minutes of treadmill running exercise for 5 days/week was performed at 75% VO2max from week 1 to week 3, at 90% VO2max from week 4 to week 6 and at 100– 110% VO2max from week 7 to week 10. From week 11 to week 18, the training consisted of 2 min of treadmill running exercise at 110–115% VO2max for 2 days/week and 3 min of treadmill running exercise at 90% VO2max for 3 days/ week.
EPR measurement
Frozen muscle samples (~50 mg wet weight) were homogenized with 500 μl of cold homogenizing buffer (~4°C) consisting of 40 mM Tris-HCl and 300 mM sucrose. Homogenized tissue samples were transferred to a quartz sample tube for EPR spectroscopy and set in the EPR cavity. All EPR measurements were carried out at room temperatures using an X-band E500 spectrometer (Bruker BioSpin, Yokohama). Spectrometer settings were maintained at: microwave power=10 mW; microwave frequency=9.85 GHz; modulation frequency=100 kHz; modulation amplitude=5.0 G; magnetic field center=3,510 G; scan width=100 G; scan time=20 sec; time constant=0.02 sec for 1 sweep. The measured spectra were subtracted from the background spectrum obtained for the quartz sample tube containing only homogenized buffer.
Histochemical analysis
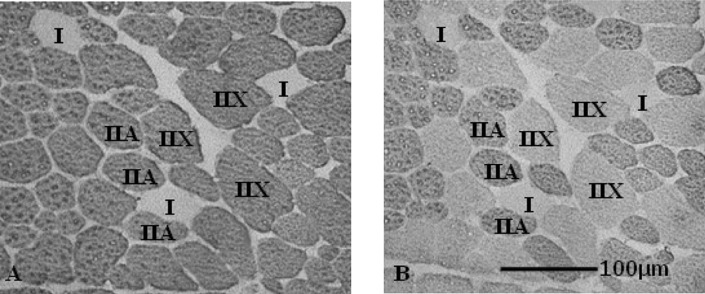
Frozen muscle samples were cut with a freezing microtome (Leica CM510; Leica Microsystems, Tokyo) into 2 transverse sections of 10 μm thickness. Based on previous studies [20, 26, 29], the two transverse sections were reacted for immunohistochemical analysis with anti-mouse IgG. The sections were allowed to warm to room temperature and then pre-incubated in normal goat serum in phosphate buffer at 25°C for 10 min. The primary monoclonal antibodies against specific myosin heavy chain (MHC) isoforms were then applied to anti-Fast Myosin (1:1,000), which specifically labels MHC-II, and SC-71 (1:1,000), which specifically labels MHC-IIa. The specificity of these monoclonal antibodies in horses has been previously demonstrated [15, 21]. The sections were incubated in primary monoclonal antibody at 25°C overnight, then washed with phosphate buffer five times, reacted with a horseradish peroxidase-labeled secondary antibody (1:1,000) at 25°C for 3 hr and washed with phosphate buffer again. Diaminobenzidine tetrahydrochloride was used as a chromogen to localize peroxidase in both primary antibodies. On the basis of examination of the immunohistochemical staining images, muscle fibers were classified into Type I, IIA and IIX fibers (Fig. 1). Type IIB fiber was not present in our horse skeletal muscle, as demonstrated in a previous study [21].
Fig. 1.
Serial transverse sections of gluteus medius muscle from a representative Thoroughbred horse stained for immunocytochemistry. The sections were stained with monoclonal antibodies to specific myosin heavy chain (MHC) isoforms. Muscle fibers were classified into Type I (expressing MHC-I), Type IIA (expressing MHC-IIa), and Type IIX (expressing MHC-IIx). A: anti-Fast Myosin (anti-MHC-II), B: SC-71 (anti-MHC-IIa).
Statistical analysis
Significant differences between the pre-experiment condition and each experimental time point were analyzed using the paired t-test. A liner regression line analysis was performed between EPR signal intensity and muscle fiber type composition. In all cases, values of p<0.05 were considered significant. All values were reported as mean ± standard deviation (SD).
Results
EPR measurement
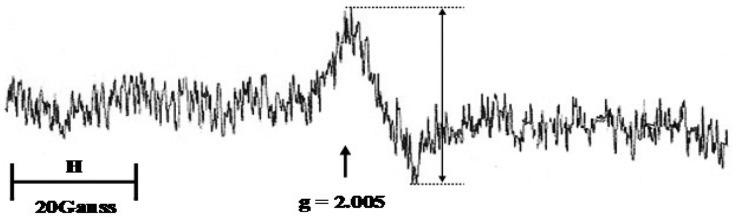
Figure 2 shows a typical EPR spectrum at room temperature. Free radical signals are characterized by their “g” value that is derived from the magnetic field strength and the microwave frequency at which the signal is seen. For all muscle samples, a free radical signal of the g value at 2.005 was seen.
Fig. 2.
Typical EPR spectrum of Thoroughbred horse skeletal muscle at room temperature. The spectra indicate a g=2.005 signal. This EPR spectrum was obtained by subtracting the background spectrum from the measured spectrum. The background spectrum was scanned from a quartz sample tube containing homogenizing buffer. Spectrometer settings: microwave power=10 mW; microwave frequency=9.85 GHz; modulation frequency=100 kHz; modulation amplitude=5.0 G; magnetic field center=3,510 G; scan width=100 G; scan time=20 sec; time constant=0.02 sec (1 sweep), H=magnetic field.
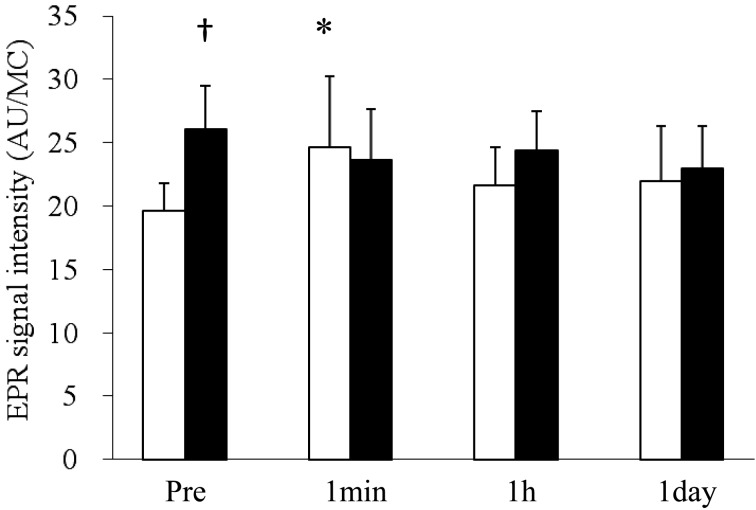
Figure 3 shows the time course of changes in EPR signal intensity. The results represent a normalized value (arbitrary unit) for muscle tissue concentration in homogenate (MC; g/l). Before the training period, a significant increase in the EPR signal intensity was observed at 1 min after the exercise test compared with the pre-exercise (Pre) value. There were no significant differences between Pre and 1 hr or 1 day after the exercise test, but the EPR signal intensities at 1 hr and 1 day did not completely recover to the Pre value. On the other hand, after training, no significant change in the EPR signal intensity was detected at any time point after the exercise test compared with Pre. EPR signal intensity at rest after training was significantly higher than the value before training.
Fig. 3.
Effects of a bout of exercise and training on the EPR signal detected in Thoroughbred horse skeletal muscle at room temperature. The values were normalized for muscle concentration (MC; g/l) and expressed as mean ± SD. * p<0.05 (compared to Pre), □: Pre-training, ■: Post-training. * p<0.05 (compared to Pre), †p<0.05 (compared to Pre-training). AU: arbitary unit.
Muscle fiber composition
We examined muscle fiber composition in the Thoroughbreds’ middle gluteal muscle before and after training (Table 1). The percentages of Type I, IIA and IIX fibers before training were 10.5 ± 4.1, 43.4 ± 3.7 and 46.1 ± 5.7%, respectively. After training, the percentages of Type I, IIA and IIX fibers changed to 12.0 ± 2.9, 57.5 ± 6.6 and 30.5 ± 7.6%, respectively. There was a significant increase and decrease in Type IIA and IIX fibers, respectively.
Table 1. Effect of training on muscle fiber type (Type I, IIA, and IIX) composition.
| Pre-training | Post-training | |||||
|---|---|---|---|---|---|---|
| Type I % | Type IIA % | Type IIX % | Type I % | Type IIA % | Type IIX % | |
| horse1 | 7.0 | 41.2 | 51.8 | 12.7 | 47.0 | 40.3 |
| horse2 | 7.3 | 36.1 | 56.6 | 8.2 | 53.4 | 38.4 |
| horse3 | 11.2 | 46.4 | 42.4 | ND | ND | ND |
| horse4 | 2.8 | 46.6 | 50.6 | 9.4 | 67.5 | 23.1 |
| horse5 | 18.2 | 42.2 | 39.6 | 14.1 | 63.7 | 22.3 |
| horse6 | 10.7 | 46.6 | 42.7 | ND | ND | ND |
| horse7 | 10.9 | 38.7 | 50.4 | 9.4 | 51.6 | 39.0 |
| horse8 | 13.7 | 44.9 | 41.4 | 15.3 | 58.2 | 26.5 |
| horse9 | 11.3 | 45.6 | 43.1 | 15.5 | 59.8 | 24.7 |
| horse10 | 12.0 | 45.6 | 42.4 | 11.1 | 59.2 | 29.7 |
| Average | 10.5 | 43.4 | 46.1 | 12.0 | 57.5* | 30.5* |
| SD | 4.1 | 3.7 | 5.7 | 2.9 | 6.6 | 7.6 |
* p < 0.05 (compared to Pre-training; n=8). ND: not determined, SD: standard deviation.
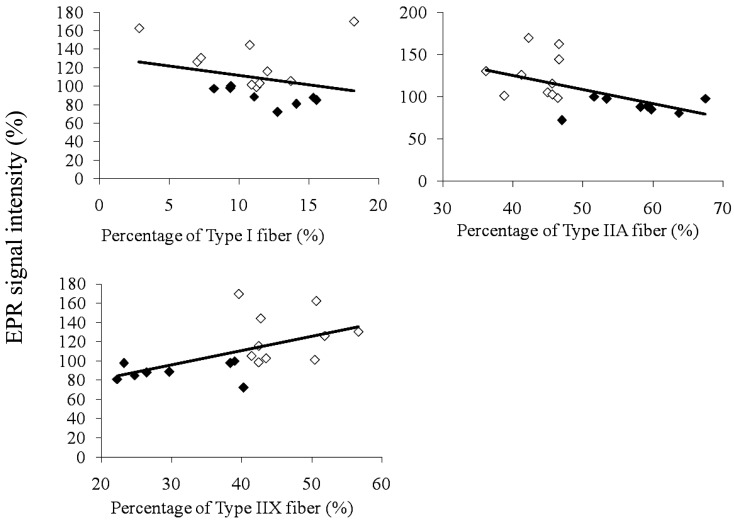
Correlations between the percentages of each muscle fiber type and change rates in EPR signal intensity at 1 min after exercise are shown in Fig. 4. The change rates in EPR signal intensity are presented as values relative to each Pre value. There was a significant negative correlation between the percentage of Type IIA fiber and change rates in EPR signal intensity at 1 min after the exercise test, and a significant positive correlation between the percentage of Type IIX fiber and change rates in EPR signal intensity at 1 min after the exercise test.
Fig. 4.
Correlations between percentages of each muscle fiber type and change rates in EPR signal intensity at 1 min after exercise. Change rates at 1 min after the exercise test are expressed relative to the pre-value. Percentages of each muscle fiber type are expressed as the ratio to all muscle fibers. ◇: Pre-training, ◆: Post-training.
Discussion
The present study is the first to report an EPR signal, a proxy for free radicals, in horse skeletal muscle. Davies et al. [7] and Jackson et al. [11] observed the EPR signal in rodent skeletal muscle, and Bailey et al. [3] also observed the signal in human skeletal muscle. They reported that the EPR signals were derived from ubiquinone, which has a g value of 2.004. EPR signals in our study had a similar g value. However, temperature conditions were set at 77K in the previous studies, whereas it was room temperature in our study. Therefore, further studies are needed to identify that the origin of free radical signals in our present study was ubiquinone radicals.
The level of free radicals increased immediately after a bout of exercise in pre-training horses. A major contributor to the formation of free radicals, especially ROS, immediately after exercise is O2 utilized during mitochondrial oxidative phosphorylation. Whole-body O2 consumption during exercise can increase by up to 30-fold in the horse [5], and increases electron leakage via the mitochondrial electron transport chain, resulting in increased formation of free radicals. At the same time, antioxidant capacity acutely increases to scavenge free radicals. However, this acute increment cannot completely compensate for the exercise-induced formation of free radicals, resulting in an increase in the level of free radicals, or oxidative stress [13, 14].
During a recovery period after exercise, leukocytes and macrophages, which are potential sources of ROS, invade damaged muscle fibers [8]. These phagocytes play a role not only in removing cell debris, but also have harmful effects (i.e. delayed muscle recovery), when phagocyte-derived ROS are overproduced beyond requirement. In the present study, exercise-induced increases in free radicals did not completely return to rest values during a 1-day recovery period in pre-training horse.
When increased generation of free radicals, especially ROS, after exercise overwhelms antioxidant capacity, muscular oxidative damage is induced via radical chain reactions. Thus, if antioxidant capacity is enhanced by training and/or mitochondria-derived ROS generation is reduced by training, these training adaptations can minimize oxidative stress. In fact, there was no increase in free radicals immediately after a bout of exercise in post-training horses. In past studies, Avellini et al. [2] reported that training can increase antioxidant capacity in both the extracellular fluids and blood cells of horses. Venditti et al. [28] reported that the mitochondrial H2O2 release rate was significantly lower in trained than in untrained rats. These reports suggest that regular long-term training could induce some important adaptations to minimize the formation of free radicals immediately after a bout of exercise.
In the present study, 18-week training induced a significant increase in the level of free radicals at rest. This result indicates that regular long-term training induces the accumulation of free radicals, or chronic oxidative stress. A similar negative training effect was reported in humans [16]. That report demonstrated that the plasma creatine kinase (CK) activity and thiobarbituric acid-reactive substances levels in weightlifters were significantly higher than those of non-athletes, and CK activity and the indices of lipid peroxidation significantly increased after 1 week of intensive training in weightlifters. Therefore, a proper recovery period after training may be necessary in order to reduce exercise-induced oxidative damage.
Regular long-term training induces oxidative fiber type shift of muscle composition within Type II fibers [22]. We found a significant increase in the percentage of Type IIA fibers and a significant decrease in the percentage of Type IIX fibers, which indicates a fiber type transformation from Type IIX to Type IIA. Rivero et al. [22] also reported a similar fiber type transformation of muscle composition. The decrease in the percentage of Type IIX in their study was similar to our result, but the increase in the percentage of Type IIA in their study was lower than ours. This inconsistency likely arises because they classified muscle fiber type into 4 types (I, IIA, IIA/X, IIX), whereas we grouped most of Type IIA and IIA/X fibers into IIA fiber.
We found a significant negative correlation between percentage of Type IIA fiber and change rates in EPR signal intensity at 1 min after a bout of intensive exercise. We also found a significant positive correlation between the percentage of Type IIX fiber and change rates in EPR signal intensity at 1 min after a bout of intensive exercise. These results indicate that the training-induced fiber type transformation from Type IIX to Type IIA plays an important role in depressing the generation of free radicals after exercise. However, mitochondrial oxygen consumption and mitochondrial enzyme content are greater in Type IIA fiber than Type IIX fiber [1, 9]. Thus, it seems that electron leakage via the mitochondrial respiratory chain during exercise is more severe in Type IIA. In spite of this fact, why did post-training horses, which possess a higher percentage of Type IIA fiber compared to pre-training horses, produce less free radicals immediately after exercise? Why did the training-induced fiber type shift from Type IIX to Type IIA depress the generation of free radicals immediately after exercise? A possible answer to these questions is the differences in mitochondrial properties between muscle fiber types. Ogata and Yamasaki [19] demonstrated that Type IIB fibers possess a thinner ultrastructure of mitochondria than other types. Anderson et al. [1] reported that Type IIB fibers appear to possess unique properties that potentiate mitochondrial O2–• generation and/or H2O2 production/emission. These studies suggest that Type IIB (Type IIX in horses) fibers might possess properties limiting the efficiency of intramitochondrial ROS scavenging, advancing the rate of ROS production/ emission. Another possible answer is the differences in antioxidant capacity among muscle fiber types. Anderson et al. [1] demonstrated that ROS removal capacity is significantly greater in rat soleus (primarily Type I fibers) > red gastrocnemius (Type IIA) > white gastrocnemius (Type IIB) muscle when expressed relative to milligrams of muscle dry weight. Hollander et al. [10] demonstrated that reinforcement by training of some antioxidant enzyme activities occurred primarily in Type IIA fibers. These reports suggest that Type IIA fibers have a higher antioxidant capacity than Type IIX fibers.
In summary, in pre-training horses, the level of free radicals was significantly increased immediately after the exercise test and did not completely recover to the pre-exercise value. After 18 weeks of training, the exercise-induced increase in free radicals was absent, but the accumulation of free radicals occurred during rest. There were significant relationships between the changes in free radicals immediately after exercise and the percentage of Type II fibers. Although further studies are needed, free radicals are a very useful indicator of muscle condition, at least in Thoroughbred horses.
Acknowledgements
We wish to thank the Management Section of the Equine Research Institute for their help throughout the experiments. This work was supported in part by a grant from The Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 21300251).
References
- 1.Anderson E.J., Neufer P.D.2006. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am. J. Physiol. 290: C844–C851 [DOI] [PubMed] [Google Scholar]
- 2.Avellini L., Chiaradia E., Gaiti A.1999. Effect of exercise training, selenium and vitamin E on some free radical scavengers in horses (Equus caballus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 123: 147–154 [DOI] [PubMed] [Google Scholar]
- 3.Bailey D.M., Lawrenson L., McEneny J., Young I.S., James P.E., Jackson S.K., Henry R.R., Mathieu-costello O., McCord J.M., Richardson R.S.2007. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radic. Res. 41: 182–190 [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom J., Hermansen L., Hultman E., Saltin B.1967. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 71: 140–150 [DOI] [PubMed] [Google Scholar]
- 5.Butler P.J., Woakes A.J., Smale K., Roberts C.A., Hillidge C.J., Snow D.H., Martin D.J.1993. Respiratory and cardiovascular adjustments during exercise of increasing intensity and during recovery in thoroughbred racehorses. J. Exp. Biol. 179: 159–180 [DOI] [PubMed] [Google Scholar]
- 6.Close G.L., Ashton T., Cable T., Doran D., MacLaren D.P.M.2004. Eccentric exercise, isokinetic muscle torque and delayed onset muscle soreness: the role of reactive oxygen species. Eur. J. Appl. Physiol. 91: 615–621 [DOI] [PubMed] [Google Scholar]
- 7.Davies K.J.A., Quintanilha A.T., Brooks G.A., Packer L.1982. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 107: 1198–1205 [DOI] [PubMed] [Google Scholar]
- 8.Duarte J.A., Carvalho F., Bastons M.L., Soares J.M.C., Appell H.J.1994. Do invading leucocytes contribute to the decrease in glutathione concentrations indicating oxidative stress in exercised muscle, or are they important for the recovery? Eur. J. Appl. Physiol. 68: 48–53 [DOI] [PubMed] [Google Scholar]
- 9.Graziotti G.H., Rios C.M., Rivero J.L.2001. Evidence for three fast myosin heavy chain isoforms in Type II skeletal muscle fibers in the adult llama (lama glama). J. Histochem. Cytochem. 49: 1033–1044 [DOI] [PubMed] [Google Scholar]
- 10.Hollander J., Flebiq R., Gore M., Bejma J., Ookawara T., Ohno H., Ji L.L.1999. Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training. Am. J. Physiol. 277: R856–R862 [DOI] [PubMed] [Google Scholar]
- 11.Jackson M.J., Edwards R.H.T., Symons M.C.R.1985. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim. Biophys. Acta 847: 185–190 [DOI] [PubMed] [Google Scholar]
- 12.Kanter M.1998. Free radicals, exercise and supplementation. Proc. Nutr. Soc. 57: 9–13 [DOI] [PubMed] [Google Scholar]
- 13.Kinnunen S., Hyyppa S., Lappalainen J., Oksala N., Venojarvi M., Nakao C., Hanninen O., Sen C.K., Atlay M.2005. Exercise-induced oxidative stress and muscle stress protein responses in trotters. Eur. J. Appl. Physiol. 93: 496–501 [DOI] [PubMed] [Google Scholar]
- 14.Kinnunen S., Hyyppa S., Lehmuskero A., Oksala N., Maenpaa P., Hanninen O., Atlay M.2005. Oxygen radical absorbance capacity (ORAC) and exercise-induced oxidative stress in trotters. Eur. J. Appl. Physiol. 95: 550–556 [DOI] [PubMed] [Google Scholar]
- 15.Klomkleaw W., Kasashima Y., Fuller G.A., Kobayashi A., Yoshihara T., Oikawa M.A., Izumisawa Y., Yamaguchi M.2002. Horse lumbrical muscle: possible structural and functional reorganization in regressive muscle. Anat. Histol. Embryol. 31: 85–98 [DOI] [PubMed] [Google Scholar]
- 16.Liu J.F., Chang W.Y., Chan K.H., Tsai W.Y., Lin C.L., Hsu M.C.2005. Blood lipid peroxides and muscle damage increased following intensive resistance training of female weightlifters. Ann. N. Y. Acad. Sci. 1042: 255–261 [DOI] [PubMed] [Google Scholar]
- 17.McArdle A., Van der Meulen J.H., Catapano M., Symons M.C.R., Faulkner J.A., Jackson M.J.1999. Free radical activity following contraction-induced injury to the extensor digitorum longus muscles of rats. Free Radic. Biol. Med. 26: 1085–1091 [DOI] [PubMed] [Google Scholar]
- 18.Mills P.C., Smith N.C., Casas I., Harris P., Harris R.C., Marlin D.J.1996. Effects of exercise intensity and environmental stress on indices of oxidative stress and iron homeostasis during exercise in the horse. Eur. J. Appl. Physiol. 74: 60–66 [DOI] [PubMed] [Google Scholar]
- 19.Ogata T., Yamasaki Y.1997. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat. Rec. 248: 214–223 [DOI] [PubMed] [Google Scholar]
- 20.Rivero J.L.L., Serrano A.L., Henckel P., Agiiera E.1993. Muscle fiber type composition and fibersize in successfully and unsuccessfully enduranceraced horses. J. Appl. Physiol. 75: 1758–1766 [DOI] [PubMed] [Google Scholar]
- 21.Rivero J.L.L., Talmadge R.J., Edgerton V.R.1996. Myosin heavy chain isoforms in adult equine skeletal muscle: an immunohistochemical and electrophoretic study. Anat. Rec. 246: 185–194 [DOI] [PubMed] [Google Scholar]
- 22.Rivero J.L.L., Ruz A., Marti-Korff S., Estepa J.C., Aguilera-Tejero E., Werkman J., Sobotta M., Lindner A.2007. Effects of intensity and duration of exercise on muscular responses to training of thoroughbred racehorses. J. Appl. Physiol. 102: 1871–1882 [DOI] [PubMed] [Google Scholar]
- 23.Sachdev S., Davies K.J.A.2008. Production, detection, and adaptive responses to free radicals in exercise. Free Radic. Biol. Med. 44: 215–223 [DOI] [PubMed] [Google Scholar]
- 24.Sastre J., Asensi M., Gasco E., Pallardo F.V., Ferrero J.A., Furukawa T., Vina J.1992. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am. J. Physiol. 263: R992–R995 [DOI] [PubMed] [Google Scholar]
- 25.Sen C.K., Packer L., Hanninen O.2000. Handbook of oxidants and antioxidants in exercise. Elsevier, Amsterdam. [Google Scholar]
- 26.Serrano A.L., Quiroz-Rothe E., Rivero J.L.L.2000. Early and long-term changes of equine skeletal muscle in response to endurance training and detraining. Pflugers Arch. 441: 263–274 [DOI] [PubMed] [Google Scholar]
- 27.Thompson D., Williams C., Garcia-Roves P., McGregor S.J., McArdle F., Jackson M.J.2003. Post-exercise vitamin C supplementation and recovery from demanding exercise. Eur. J. Appl. Physiol. 89: 393–400 [DOI] [PubMed] [Google Scholar]
- 28.Venditti P., Masullo P., Di Meo S.1999. Effect of training on H2O2 release by mitochondria from rat skeletal muscle. Arch. Biochem. Biophys. 372: 315–320 [DOI] [PubMed] [Google Scholar]
- 29.Yamano S., Eto D., Sugiura T., Kai M., Hiraga A., Tokuriki M., Miyata H.2002. Effect of growth and training muscle adaptation in Thoroughbred horses. Am. J. Vet. Res. 63: 1408–1412 [DOI] [PubMed] [Google Scholar]