Abstract
This study was conducted to determine the prevalence and significance of parasites of horses in northern Nigeria. Blood and faecal samples were randomly collected from 243 horses from different stables in some states of northern Nigeria for laboratory analyses. Fifty-seven horses (23.5%) were found infected with parasites. The hemoparasites detected, 21 (8.6%), include Theileria equi, Babesia caballi, Trypanosoma vivax and Trypanosoma evansi. The endoparasites encountered, 29 (11.9%) were Strongylus spp., Strongyloides spp., Oxyuris equi, Parascaris equorum, Paragonimus spp. and Dicrocoelium spp., 3 (1.2%) was Eimeria spp. Four horses (1.6%) had mixed infection of hemo- and endoparasites. This preliminary finding shows that parasitism is a problem in the horse stables examined, and calls for proper stable hygiene, routine tick control and regular deworming programme.
Keywords: horses, parasites, Nigeria, stables
Parasite represents a significant threat to the health of animals [1, 9]. The horse is susceptible to more than 60 parasites, and may harbour several species of worms at any time [3]. The effects of parasitic infection are more evident in young and undernourished horses. Parasitic disease have been reported to be the most prevalent disease of horses in Zaria, a part of northern Nigeria, accounting for 82.3% of cases presented in a veterinary clinic over a period of 28 years. Other parasitic condition recorded were trypanosomosis (0.10%) and coccidiosis (1.22%) [15]. Indigenous horses have been used by institutional and private owners in Nigeria for pleasure riding, polo games, ceremonies, crowd control, entertainment and research [6, 15]. These uses have encouraged horse owners to import exotic breeds to overcome the limitations of the available local breeds [13]. Horses are associated with royalty and some special traditional festivals in the northern parts of Nigeria [2]. Little is known on the parasitic infection of horses in this part of the country. The aim of this study was to determine the prevalence and significance of parasitic infection of horses in three states (Bauchi, Kaduna and Plateau) of northern Nigeria. Horse stables belonging to institutional (research institutes, agricultural colleges, security agencies); traditional (Emirs, local chiefs) and private (elites) owners were used for this study. Routine veterinary care was provided mainly for the institutional and private stables. Two hundred and forty-three samples were randomly collected from horses in institutional (n=144), private (n=58) and traditional (n=41) stables in three states in northern Nigeria. Under proper restraint, blood samples were collected from each selected horse using vacutainers into labelled bijou bottles containing ethylene diamine tetra-acetic acid (EDTA), and faecal samples were collected directly from the rectum into labelled airtight plastic containers. These samples were transported daily to the Parasitology Laboratory, National Veterinary Research Institute, Vom for analyses. Blood samples were examined for the presence of hemoparasites [12], and the packed cell volume (PCV) was determined using a hematocrit reader (Hawksley), and buffy coat was examined for motile parasites [16]. Faecal samples were processed by the simple floatation and formaldehyde-ether sedimentation methods [7, 8]. Identification of parasite egg was done according to Cole [4] and Soulsby [14]. Data obtained were analyzed using OpenEpi software [5]. χ2 test and confidence interval (CI) limits was used to test for significance. p-values less than 0.05 were considered significant.
Fifty-seven (23.5%) of the 243 horses examined had infection with hemoparasites, endoparasites and coccidia (Table 1). The prevalence of hemoparasites was 21 (8.6%), endoparasites was 29 (11.9%) and that of coccidia was 3 (1.2%), except the mixed infection with hemoparasites and endoparasites 4 (1.6%). Highest prevalence was recorded in horses from traditional stables 31 (75.6%) while the institutional and private stables had prevalence of 21 (14.6%) and 5 (8.6%) parasitic infection, respectively. This was statistically significant (p<0.05). Table 2 shows the prevalence of parasites of horses in relation to breed and sex. Parasites detected in 52 (28%) of the local/indigenous horses examined (n=185) was significantly higher (p<0.05) compared to 5 (8.6%) detected in the exotic breed (n=58). The difference between parasites detected in male, 33 (16.8%) and female, 24 (51.1%) was statistically significant (p<0.05). The equine parasites detected and their effects on the mean PCV are shown in Table 3. Hemoparasites detected includes Theileria equi (n=10), Babesia caballi (n=6), Trypanosoma vivax (n=1) and Trypanosoma evansi (n=4). Strongylus spp. (n=8), Strongyloides spp. (n=4), Parascaris equorum (n=7), Oxyuris equi (n=6), Paragonimus spp. (n=2), Dicrocoelium spp. (n=2) and Eimeria spp. (n=3) were the endoparasites and coccidia detected from faecal analysis. A few horses (n=4) had mixed infection (hemoparasites and endoparasites). The packed cell volume of the horses ranged from 18–52%. The mean PCV of uninfected horses (n=190) was 46% ± 5.5. The lowest mean PCV (24%) was detected in horses infected with T. vivax including mixed infection with Strongylus spp. Horses infected with Eimeria spp. and Dicrocoelium spp. had PCV comparable with the uninfected horses. The mean PCV of horses infected with hemoparasites and P. equorum was significantly lower (p<0.05) compared with the mean PCV of uninfected horses.
Table 1. Prevalence of parasitic infections in horses in northern Nigeria (Percentages of positive were shown in parentheses).
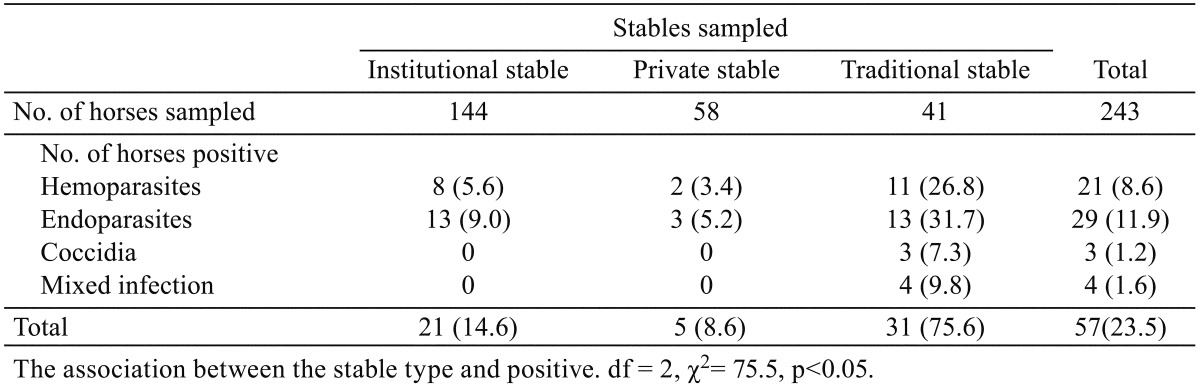
Table 2. Prevalence of parasites of horses in relation to breed and sex of horses in northern Nigeria.
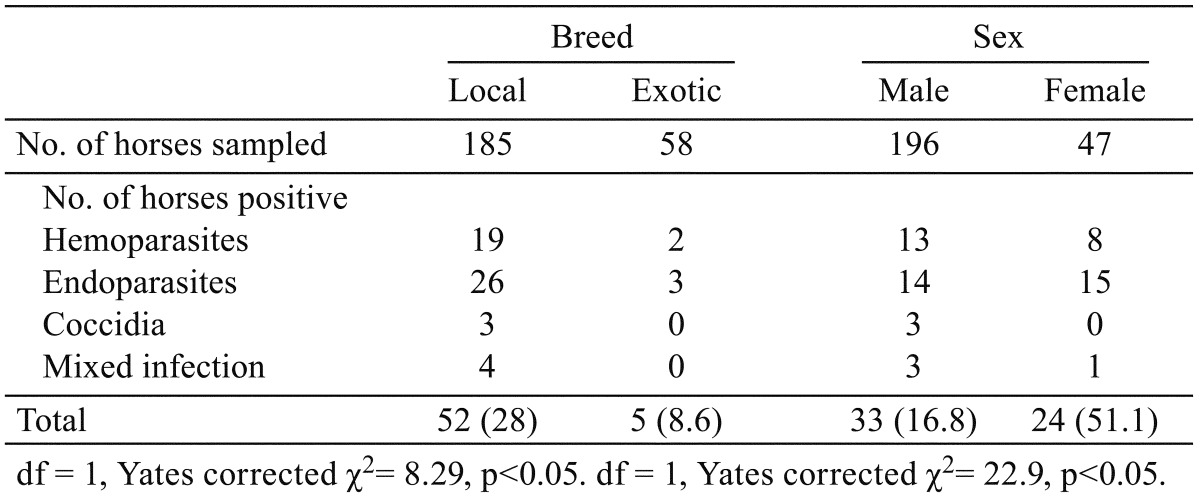
Table 3. Parasites detected in horses and their effects on mean packed cell volume (PCV).
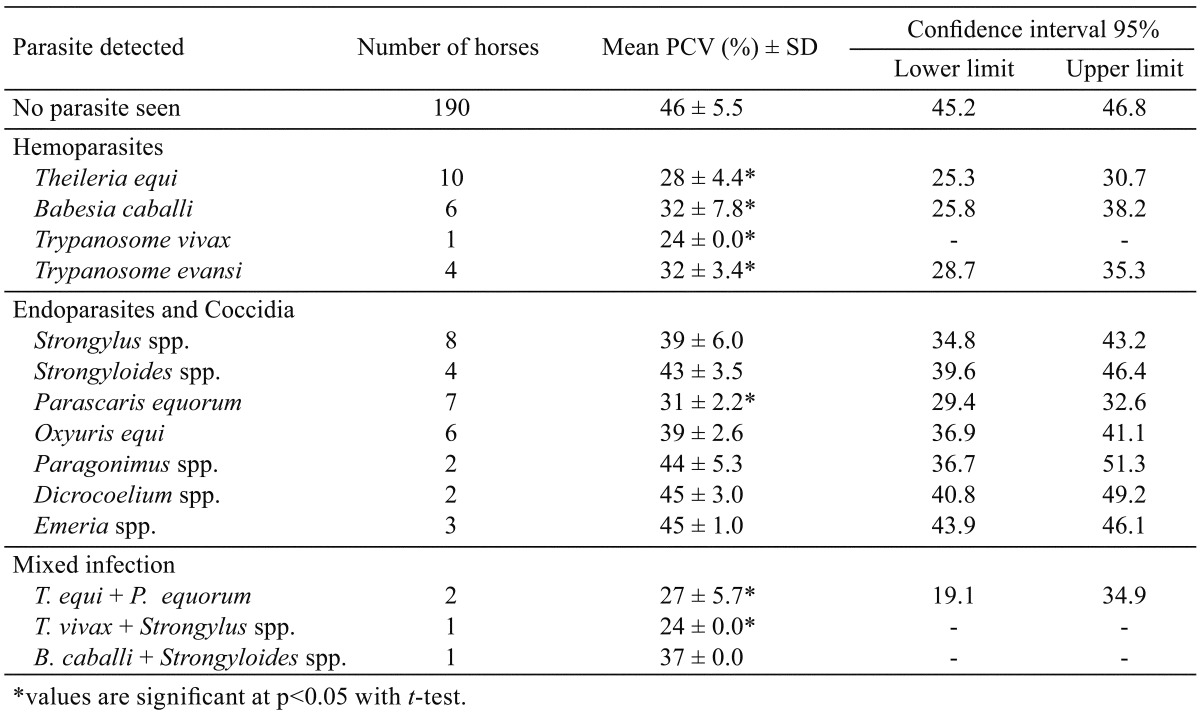
Parasites can cause irreparable internal damages. The nature and extent of damage varies with the type of parasite [9]. Despite the attention given to horse management in northern Nigeria compared to domestic livestocks, our finding shows that parasitism is a problem in the horse stables examined. From our investigation on management system practiced in the stables examined we conclude that stable management system appears to play a role in the prevalence of parasitism. The finding that endoparasites is most prevalent (11.9%) in this study is consistent with previous report [15]. The low prevalence observed in exotic breed compared to local breed of horses may also be related to management practices which often are better in stables housing exotic breeds. However, the significant difference in the prevalence in sex observed in this study cannot be explained. Hemoparasites had significant effect on the PCV of infected horses. The favourable environmental conditions for the survival and transmission dynamics of the vectors (tick and tsetse-fly) could influence the burden and severity of hemoparasitic infections. Infection with T. equi appeared to produce severe effect on mean PCV than B. caballi as corroborated by Soulsby [14]. T. vivax appears less common, but had marked reduction in the mean PCV (24%) compared with uninfected horses (46%). This agrees with the findings of previous investigators [10, 11]. Furthermore, we observed that endoparasites does not result in severe reduction of the mean PCV of infected horses, but in a mixed infection with hemoparasite, anemia may be seen. Though more prevalent, endoparasitic infection alone may not constitute a serious threat to horses compared to hemoparasitic infection. This preliminary finding adds to our understanding of parasitic infection in horses in northern Nigeria and calls for the improvement in the management of horses, particularly under the traditional set-up. Considering the importance of horses in the area studied, routine tick control and deworming programmes using efficacious acaricides and anthelmintic is recommended to reduce the menace of parasitic infection and infestation in horse stables.
Acknowledgements
The authors sincerely appreciate the stable attendants for their support and the technical assistance of the staff in the Parasitology Department, National Veterinary Research Institute, Vom, Nigeria.
References
- 1.Ademola I.O., Fagbemi B.O., Idowu S.O.2004. Evaluation of the anthelmintic activity of Khaya senegalensis extract against gastrointestinal nematodes of sheep: in-vtro and in-vivo studies. Vet. Parasitol. 122: 151–164 [DOI] [PubMed] [Google Scholar]
- 2.Bukar M.M., Sadiq M.A., Geidam Y.A.2007. A survey of cutaneous neoplasms among horses used for cultural festivals in Borno state, Nigeria. Niger. Vet. J. 28: 27–33 [Google Scholar]
- 3.Charles L.S., Clare H.P.2003. Internal parasite of horses, V453 http://www.ag.ndsu.nodak.edu Accessed June 13, 2011
- 4.Coles E.H.1986. Veterinary Clinical Pathology, 4th ed. pp. 374–400. W.B.Saunders, Comp. [Google Scholar]
- 5.Dean A.G., Sullivan K.M., Soe M.M.2010. OpenEpi: open source epidemiologic statistics for public health, version 2.3.1. http://www.openepi.com Accessed September 27, 2011
- 6.Ehizibolo D.O., Gusi A.M., Ehizibolo P.O., Mbuk E.U., Ocholi R.A.2011. Serologic prevalence of brucellosis in horse stables in two northern states of Nigeria. J. Equine Sci. 22: 17–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmer R.N., Glenn A.N.1982. Parasitology: the biology of animal parasites, 5th ed. pp. 127–211. Lea and Fediger, Philadelphia. [Google Scholar]
- 8.Franklin A.N., Harold W.B.1994. Basic Clinical Parasitology, 6th ed. pp. 317–343. Prentice Hall International (UK) limited, London. [Google Scholar]
- 9.Front Range Frenzy Home2011. Basic horse care: parasites of the horse. http://www.frontrangefrenzy.com/horseparasites Accessed June 13, 2011.
- 10.Friedhof K.T., Tenter A.M., Muller I.1990. Haemoparasites of equines: impact on international trade of horses. Rev. Sci. Tech. 9: 1187–1194 [PubMed] [Google Scholar]
- 11.Hutchens D.E., Paul A.J., Dipietro J.A.1999. Treatment and control of gastrointestinal parasites. pp. 561–571. In: Veterinary Clinics of North America, Equine Practice, Vol.155 (Witthem, T. ed.), W.B. Saunders, Philadelphia. [DOI] [PubMed]
- 12.Saal J.R.1964. Giemsa stain for the diagnosis of bovine babesiosis. II. Changes in erythrocytes infected with Babesia bigemina and B. Argentina. J. Protozool. 11: 582–585 [DOI] [PubMed] [Google Scholar]
- 13.Saror D.I.1976. Haematological values in Nigerian part-arab Stallions. Vet. Rec. 99: 397–398 [DOI] [PubMed] [Google Scholar]
- 14.Soulsby E.J.L.1982. Helminths arthropods and protozoa of domesticated animals, 7th ed. p. 771. Bailliere Tindall, London. [Google Scholar]
- 15.Useh N.M., Oladele S.B., Ibrahim N.D., Nok A.J., Esievo K.A.2005. Prevalence of equine diseases in the northern Guinea Savannah of Zaria, Nigeria. J. Equine Sci. 16: 27–28 [Google Scholar]
- 16.Woo P.T.1971. Evaluation of the heamatocrit centrifuge and other techniques for the field diagnosis of human trypanosomiasis and filariasis. Acta Trop. 28: 298–303 [PubMed] [Google Scholar]


