Abstract
In vitro cell studies might be a useful tool for studying tendon pathology, but no suitable in vitro models exist for tendon disorders. The purpose of this study was to confirm whether cell scratch culture using tendon-derived fibroblasts can provide a suitable in vitro tendon disorder model. Extracellular matrix components were examined immunohistochemically in tendon tissue, and then their related gene expression levels were analyzed by conventional reverse transcription polymerase chain reaction (RT-PCR) and/or quantitative real-time RT-PCR in tissues and cells. Collagen type I (Col I), collagen type III (Col III), tenascin-C (TN-C) and cartilage oligomeric matrix protein (COMP) were detected in tendon tissue sections, and RT-PCR confirmed their expression in tendon tissue and cells. Cells that had been cultured from explanted tendon tissue maintained the characteristics of in vivo tendon cells. The combination of TN-C and COMP might be a useful marker of tendon cells because they display more tendon-specific expression than Col I and III. In particular, the significant increase of TN-C mRNA expression in the scratch wound assay, at 12 hr after scratching, concomitant with the regeneration of the cell sheet, indicates its crucial role in tendon cell proliferation and migration. Thus, TN-C appears to be a key factor in tendon wound healing. In vitro cell scratch assays using tendon cells appear to mimic the repair of tendon tissue after injury.
Keywords: cartilage oligomeric matrix protein, collagen, equine tendon, scratch wound assay, tenascin-C
Tendons are an important tissue in equines; however, they are fragile, and racehorses often suffer tendon problems. Recovering from injury is a key part of life for racehorses; however, there is limited information about equine recovery from tendon disorders. Although various tendon components, such as collagens, tenascin-C (TN-C), cartilage oligomeric matrix protein (COMP), scleraxis and other matrix components, have been determined [3, 6, 19, 27], information about the many recovery processes of equine tendonitis is very limited. In vitro cell culture systems provide a convenient tool for studying the functions of cells in specific tissues or organs, and the specificity of equine tendon cell lines has been examined [10, 11, 26]. About 60–68% of the dry weight of tendons is collagen [15], and collagen type I (Col I) arranged in tensile-resistant fiber comprises about 60% of the total collagen content. Collagen type III (Col III), which makes up less than 10%, is the second largest component of tendon tissue [15]. In addition, other components, such as TN-C and COMP, have been reported to be tendon-specific [4, 7, 12, 24]. TN-C is a disulfide-linked hexamer glycoprotein with subunits that range from 190 to 300 kDa in size [1]. It has been suggested to play a crucial role in embryogenesis, wound healing, tumorigenesis, and the loading of tendon-like tissues during physical exercise [1, 12, 13]. COMP was initially found in cartilage [9] and has subsequently been isolated from tendons [4, 24]. It is a pentameric non-collagenous glycoprotein that belongs to the thrombospondin gene family of extracellular calcium-binding proteins [9, 17, 20]. It is suggested to be involved in the regulation of tendon formation [24].
The purpose of this study was to assess changes in the expression profiles of Col I, Col III, TN-C and COMP following cell damage as a preliminary in vitro model of tendon disorders. Prior to the main study, the general characteristics of the equine tendon were reconfirmed by gene expression profiling and immunohistochemistry.
Materials and Methods
Animals and tissue collection
Superficial digital flexor tendon and other tissues were collected from 5 Thoroughbred horses at the time of their slaughter for pathological examination (6.4 ± 4.9 years old, mean ± SD). No significant clinical tendonitis was observed in any of the horses. The tendons were collected, and the middle portion was used for molecular biological, immunohistochemical, and cell culture studies. Connective tissues (subdermic), skeletal muscle, patella from the anterior half of the body, myocardium, the atrioventricular valve, and liver were simultaneously collected for the evaluation of gene expression. All animal experimental procedures were approved by the Animal Care and Use Committee of Iwate University.
Immunohistochemical analysis of equine tendons
To immunohistochemically detect Col I, Col III and COMP, the tendon was immediately embedded into optimal cutting temperature (O.C.T.) compound (Tissue-Tek, SAKURA, Tokyo, Japan), frozen in liquid nitrogen and stored at –80°C until it was processed. The embedded tissues were serially cryosectioned at 7 µm and mounted on glass slides. To immunohistochemically detect TN-C, the tendon was embedded in paraffin after being fixed with formalin. The embedded tissues were serially sectioned at 5 µm. Endogenous peroxidase activity was blocked with methanol containing 0.3% H2O2 for 20 min at room temperature. After the sections had been washed with phosphate-buffered saline (PBS), they were incubated for 30 min with 2% BSA, and then immunolabeled with a panel of monoclonal antibodies to Col I, Col III, TN-C and COMP. Full details of the antibodies are provided in Table 1, together with the pretreatment procedures. After incubation, the sections were washed with PBS and treated with biotinylated antibody to mouse (Vector Laboratories, Burlingame, CA, USA) or rat IgG (Jackson ImmunoResearch, West Grove, PA, USA), and the avidin-biotin-peroxidase complex (Elite ABC kit, Vector Laboratories), for 30 min each at room temperature. The immunoreactive sites were visualized by incubation with Tris-HCl buffer (0.15 M NaCl, 50 mM Tris-HCl, pH 7.5) containing 0.02% 3, 3’-diaminobenzidine. They were then lightly counterstained with hematoxylin, dehydrated in ethyl alcohol, cleared with xylene and sealed with coverslips. Tendon-derived cells cultured in vitro were stained with the abovementioned anti-TN-C antibody without counterstaining. Other cultures of these cells were also stained with anti-vimentin antibody (1:1,000, Vector Laboratories) and detected with FITC-labeled anti-mouse antibody (Jackson ImmunoResearch) under fluorescence microscopy (Nikon ECLIPSE, Tokyo, Japan).
Table 1. Primary antibodies used in the present study.
| Antibody against | Clone number | Host | Pretreatment | Dilution | Source |
|---|---|---|---|---|---|
| Collagen type I | COL-1 | Mouse | 0.1% Trypsin, 5 min | 1:1,000 | A |
| Collagen type III | FH-7A | Mouse | 0.1% Trypsin, 5 min | 1:2,000 | A |
| Tenascin-C | 578 | Rat | Citric acid buffer, microwave, 5 min | 1:50 | B |
| COMP | MA37C94 | Rat | 0.1% Trypsin, 5 min | 1:20 | C |
A: Sigma-Aldrich (Sigma, St. Louis, MO, USA), B: RD Systems (Minneapolis, MN, USA), C: Serotec (Oxford, UK).
Explant cell cultures
Tendon cells were prepared from an explant culture of superficial digital flexor tendon tissue. The tendon was cut into small pieces (5 × 5 × 5 mm) and treated with 0.1% type I collagenase (Worthington Biochemical Corporation, Lakewood, NJ, USA) at 37°C for 20 min to facilitate the release of cells from the matrix components. The tissue pieces were then placed in culture dishes and cultured in Dulbecco’s modified Eagle medium (GIBCO, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (Moregate Biotech, Bulimba, Australia), 100 U/ml penicillin G and 100 µg/ml streptomycin solution (Sigma-Aldrich, St. Louis, MO, USA) in an atmosphere of humidified air (5% CO2) at 37°C. After reaching a sub-confluent state, the primary cultures were dispersed with 0.1% trypsin in PBS and sub-cultured in the abovementioned medium. Some subcultured cells were subjected to immunohistochemical examination and scratch wound assay. Cells were cultured close to confluence (normally takes 3–4 days) in 12-well plates (Sanplatec, Tokyo, Japan), and fixed with Zamboni solution (3.6 g NaH2PO4/2H2O, 27.5 g NaHPO4, 40 g paraformaldehyde, 5 g picric acid/l) for 30 min. After washing with PBS, the cells were incubated with normal donkey sera to block non-specific reactions. They were then subjected to immunohistochemical examination.
RNA isolation and conventional reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from various equine tissues and cultured tendon cells using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse transcription (RT) PCR was carried out as described previously [21]. The PCR amplification was performed with AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA). After the reaction, products were analyzed by electrophoresis in 1.5% agarose gels with ethidium bromide staining. The primers used for our RT-PCR experiments are listed in Table 2.
Table 2. Sequences of the RT-PCR primer sets used in this study.
| Primer | Sequence | Product size (bp) |
|---|---|---|
| Collagen I alpha I | 5’-ACCTGCATACAGGACGGCCTC-3’
(Forward) 5’-GCCAGGCACGGAAATTCCAGC-3’ (Reverse) |
420 |
| Collagen III alpha I | 5’-CACAAAGAGTCTCATGTCTGA-3’
(Forward) 5’-GGTCACCATTTCTCCCAGGA-3’ (Reverse) |
397 |
| Tenascin-C | 5’-GGACAATGAGATGCGGGTCAC-3’
(Forward) 5’-GTCTGTGACATCTTTCACCTC-3’ (Reverse) |
520 |
| COMP | 5’-GACAGTCGGGACAACTGCCG-3’
(Forward) 5’-CCAGCTCAGGGCCCTCATAG-3’ (Reverse) |
682 |
| GAPDH | 5’-GACCACAGTCCATGCCATCAC-3’
(Forward) 5’-GTCCACCACCCTGTTGCTGTA-3’ (Reverse) |
454 |
Real-time RT-PCR
Total RNA (100 ng) was reverse transcribed into cDNA and used for real-time RT-PCR, as described previously [14]. The primers were designed using Primer Express software, version 3.0 (Applied Biosystems) according to the manufacturer’s instructions, and the primers for 18s rRNA were purchased from Applied Biosystems. Real-time RT-PCR detection was performed using an ABI PRISM 7300 sequence detector with the bundled software (version 1.3; Applied Biosystems). The reaction mixture was dispensed into a 96-well plate and amplified. The thermal cycling conditions included 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Standard curves were generated for each gene via serial dilution of plasmids containing Col I, Col III, TN-C or COMP cDNA, and used to quantify mRNA concentrations. The ratio of each mRNA to 18s rRNA was calculated to adjust for any variations in the RT-PCR reaction.(Table 3)
Table 3. Sequences of the real-time RT-PCR primer sets used in this study.
| Primer | Sequence | Product size (bp) |
|---|---|---|
| Collagen I alpha I | 5’-TGGCCTCGGAGGAAACTTT-3’
(Forward) 5’-GCACGGAAATTCCAGCAAAT-3’ (Reverse) |
71 |
| Collagen III alpha I | 5’-CCAGAGATTCCATTTGGAGAATG-3’
(Forward) 5’-AGGGCGAGTAGGAGCTGTTG-3’ (Reverse) |
66 |
| Tenascin-C | 5’-CGGCCTGGAAATGCAGTT-3’
(Forward) 5’-CCTGGATGATGGTGGATGTCT-3’ (Reverse) |
56 |
| COMP | 5’-GAGATCGTGCAAACAATGAACAG-3’
(Forward) 5’-TGGAACGTGCCTTCGAAGTC-3’ (Reverse) |
86 |
Scratch wound assay
Tendon cells were cultured until they were approximately 90% confluent. Then, the cell sheets were scratched with blue pipette tips (volume: 1,000 µl, the sharp end measured about 1.0 mm in width). Five horizontal scratches were made, then the cell sheets were washed with PBS [25]. After 0, 12, 24, 48 and 78 hr of continuous culture, total RNA was extracted and used to analyze the gene expression profiles of the cells.
Statistical analysis
Statistical comparisons among gene expression levels of each culture period were carried out using Dunnett’s method. All data are presented as mean ± SD of triplicate cultures. P values less than 0.05 were considered significant.
Results
Immunolocalization of Col I, Col III, TN-C and COMP protein in the equine tendon
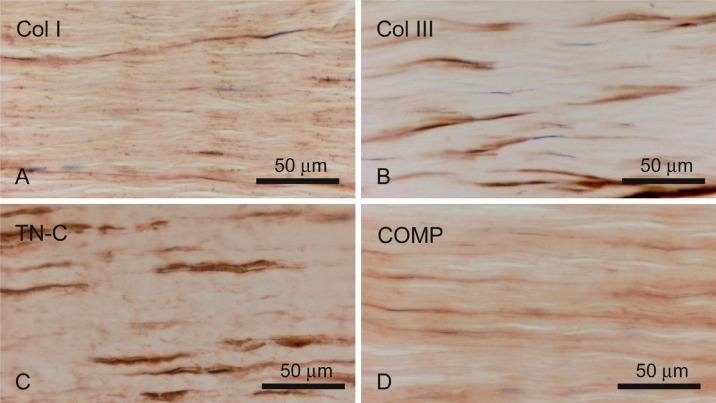
Col I was detected weakly in whole tendon sections, and intense expression was found in the collagen fibrils within the tendon. In addition, Col III was mainly detected in the endotendon together with Col I. However, no collagen protein was detected in the tendon cells. TN-C was localized at the interface of the tendon cells and collagen fibers. COMP was exhibited throughout the tendon, with stronger staining detected in the interfibrillar regions (Fig. 1). In cultured cells, only TN-C was found positively within the hill area but its intensity was rather weak (Fig. 2). These cells, which had a fibroblast-like shape, were clearly stained with anti-vimentin antibody.
Fig. 1.
Immunohistochemical detection of matrix proteins. Immunoreactivity of Col I (A), Col III (B), TN-C (C) and COMP (D) in longitudinal sections of the equine tendon.
Fig. 2.
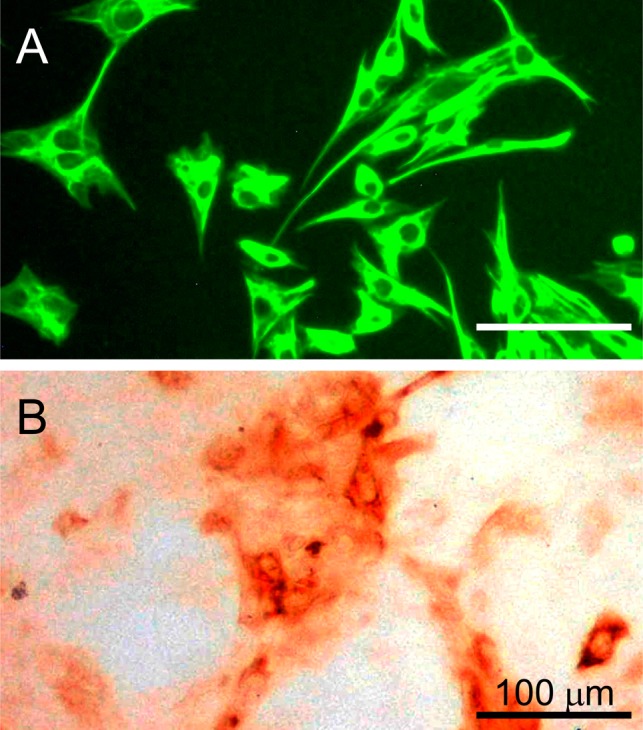
Vimentin and TN-C immunoreactivity in cultured equine tendon cells. A: Vimentin staining, B: TN-C staining. Different specimens were used independently for each staining method; the same magnification was used in all cases.
Col I, Col III, TN-C and COMP mRNA expressions in tendons and cultured cells
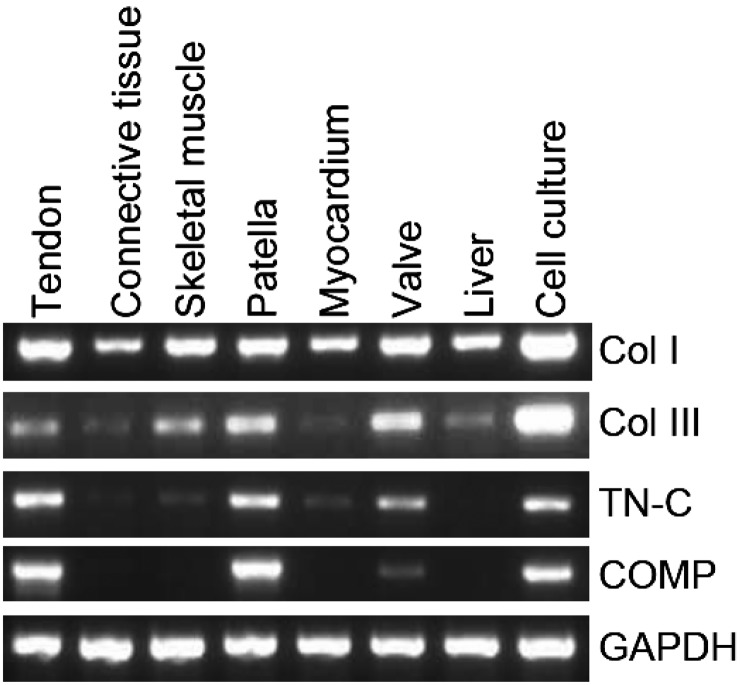
Although conventional RT-PCR (Fig. 3) detected Col I and III mRNA in all examined tissues, the intensity of Col III mRNA expression depended on the tissue: the tendon, skeletal muscle, patella and atrioventricular valve showed higher expression, while lower expression was detected in connective tissue, the myocardium and liver. The cultured tendon cells markedly expressed Col I and III mRNA. TN-C mRNA was also detected in all of the examined tissues, except the liver, but its intensity was particularly stable in the tendon, patella and atrioventricular valve. COMP mRNA was expressed in a more specific pattern than the other molecules: it was found only in the tendon, patella, atrioventricular valve and cultured tendon cells.
Fig. 3.
Analysis of TN-C expression in various tissues. RNA was extracted from the tendon, connective tissue, skeletal muscle, patella, ventricular myocardium, atrioventricular valve, and liver. The primer sets used for the RT-PCR are described in Table 2.
Effects of scratching on the gene expression profiles of cultured tendon cells
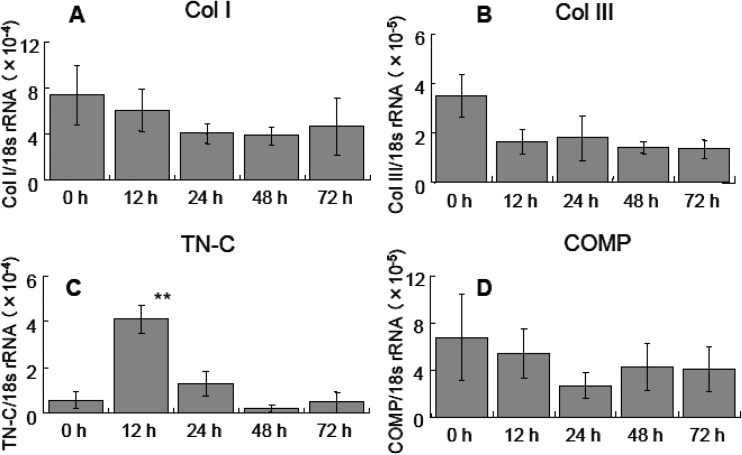
We investigated the changes in the expression profiles of the abovementioned genes in a scratch wound assay involving cultured tendon-derived cells. The cell monolayer was mechanically scratched after reaching 90% confluence, after which the cells gradually proliferated and migrated into the space made by the scratch. The cell sheets almost recovered their confluent status in 7 days. During this process, the mRNA expression levels of Col I, Col III and COMP did not change significantly. However, TN-C mRNA expression was significantly upregulated after 12 hr (Fig. 4).
Fig. 4.
Quantitative expression of Col I, Col III, TN-C, and COMP mRNA in scratch wound healing assay. Col I, Col III and COMP did not display significant increases in their expression at any time point, but TN-C expression was significantly increased at 12 hr compared with that observed at 0 hr (control). The data are the averages of triplicate experiments. ** P<0.01, n=3, mean ± SD.
Discussion
Immunochemical analysis demonstrated that the collagen fibrils within the examined tendons contained Col I. Thus, Col I might contribute to tendon strength. COMP was also found throughout the tendon, although its expression was higher in the interfibrillar regions. This result agrees with the findings of a previous report [24]. It is known that COMP binds to Col I [22] and is involved in tensile stress [5], because COMP plays an important role in the precise establishment of tendon structure. We also detected the co-distribution of Col III and Col I, as was observed in a previous study [3]. Col I participates in tension development, whereas Col III expression is increased at tendon rupture sites [2, 8]. Therefore, Col III could be a fundamental factor in the recovery process following tendon injuries and during the remodeling of tendon structures during tendonitis.
In this study, the TN-C protein was detected at the interface between tendon cells and collagen fibers. This was consistent with the results of a previous study of rats [12], and TN-C may bind to the tendon cell membrane as reported [1]. The expression of TN-C is regulated by mechanical loading, and TN-C provides elasticity for the tendons during heavy tensile loading [12]. In the present study, cultured cells derived from tendon explants maintained the characteristics of in vivo tendon cells; i.e., they produced the TN-C protein and expressed Col I, III, TN-C and COMP mRNA in vitro. The combination of TN-C and COMP might be useful as a specific marker of tendon cells because they display more tendon-specific expression than Col I and Col III. The specificity of these molecules was supported by their mRNA expression: they were found in tendon tissue and cultured cells, but were not detected in connective tissue and the liver. In vitro cultured cells had fibroblast-like shape and morphology, and expressed the TN-C protein but not the COMP protein. These in vitro cultured cells expressed both the TN-C and COMP genes. A previous study showed that NIH3T3 fibroblast cells display characteristics different from tendon cells [23], and COMP mRNA expression was confirmed in the present study. These results suggest that the tandem expression of TN-C and COMP could be a reliable marker of tendon cells.
The scratch wound assay is a bioassay method in which cell populations are damaged by scratching and then have their subsequent restoration process monitored. In the present study, TN-C expression increased around 12 hr after scratching, and cells from both sides of the scratch migrated into the damaged area. Positive TN-C immunoreactivity was mainly found in the hill area of the cell clump, indicating that TN-C may be expressed in actively proliferating and migrating cells. TN-C may play a crucial role during the recovery period by enhancing cell migration and proliferation. In a previous report, TN-C protein secretion peaked at 12 hr after scratching in a scratch wound assay involving cultured astrocytes [18]. Similar cell behavior and response for TN-C were found in current and previous studies using different cells [16]. These results suggest that scratch wound assays are a feasible model of tendon tissue repair after injury, and that the in vitro model used in the present study mimics the status of in vivo tendon injury. The expression levels of Col I, Col III and COMP were not significantly altered in the scratching assay. Under in vitro conditions, Col III and COMP expression levels were stable and prominent in cultured tendon-derived cells compared to those in tendon tissue, but decreased to half the original level after scratching. The expression of Col I, Col III and/or COMP may not be correlated with cell proliferation and migration. Although tendon cells may produce these proteins during the acute processes of tissue remodeling and recovery, cells may first have to migrate and proliferate with the production of matrix metalloproteinases [28]. Therefore, the production of these proteins is lower in the early period of the recovery process as shown in the scratch wound assay. During the early period of recovery following tendon injuries, the expression of TN-C increased rapidly. Therefore, the increase in the expression of this gene and protein before producing matrix proteins and related enzymes may be a necessary process in the remodeling of tissues and recovery from injuries. Although a more detailed examination is necessary to clarify the roles of TN-C during tendon recovery, TN-C may be involved in the recovery process after tendon injury, as previously reported [26].
In conclusion, 1) mRNA and protein expression of Col I, Col III, TN-C and COMP were detected in horse tendon tissue; 2) the combined detection of TN-C and COMP specifically identifies tendon cells; and 3) the scratched cell sheet method may supply an excellent model for the study of tendon injury and healing.
Acknowledgments
We thank Professor Masanobu Goryo and the staff of the Laboratory of Veterinary Pathology, Iwate University, for helping with equine tissue collection.
References
- 1.Chiquet-Ehrismann R., Tucker R.P.2004. Connective tissues: signalling by tenascins. Int. J. Biochem. Cell Biol. 36: 1085–1089 [DOI] [PubMed] [Google Scholar]
- 2.Dahlgren L.A., Brower-Toland B.D., Nixon A.J.2005. Cloning and expression of type III collagen in normal and injured tendons of horses. Am. J. Vet. Res. 66: 266–270 [DOI] [PubMed] [Google Scholar]
- 3.Dahlgren L.A., Mohammed H.O., Nixon A.J.2005. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J. Orthop. Res. 23: 84–92 [DOI] [PubMed] [Google Scholar]
- 4.DiCesare P., Hauser N., Lehman D., Pasumarti S., Paulsson M.1994. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 354: 237–240 [DOI] [PubMed] [Google Scholar]
- 5.Dowling B.A., Dart A.J.2005. Mechanical and functional properties of the equine superficial digital flexor tendon. Vet. J. 170: 184–192 [DOI] [PubMed] [Google Scholar]
- 6.Dowling B.A., Dart A.J., Hodgson D.R., Smith R.K.2000. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 32: 369–378 [DOI] [PubMed] [Google Scholar]
- 7.Edom-Vovard F., Schuler B., Bonnin M.A., Teillet M.A., Duprez D.2002. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247: 351–366 [DOI] [PubMed] [Google Scholar]
- 8.Eriksen H.A., Pajala A., Leppilahti J., Risteli J.2002. Increased content of type III collagen at the rupture site of human Achilles tendon. J. Orthop. Res. 20: 1352–1357 [DOI] [PubMed] [Google Scholar]
- 9.Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosa-Pimentel E., Sommarin Y., Wendel M., Oldberg Å., Heinegård D.1992. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 267: 6132–6136 [PubMed] [Google Scholar]
- 10.Hosaka Y.Z., Takahashi H., Uratsuji T., Tangkawattana P., Ueda H., Takehana K.2010. Comparative study of the characteristics and properties of tendinocytes derived from three tendons in the equine forelimb. Tissue Cell 42: 9–17 [DOI] [PubMed] [Google Scholar]
- 11.Hosaka Y.Z., Uratsuji T., Ueda H., Uehara M., Takehana K.2010. Comparative study of the properties of tendinocytes derived from three different sites in the equine superficial digital flexor tendon. Biomed. Res. 31: 35–44 [DOI] [PubMed] [Google Scholar]
- 12.Järvinen T.A., Józsa L., Kannus P., Järvinen T.L., Hurme T., Kvist M., Pelto-Huikko M., Kalimo H., Järvinen M.2003. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J. Cell Sci. 116: 857–866 [DOI] [PubMed] [Google Scholar]
- 13.Jones F.S., Jones P.L.2000. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev. Dyn. 218: 235–259 [DOI] [PubMed] [Google Scholar]
- 14.Kizaki K., Nakano H., Takahashi T., Imai K., Hashizume K.2001. Expression of heparanase mRNA in bovine placenta during gestation. Reproduction 121: 573–580 [PubMed] [Google Scholar]
- 15.Kjaer M.2004. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84: 649–698 [DOI] [PubMed] [Google Scholar]
- 16.McKean D.M., Sisbarro L., Ilic D., Kaplan-Alburquerque N., Nemenoff R., Weiser-Evans M., Kern M.J., Jones P.L.2003. FAK induces expression of Prx1 to promote tenascin-C-dependent fibroblast migration. J. Cell Biol. 161: 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mörgelin M., Heinegård D., Engel J., Paulsson M.1992. Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J. Biol. Chem. 267: 6137–6141 [PubMed] [Google Scholar]
- 18.Nishio T., Kawaguchi S., Yamamoto M., Iseda T., Kawasaki T., Hase T.2005. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience 132: 87–102 [DOI] [PubMed] [Google Scholar]
- 19.Oikawa M., Kasashima Y.2002. The Japanese experience with tendonitis in racehorses. J. Equine Sci. 13: 41–56 [Google Scholar]
- 20.Oldberg Å., Antonsson P., Lindblom K., Heinegård D.1992. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J. Biol. Chem. 267: 22346–22350 [PubMed] [Google Scholar]
- 21.Oonuma T., Morimatsu M., Ochiai K., Syuto B.2003. Properties of the tumor suppressor gene brca2 in the cat. J. Vet. Med. Sci. 65: 1123–1126 [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg K., Olsson H., Morgelin M., Heinegard D.1998. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 273: 20397–20403 [DOI] [PubMed] [Google Scholar]
- 23.Salingcarnboriboon R., Yoshitake H., Tsuji K., Obinata M., Amagasa T., Nifuji A., Noda M.2003. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp. Cell Res. 287: 289–300 [DOI] [PubMed] [Google Scholar]
- 24.Smith R.K., Zunino L., Webbon P.M., Heinegard D.1997. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 16: 255–271 [DOI] [PubMed] [Google Scholar]
- 25.Stoll S.W., Kansra S., Elder J.T.2003. Keratinocyte outgrowth from human skin explant cultures is dependent upon p38 signaling. Wound Repair Regen. 11: 346–353 [DOI] [PubMed] [Google Scholar]
- 26.Taylor S.E., Vaughan-Thomas A., Clements D.N., Pinchbeck G., Macrory L.C., Smith R.K., Clegg P.D.2009. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet. Disord. 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.H.2006. Mechanobiology of tendon. J. Biomech. 39: 1563–1582 [DOI] [PubMed] [Google Scholar]
- 28.Xue M., Smith M.M., Little C.B., Sambrook P., March L., Jackson C.J.2009. Activated protein C mediates a healing phenotype in cultured tenocytes. J. Cell. Mol. Med. 13: 749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]