Summary
PSMs are a recently discovered family of short, amphipathic, α-helical peptides in staphylococci. Several PSMs are key virulence determinants, particularly in highly virulent Staphylococcus aureus strains. PSMα peptides of S. aureus facilitate neutrophil lysis after phagocytosis, and are key contributors to several infection types, including skin infection and bacteremia. Furthermore, all PSMs contribute to biofilm structuring and the dissemination of biofilm-associated infection. Cytolytic PSMs as produced by S. aureus appear to have evolved from original functions in the non-infectious lifestyle of staphylococci. The surfactant properties of PSMs, which they all share, are believed to facilitate growth on epithelial surfaces. The basic role of PSMs in staphylococcal physiology is underscored, for example, by their exceptionally strict and direct control by quorum-sensing and the presence of a dedicated secretion system. Targeting PSMs for anti-staphylococcal drug development may be a promising approach to overcome the problems associated with widespread antibiotic resistance in staphylococci.
Keywords: Phenol-soluble modulins, Staphylococcus aureus, Staphylococcus epidermidis, cytolysis, toxins, leukotoxin, neutrophils, biofilm
Introduction
Phenol-soluble modulins (PSMs), a family of amphipathic, α-helical peptides found in staphylococci, have recently drawn much attention owing to the key contribution of some PSM peptides to staphylococcal virulence, in particular in highly virulent Staphylococcus aureus (Wang et al., 2007). However, it is often overlooked that only some PSMs have aggressive properties that make them virulence determinants. Many PSMs, especially those found in less pathogenic species, appear to have different roles in staphylococcal physiology (Periasamy et al., 2012a), which is why in order to understand the evolution of PSMs, it is crucial to consider both commensal and pathogenic species, or lifestyles, of staphylococci.
Staphylococci are commensals on the epithelia of humans and mammals (Kloos and Musselwhite, 1975). While there is a certain specification among staphylococci regarding which niches on the human body they prefer, all have to deal with the challenges that colonization of surfaces with frequently changing environmental conditions entails. In addition to having to be able to endure high osmotic stress and changing temperatures, staphylococci have to live and acquire nutrients in an environment that contains a high number of hydrophobic molecules, such as lipids and waxes. This is especially true for life in and around sebaceous glands or hair follicles, which are preferred niches for many staphylococci (Grice et al., 2009). There are reports on staphylococci expressing enzymes that detoxify harmful fatty acids; and many staphylococci secrete lipases, which may have a function in degrading lipids for nutrient acquisition (Otto, 2004). However, how staphylococci manage to live in such an environment with the specific challenges regarding aqueous/oily interfaces is poorly understood.
For many opportunistic pathogens among the staphylococci, such as S. epidermidis and others, infection may be regarded as an “accident” rather than a program (Otto, 2009). Many molecules that these species produce may rise to additional benefit during infection, but judging from the fact that they usually have additional, or rather original, roles in their commensal lifestyles, they appear not to have evolved for a role in pathogenesis. Examples are the polyglutamate capsule of S. epidermidis (Kocianova et al., 2005) or surface proteins needed to attach to epithelial surfaces (Bowden et al., 2005). In contrast, S. aureus produces a large series of molecules whose production is directly related to infection (Foster, 2005; Kim et al., 2012; Lowy, 1998). Most of these subvert mechanisms of host defense. It is poorly understood why S. aureus is largely immune to elimination by antibody-mediated mechanism of the acquired immune system. In contrast, many mechanisms are known by which S. aureus escapes the innate immune system, including complement and phagocytosis by leukocytes (Rooijakkers et al., 2005).
Here, the genetics, biochemistry, and roles of PSMs in the commensal and infectious lifestyles of staphylococci will be reviewed. It will also be discussed whether and how PSMs could be targeted for anti-staphylococcal drug development.
PSMs are widespread in staphylococci
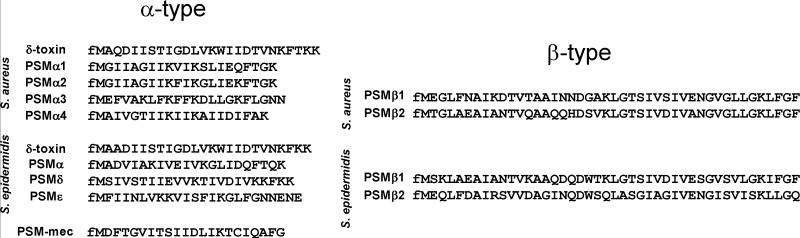
The term “phenol-soluble modulin” was coined by the group of Seymour Klebanoff. This group isolated what they described as a complex of three peptides from S. epidermidis culture filtrate by hot phenol extraction (Mehlin et al., 1999). The peptides were named PSMα, PSMβ, and PSMγ, with PSMγ being identical to the long-known δ-toxin (McKevitt et al., 1990). Afterwards, the PSM composition of S. epidermidis and then S. aureus was analyzed more systematically, using the exceptionally late elution behavior of PSMs on reversed-phase columns as an analytical and preparative tool (Vuong et al., 2004a; Wang et al., 2007; Yao et al., 2005). PSMs can be grouped into the smaller (~20-25 amino acids) α-type PSMs and the longer (~44 amino acids) β-type PSMs (Fig. 1).
Fig. 1.
Amino acid sequences of PSMs in S. aureus and S. epidermidis. PSMs can be divided in the shorter (~20-25 amino acids) α-type and the longer (~44 amino acids) β-type. Except for PSM-mec, all PSMs are genome-encoded. Note that α-type PSMs need to be identified directly by isolation and amino acid sequence determination, while β-type PSMs can be found in many staphylococcal genomes by sequence comparison. The β-type PSMs recently received a pfam identifier (pfam05480, Staphylococcus haemolytic protein).
As many psm genes are smaller than the open reading frame cutoff length used in most genome annotations, similarity searches such as by BlastP or BlastN do not reliably find psm genes. This approach has worked only to identify the somewhat larger β genes; and many genes encoding PSMβ-like peptides were found in the genomes of recently sequenced coagulase-negative staphylococci. These PSMβ-like peptides from different staphylococcal species show relatively strong similarity. Unfortunately, they were not always annotated as PSMβ peptides. Recently, they received a pfam group identifier: pfam05480 (Staphylococcus haemolytic protein). In contrast, for α-type PSMs, isolation of the PSM in question, N-terminal sequencing to obtain the amino acid sequence, and search of available whole-genome databases to identify the psm gene(s) was required in most cases. Using a combination of these approaches, it was found that S. aureus produces 7 PSMs named PSMα1-α4, PSMβ1 and PSMβ2, and the S. aureus δ-toxin (Wang et al., 2007) (Fig. 1). S. epidermidis also produces 7 PSMs, named PSMα, PSMβ1 and PSMβ2, PSMδ, PSMε, and the S. epidermidis δ-toxin (McKevitt et al., 1990; Mehlin et al., 1999; Vuong et al., 2004a; Yao et al., 2005). While all these PSMs are core genome-encoded, specific staphylococcal cassette chromosome (SCC)mec elements, which carry methicillin resistance genes, encode a PSM that was termed PSM-mec (Queck et al., 2009). It is found in SCCmec types II, III, and VIII, which are present in many MRSA and MRSE (methicillin-resistant S. aureus and S. epidermidis, respectively) strains (Chatterjee et al., 2011). The psm-mec gene also encodes a regulatory RNA that works via inhibition of translation of AgrA (Kaito et al., 2013). Notably, PSMs are different in different species, even when they carry the same name (such as for example S. epidermidis versus S. aureus PSMβ1). It is thus best to always identify a PSM peptide by the producing species in addition to its name.
In other staphylococcal species, psm gene sequences have not been systematically searched for. Some peptides that were described before the term PSM was coined are now known to belong to the PSM family, such as the PSMβ-like SLUSH peptides from Staphylococcus lugdunensis or the gonococcal growth inhibitor (GGI) peptides from Staphylococcus haemolyticus (Donvito et al., 1997; Frenette et al., 1984). Analysis of the culture filtrates of a series of staphylococcal species by reversed-phase high-pressure chromatography/mass spectrometry (RP-HPLC/MS), which is the optimal method to measure PSM production, showed that many staphylococci produce PSMs (Rautenberg et al., 2011). However, only the masses of these peptides were identified; further analyses to obtain amino acid sequences and psm genes, as performed in S. aureus and S. epidermidis, have not yet been undertaken. Based on these findings, the sequence similarity-based discovery of PSMβ-like peptides in virtually every sequenced staphylococcal genome, and the recent discovery of a PSM-specific secretion system that is present in all staphylococcal genomes sequenced so far, it is evident that most staphylococcal species produce a species-specific repertoire of PSMs (Chatterjee et al., 2013).
The “original” role of PSMs in surface colonization
With the focus of research on staphylococci being pathogenesis, it is understandable that in general as well as specifically for the roles of PSMs, our knowledge on the commensal lifestyle of staphylococci is relatively poor. However, several observations support an evolutionarily old and conserved role of PSMs in staphylococcal surface colonization (Fig. 2). First, all PSMs have a pronounced amphipathic α-helical structure (Cheung et al., 2010; Wang et al., 2007), optimally suited to produce surfactant-like characteristics that facilitate growth in environments with oil/water interfaces, such as on the skin. As is necessary for this basic physiological role, PSMs are produced in extraordinarily high amounts. In S. aureus, it was demonstrated that they represent more than half of the protein mass secreted into the media (Chatterjee et al., 2013). Second, PSMs and their dedicated secretion system are encoded on the core genome, their regulation by Agr is exceptionally direct (Queck et al., 2008) (see below), and they appear in most species (Rautenberg et al., 2011). This clearly contrasts many other toxins, which often are encoded on mobile genetic elements (MGEs) and are highly specific for certain strains and species (Novick et al., 2001). Third, there is experimental evidence indicating that PSMs facilitate spreading on surfaces (Tsompanidou et al., 2013), owing to their surfactant properties, which is one example underlining their assumed role in epithelial colonization (Fig. 2). Fourth, PSMs contribute to biofilm development, a phenotype believed to be crucial for staphylococcal colonization (Periasamy et al., 2012b; Schwartz et al., 2012; Wang et al., 2011) (Fig. 2). Thus, the toxic functions of PSMs that most research has focused on so far appear to have evolved from an original role of PSMs in the non-infectious lifestyle of staphylococci.
Fig. 2.
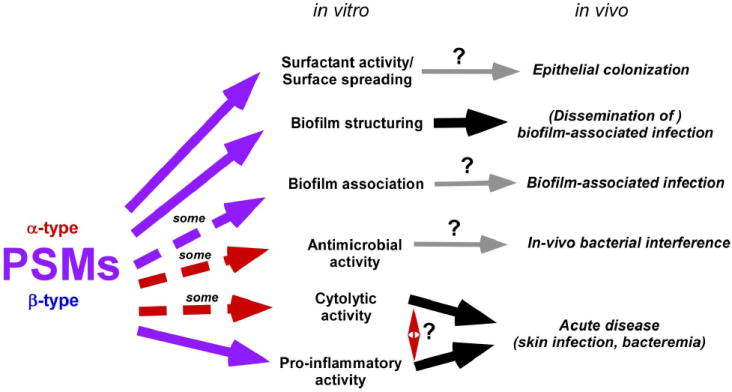
In-vitro and in-vivo functions of PSMs. Demonstrated and putative functions of PSMs in vitro and in vivo are listed. Red arrows stand for activities by α-type, purple arrows for activities by all (α- and β-type) PSMs. Dashed arrows are used when only some PSMs are known to have such activity. Confirmed in-vivo functions are depicted by thick black, speculated in-vivo functions by thin grey arrows. Both cytolytic and pro-inflammatory activities are assumed to impact disease pathogenesis facilitated by PSMs, but their relative contribution is unknown.
PSMs in pathogenesis
Cytolysis
Cytolysis by PSMs most likely occurs in a non-specific, receptor-independent manner (Fig. 2). This is supported by the fact that the PSM receptor FPR2 that mediates PSM pro-inflammatory PSM activities (see below) is not necessary for cytolysis (Kretschmer et al., 2010). In a way similar to what has been described for δ-toxin (Talbot et al., 2001), other cytolytic PSMs are also assumed to destroy membrane integrity by initial membrane attachment and membrane perturbation at high peptide density. Cytolytic properties of PSMs were analyzed mainly for human neutrophils and sheep or human erythrocytes (Cheung et al., 2012; Wang et al., 2007). S. aureus PSMα3 and S. epidermidis PSMδ have exceptionally high capacities to lyse human neutrophils (Cheung et al., 2010; Wang et al., 2007). Interestingly, the genetic location encoding S. epidermidis PSMδ (and PSMα) corresponds to that of the S. aureus psmα operon, indicating that they have a common ancestor. This operon thus appears primarily responsible for the evolution of cytolytic PSMs. Furthermore, in case of the S. aureus psmα operon, the evolution of PSMs toward cytolytic functions can be followed, as among the four PSMα peptides of S. aureus, which are quite similar to each other and apparently arose from gene duplication events, only one, PSMα3, has high cytolytic activity, whereas the others are only moderately (PSMα1, PSMα2) or not cytolytic (PSMα4). The unrelated S. epidermidis PSMε, and the two δ-toxins of S. aureus and S. epidermidis have moderate cytolytic capacities toward human neutrophils. Other PSMs in S. aureus and S. epidermidis, most notably all PSMβ peptides, are barely or not cytolytic to that cell type (Cheung et al., 2010; Wang et al., 2007).
Highly virulent S. aureus, such as community-associated methicillin-resistant S. aureus (CA-MRSA) strains, have an extraordinary capacity to escape elimination by the innate immune system by leading to neutrophil killing after phagocytosis (Voyich et al., 2005). Notably, this phenotype is believed to be mainly responsible for the high pathogenic potential of CA-MRSA. Using isogenic mutants in CA-MRSA strains, it was shown that almost the entire pronounced capacity of those strains to lyse human neutrophils is dependent on the psmα operon (Wang et al., 2007). Plasmid-based complementation of the hospital-associated (HA-) MRSA strain 252 with the psmα operon, leading to PSMα production at levels similar to those found in CA-MRSA wild-type strains, led to capacity to lyse human neutrophils comparable to that of CA-MRSA strains. This indicates that PSMα peptides primarily are responsible for this key virulence phenotype that underlies the exceptional virulence potential of CA-MRSA strains. Notably, PSMα3 alone was almost entirely responsible for those effects (Wang et al., 2007).
Phagocyte lysis after phagocytosis
Highly virulent S. aureus, in particular CA-MRSA strains, have a high capacity to lyse neutrophils after phagocytosis. However, the mechanisms underlying that phenotype have remained poorly understood until recently. The finding that serum lipoproteins reduce the cytolytic activity of PSMs in human serum suggested that PSMs exert their contribution to pathogenesis by intracellular killing and participate in neutrophil killing after phagocytosis (Surewaard et al., 2012). Three recent reports directly identified the PSMα peptides of S. aureus, in contrast to the δ-toxin and PSMβ peptides, as responsible for killing after phagocytosis in neutrophils (Chatterjee et al., 2013; Geiger et al., 2012; Surewaard et al., 2013). In one report, PSMβ peptides over-expressed from a plasmid also showed a contribution (Geiger et al., 2012), which contradicts the absence of lytic capacity when these peptides are incubated with neutrophils in their pure form. Among the classic staphylococcal bicomponent leukotoxins, the Panton-Valentine leukocidin (PVL) does not show such activity (Surewaard et al., 2013), in contrast to the recently described LukAB (LukGH) (DuMont et al., 2013; Ventura et al., 2010). Thus, neutrophil killing after phagocytosis as a key component of the pathogenesis program of highly virulent S. aureus appears to be mediated by a combined action of PSMα peptides and at least some of the bicomponent leukocidins. The relative importance of PSMs versus leukocidins in that mechanism remains to be analyzed. Interestingly, the stringent response appears to be the trigger for enhanced PSM expression within neutrophil phagosomes rather than up-regulation of Agr (Geiger et al., 2012), as previously assumed. In theoretical models, Agr was predicted to be up-regulated in this confined environment owing to the phenomenon of diffusion sensing (Carnes et al., 2010), but in experimental settings Agr was not significantly up-regulated after ingestion of S. aureus by human neutrophils (Voyich et al., 2005).
Lysis of erythrocytes and other cells
The lytic capacity of PSMs toward human erythrocytes only partially follows the same pattern as that toward neutrophils. For example, S. aureus PSMβ1, PSMα1 and PSMα2 are equally as cytolytic to that cell type as S. aureus PSMα3 (Cheung et al., 2012). Similarly, the pattern of PSM-dependent lysis of synthetic phosphatidylglycerol vesicles is different from that observed for neutrophils, inasmuch as especially PSMβ peptides lyse synthetic vesicles (Duong et al., 2012), while they are non-lytic to neutrophils (Cheung et al., 2010; Wang et al., 2007). This indicates that yet unidentified components of cell membranes – such as differential phospholipid or protein composition – may lead to differential susceptibility of different cell types to the cytolytic action of PSMs.
For other cell types a systematic analysis of PSM cytolytic capacities using synthetic, pure peptides has not yet been performed. However, findings achieved with isogenic deletion strains of the S. aureus psmα operon indicate that other cell types such as osteoblasts and monocytes are also susceptible (Rasigade et al., 2013; Wang et al., 2007). Finally, several PSMs contribute to synergistic hemolysis, a phenotype of strong hemolytic behavior facilitated by synergistic action with the sphingomyelinase β-toxin, previously believed to occur only with δ-toxin (Cheung et al., 2012).
Pro-inflammatory activity
PSMs cause cytolysis at micromolar concentrations, but at much lower, nanomolar concentrations, they elicit inflammatory responses (Wang et al., 2007). They attract and stimulate, and cause a specific release of cytokines in human neutrophils (Wang et al., 2007). These activities are mediated, in contrast to cytolysis, by interaction with the formyl receptor 2 (FPR2) (Kretschmer et al., 2010). While formyl peptide receptors are classified by their response to N-formylated peptides, it is important to stress that – despite PSMs usually being secreted with an N-terminal N-formyl methionine – N-formylation increases, but is not absolutely necessary for FPR2 stimulation by PSMs. FPR2 activation may form part of the S. aureus pathogenic program, as exorbitant neutrophil influx may lead to tissue destruction that is beneficial to the bacteria; however, it is more likely that this interaction evolved as a means for the innate immune system to recognize highly virulent staphylococcal invaders.
FPR2 is also present on other cell types, for example dendritic cells (DCs). These cells connect the innate and adaptive part of the host immune system. While PSMs lead to DC attraction, possibly via FPR2, they also induce a tolerogenic phenotype in DCs by inducing IL-10 and reducing the release of other pro-inflammatory cytokines, probably in an FPR2-independent manner (Schreiner et al., 2013). This interaction is believed to enhance S. aureus immune evasion, because it leads to Th1 differentiation and induction of regulatory T cells.
Antimicrobial activity
Some PSMs were reported to have antimicrobial activities. For example, PSMδ and the δ-toxin of S. epidermidis kill Streptococcus pyogenes (Cogen et al., 2010). This capacity may have importance for bacterial interference between these two bacteria in the human host. However, the concentrations at which killing was demonstrated are relatively high. Furthermore, proteolytically processed products of S. aureus PSMα1 and PSMα2 also have anti-streptococcal activity, but these products are only present in small amounts in S. aureus culture filtrates (Joo et al., 2011). Whether PSM peptides contribute to bacterial interference in vivo is not known. These observations and the fact that staphylococci may produce much more potent bacteriocins suggest that PSMs did not evolve to achieve antimicrobial activities. They may represent a side effect of the general surfactant-based functions of PSMs.
Biofilm development
Biofilms are surface-attached bacterial commmunities with a characteristic three-dimensional structure, which comprises channels to allow nutrient delivery to deeper layers (O’Toole et al., 2000). Many bacterial infections develop with the formation of biofilms (Costerton et al., 1999). While in many bacteria, the molecules that attach bacteria together in biofilms have been analyzed in great detail; the molecular factors that facilitate channel formation and biofilm structuring have remained unknown for a long time. In several bacteria, these were recently identified to be surfactant-like molecules, such as rhamnolipid in Pseudomonas aeruginosa (Boles et al., 2005) or surfactin in Bacillus subtilis (Angelini et al., 2009). In S. aureus and S. epidermidis, PSMs were found to fulfill this function (Otto, 2013; Periasamy et al., 2012b; Wang et al., 2011). All PSMs of S. aureus were shown to have biofilm-structuring activity, while in S. epidermidis, the PSMβ peptides were identified to structure biofilms. However, other S. epidermidis PSMs, owing to the difficulties in producing isogenic gene deletion mutants in S. epidermidis, were not yet analyzed for that function. Because the biofilm-structuring activities of PSMs are presumably dependent on their common surfactant properties, it is likely that they also contribute to biofilm structuring. The cell-cell disruptive capacities of PSMs, which are assumed to be dependent on the disruption of hydrophobic or hydrophilic interactions between bacterial surface molecules, may also ultimately lead to biofilm dispersal, i.e. the detachment of cells or cell clusters from the biofilm surface (Otto, 2013). During biofilm-associated infection, this mechanism is crucial for the dissemination of cells to secondary infection sites.
Absence of PSMs or the quorum-sensing regulator Agr, which is in strict control of PSM production (Queck et al., 2008; Vuong et al., 2004a), leads to compact and thickened biofilms without channels (Periasamy et al., 2012b; Vuong et al., 2003; Vuong et al., 2004b; Vuong et al., 2000; Wang et al., 2011). It may be primarily for that reason that many staphylococcal strains showing strong biofilm formation in in-vitro assays, such as S. aureus SA113 or S. epidermidis O47 are functionally deficient in Agr, as this leads to lack of PSM production (Vuong et al., 2003; Vuong et al., 2000). Mutation in Agr and the concomitant suppression of PSM production is observed quite frequently in clinical strains and also when laboratory strains are cultured over extended periods (Fowler et al., 2004; Goerke et al., 2000; Shopsin et al., 2008; Somerville et al., 2002; Traber et al., 2008; Vuong et al., 2000). This adaptation may be beneficial for the bacteria under certain physiological conditions that require the pronounced formation of biofilms. In in-vivo infectious biofilms, this may be advantageous for biofilm formation on a catheter, but as it is an essentially irreversible process, such bacterial agglomerations have lost the capacity to disseminate (Joo and Otto, 2012; Periasamy et al., 2012b).
More recently, PSMs were also found to form fibril-like structures that facilitate cell-cell association in biofilms (Schwartz et al., 2012). However, this phenotype was only observed in a specific growth medium and after prolonged growth in the biofilm mode. How the biofilm-associative and biofilm-disruptive functions of PSMs come together during biofilm development is not yet understood. As PSM absence leads to thicker biofilms, notably including in in-vivo settings, the disruptive function described above appears to dominate, at least under the conditions that were investigated (Periasamy et al., 2012b).
PSMs in infection models
The psmα and psmβ operons and the δ-toxin locus were analyzed for their contributions to infection in murine infection models using isogenic mutants (Wang et al., 2007). In case of the hld gene encoding δ-toxin, only the start codon was altered, abolishing δ-toxin production, so as not to interfere with the function of RNAIII, in which hld is embedded. Bacteremia was tested using mutants in the USA400 CA-MRSA strain MW2 and skin infection using the USA300 CA-MRSA strain LAC. In both models, psmα had a strong contribution to the outcome of infection, whereas the δ-toxin had a moderate effect and the psmβ operon had none. Using a mouse peritonitis model, psmα shown to lead to increased neutrophil and monocyte chemotaxis, and subsequent killing of those cells, confirming the key contribution of PSMs to leukocyte killing found in vitro (Wang et al., 2007).
There has been a great debate in recent years as to which virulence determinants underlie the pronounced virulence capacity of CA- as compared to HA-MRSA strains (Otto, 2010). Owing to an epidemiological association of PVL with CA-MRSA, this leukotoxin was initially believed to significantly determine CA-MRSA virulence (Vandenesch et al., 2003). Earlier mouse infection models were inconclusive, because it was recently found that the PVL receptor is only present in human and, among common test animals, rabbits; and only neutrophils from those species are therefore sensitive to PVL (Loffler et al., 2010). In a rabbit skin infection model directly comparing the contribution of PVL, α-toxin, and PSMα peptides to that main manifestation of CA-MRSA-associated disease, α-toxin and PSMα peptides showed a strong, whereas PVL had no effect (Kobayashi et al., 2011). These findings identify PSMα peptides as premier contributors to CA-MRSA virulence, next to α-toxin, which does not lyse neutrophils (Valeva et al., 1997), but erythrocytes, for example, and has many other roles in pathogenesis (Bhakdi and Tranum-Jensen, 1991; Inoshima et al., 2011). As both α-toxin and PSMα peptides are produced by virtually all S. aureus strains, these two toxins can be regarded as the main reason underlying basic S. aureus toxicity, further increased in certain strains by MGE-encoded toxins.
In biofilm-associated infection, PSMs contribute to the dissemination from an established biofilm to secondary infection sites (Periasamy et al., 2012b; Wang et al., 2011). In accordance with the in-vitro findings on biofilm-structuring, all PSMs appear to have such capacities. In S. epidermidis, antibodies against PSMβ1 and PSMbβ peptides, which are strongly produced in S. epidermidis (Cheung et al., 2010), had the potential to block dissemination (Wang et al., 2011).
Conclusions
From original functions in the commensal lifestyle of staphylococci, which probably include emulsification of nutrients, colony spreading and colony structuring, several PSMs evolved to become virulence factors. The PSMα peptides of S. aureus are the clearest example of that evolution, representing key virulence determinants in S. aureus. Highly toxic PSMs, for example S. epidermidis PSMδ, appear to have evolved also in less pathogenic species, but in accordance with the less pathogenic lifestyle of S. epidermidis, they are not produced at considerable levels, at least in vitro. Whether they are expressed at increased levels during infection is not known. Currently, the precise roles of the PSMβ and PSMβ -like peptides found in all staphylococci are unknown; it is possible that they simply represent PSMs that did not evolve to achieve further roles in addition to the surface-active surfactant and biofilm-structuring functions likely common to all PSMs. However, some of them are cytolytic to erythrocytes and these PSMβ peptides may thus have evolved to target a specific, different cell type. The δ-toxin, often produced at considerable amounts, frequently contributes moderately, but not extensively, to PSM-mediated phenotypes. Whether there is a more dedicated role of δ-toxin remains to be analyzed.
Similar to α-toxin, PSMs appear to be promising targets for anti-staphylococcal drug development that is aimed at reducing virulence, a modern approach prompted by widespread antibiotic resistance in staphylococci (Alksne and Projan, 2000). However, the fact that they are a family of peptides with highly divergent amino acid sequences makes such efforts problematic. While targeting the exceptionally toxic PSMα3 of S. aureus may be one option, the discovery of the PSM transporter system Pmt may provide a way to simultaneously target all PSMs. The fact that Pmt absence leads to intracellular accumulation of PSMs and thus direct killing of the bacteria may further strengthen the potential of a drug blocking Pmt. Efforts to target PSMs for anti-staphylococcal drug development have not begun yet, but it is likely that we will see many efforts in that direction in the time to come.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alksne LE, Projan SJ. Bacterial virulence as a target for antimicrobial chemotherapy. Curr Opin Biotechnol. 2000;11:625–636. doi: 10.1016/s0958-1669(00)00155-5. [DOI] [PubMed] [Google Scholar]
- Angelini TE, Roper M, Kolter R, Weitz DA, Brenner MP. Bacillus subtilis spreads by surfing on waves of surfactant. Proc Natl Acad Sci U S A. 2009;106:18109–18113. doi: 10.1073/pnas.0905890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Thoendel M, Singh PK. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol. 2005;57:1210–1223. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Chen W, Singvall J, Xu Y, Peacock SJ, Valtulina V, Speziale P, Hook M. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology. 2005;151:1453–1464. doi: 10.1099/mic.0.27534-0. [DOI] [PubMed] [Google Scholar]
- Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, Brinker CJ. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol. 2010;6:41–45. doi: 10.1038/nchembio.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Chen L, Joo HS, Cheung GY, Kreiswirth BN, Otto M. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6:e28781. doi: 10.1371/journal.pone.0028781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, Otto M. Essential Staphylococcus aureus toxin export system. Nat Med. 2013;19:364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Duong AC, Otto M. Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect. 2012;14:380–386. doi: 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, Gallo RL. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Donvito B, Etienne J, Denoroy L, Greenland T, Benito Y, Vandenesch F. Synergistic hemolytic activity of Staphylococcus lugdunensis is mediated by three peptides encoded by a non-agr genetic locus. Infect Immun. 1997;65:95–100. doi: 10.1128/iai.65.1.95-100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun. 2013;81:1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong AC, Cheung GY, Otto M. Interaction of phenol-soluble modulins with phosphatidylcholine vesicles. Pathogens. 2012;1:3–11. doi: 10.3390/pathogens1010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190:1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- Frenette M, Beaudet R, Bisaillon JG, Sylvestre M, Portelance V. Chemical and biological characterization of a gonococcal growth inhibitor produced by Staphylococcus haemolyticus isolated from urogenital flora. Infect Immun. 1984;46:340–345. doi: 10.1128/iai.46.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 2012;8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke C, Campana S, Bayer MG, Doring G, Botzenhart K, Wolz C. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect Immun. 2000;68:1304–1311. doi: 10.1128/iai.68.3.1304-1311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HS, Cheung GY, Otto M. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J Biol Chem. 2011;286:8933–8940. doi: 10.1074/jbc.M111.221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C, Saito Y, Ikuo M, Omae Y, Mao H, Nagano G, Fujiyuki T, Numata S, Han X, Obata K, Hasegawa S, Yamaguchi H, Inokuchi K, Ito T, Hiramatsu K, Sekimizu K. Mobile genetic element SCCmec-encoded psm-mec RNA suppresses translation of agra and attenuates MRSA virulence. PLoS Pathog. 2013;9:e1003269. doi: 10.1371/journal.ppat.1003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol. 2012;15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975;30:381–385. doi: 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, DeLeo FR. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocianova S, Vuong C, Yao Y, Voyich JM, Fischer ER, DeLeo FR, Otto M. Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J Clin Invest. 2005;115:688–694. doi: 10.1172/JCI23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- McKevitt AI, Bjornson GL, Mauracher CA, Scheifele DW. Amino acid sequence of a deltalike toxin from Staphylococcus epidermidis. Infect Immun. 1990;58:1473–1475. doi: 10.1128/iai.58.5.1473-1475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlin C, Headley CM, Klebanoff SJ. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med. 1999;189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Schlievert P, Ruzin A. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 2001;3:585–594. doi: 10.1016/s1286-4579(01)01414-9. [DOI] [PubMed] [Google Scholar]
- O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- Otto M. Virulence factors of the coagulase-negative staphylococci. Front Biosci. 2004;9:841–863. doi: 10.2741/1295. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis - the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2013;64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- Periasamy S, Chatterjee SS, Cheung GY, Otto M. Phenol-soluble modulins in staphylococci: What are they originally for? Commun Integr Biol. 2012a;5:275–277. doi: 10.4161/cib.19420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A. 2012b;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, DeLeo FR, Otto M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009;5:e1000533. doi: 10.1371/journal.ppat.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasigade JP, Trouillet-Assant S, Ferry T, Diep BA, Sapin A, Lhoste Y, Ranfaing J, Badiou C, Benito Y, Bes M, Couzon F, Tigaud S, Lina G, Etienne J, Vandenesch F, Laurent F. PSMs of hypervirulent Staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS ONE. 2013;8:e63176. doi: 10.1371/journal.pone.0063176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenberg M, Joo HS, Otto M, Peschel A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. Faseb J. 2011;25:1254–1263. doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005;13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol. 2013;190:3417–3426. doi: 10.4049/jimmunol.1202563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin B, Drlica-Wagner A, Mathema B, Adhikari RP, Kreiswirth BN, Novick RP. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis. 2008;198:1171–1174. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- Somerville GA, Beres SB, Fitzgerald JR, DeLeo FR, Cole RL, Hoff JS, Musser JM. In vitro serial passage of Staphylococcus aureus: changes in physiology, virulence factor production, and agr nucleotide sequence. J Bacteriol. 2002;184:1430–1437. doi: 10.1128/JB.184.5.1430-1437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewaard B, de Haas C, Vervoort F, Rigby K, DeLeo F, Otto M, van Strijp J, Nijland R. Staphylococcal alpha-Phenol Soluble Modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013;15:1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, van Strijp JA. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 2012;8:e1002606. doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JC, Thiaudiere E, Vincent M, Gallay J, Siffert O, Dufourcq J. Dynamics and orientation of amphipathic peptides in solution and bound to membranes: a steady-state and time-resolved fluorescence study of staphylococcal delta-toxin and its synthetic analogues. Eur Biophys J. 2001;30:147–161. doi: 10.1007/s002490000118. [DOI] [PubMed] [Google Scholar]
- Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. agr function in clinical Staphylococcus aureus isolates. Microbiology. 2008;154:2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsompanidou E, Denham EL, Becher D, de Jong A, Buist G, van Oosten M, Manson WL, Back JW, van Dijl JM, Dreisbach A. Distinct roles of phenol-soluble modulins in spreading of Staphylococcus aureus on wet surfaces. Appl Environ Microbiol. 2013;79:886–895. doi: 10.1128/AEM.03157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeva A, Walev I, Pinkernell M, Walker B, Bayley H, Palmer M, Bhakdi S. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci U S A. 1997;94:11607–11611. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS ONE. 2010;5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- Vuong C, Durr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 2004a;6:753–759. doi: 10.1111/j.1462-5822.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis. 2003;188:706–718. doi: 10.1086/377239. [DOI] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis. 2004b;190:1498–1505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wang R, Khan BA, Cheung GY, Bach TH, Jameson-Lee M, Kong KF, Queck SY, Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Sturdevant DE, Otto M. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis. 2005;191:289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]