Abstract
Our studies showed that tumor-infiltrating dendritic cells (DC) in breast cancer drive inflammatory T helper 2 (iTh2) cells and protumor inflammation. Here we show that intratumoral delivery of the β-glucan curdlan, a ligand of dectin-1, blocks the generation of iTh2 cells, and prevents breast cancer progression in vivo. Curdlan reprograms tumor-infiltrating DC via the ligation of dectin-1, enabling the DC to become resistant to cancer-derived thymic stromal lymphopoietin (TSLP), to produce IL12p70, and to favor the generation of T helper 1 (Th1) cells. DC activated via dectin-1, but not those activated with TLR-7/8 ligand or poly IC, induce CD8+ T cells to express CD103 (αE integrin), a ligand for cancer cells E-cadherin. Generation of these mucosal CD8+ T cells is regulated by DC-derived integrin αvβ8 and TGF-β activation in a dectin-1-dependent fashion. These CD103+CD8+ mucosal T cells accumulate in the tumors thereby increasing cancer necrosis and inhibiting cancer progression in vivo in a humanized mouse model of breast cancer. Importantly, CD103+CD8+ mucosal T cells elicited by reprogrammed DC can reject established cancer. Thus, reprogramming tumor-infiltrating DC represents a new strategy for cancer rejection.
Keywords: breast cancer, dendritic cells, CD8+ T cells, type 2 immunity, dectin-1, curdlan
INTRODUCTION
In recent years we have witnessed an improved understanding of the critical roles that the tumor microenvironment plays in cancer growth, evasion from host immunity, and resistance to therapeutic agents (1). A better definition of the molecular and cellular components of the tumor microenvironment will enhance the clinical efficacy of current immunotherapy approaches and enable tailoring of specific therapeutic strategies. Breast and pancreatic cancer are characterized by infiltration of inflammatory Th2 (iTh2) cells, which coexpress interleukin (IL)-4/IL-13 and tumor necrosis factor (TNF)-α but not IL-10 (2, 3). Clinically, the Th2 signature in breast cancer (4, 5) and the expression of the Th2 master regulator GATA-3 in pancreatic cancer (6) are associated with poor outcomes.
Experimentally, iTh2 cells accelerate tumor development in humanized mouse models of breast cancer through the activity of IL-13 (2). In genetically engineered mouse models of mammary cancer, iTh2 cells accelerate the development of pulmonary metastasis via IL-4 (7). IL-4 and IL-13 exert protumor activity through several pathways including: 1, the triggering of transforming growth factor (TGF)-β secretion (8); 2, the up-regulation of anti-apoptotic pathways in cancer cells (9); and 3, the generation of type-2-polarized macrophages that foster tumor growth directly via the secretion of growth factors, and indirectly via the inhibitory effects on CD8+ T cell function (10). Indeed, CD8+ T cells are essential for tumor rejection through the generation of cytotoxic effectors. The presence of CD8+ T cells in primary tumors is associated with the long-term survival of patients with colorectal and breast cancer (10, 11). Thus, iTh2 cells have a broad and profound impact on the tumor microenvironment and cancer progression.
The generation of iTh2 cells in breast cancer depends on the presence of mature tumor-infiltrating OX40L+ dendritic cells (DC) (3). In experimental models of breast cancer this DC phenotype is driven by cancer-derived thymic stromal lymphopoietin (TSLP) (3, 12). Previous studies have demonstrated that dectin-1, an innate immune receptor with activating motifs (ITAM), can reprogram DC from inducing Th2 responses into Th1 responses (13, 14). We therefore investigated whether curdlan, a natural ligand of dectin-1 (15), could reprogram the function of breast tumor-infiltrating DC to enable cancer rejection.
MATERIALS AND METHODS
Cells, tissues, and reagents
Breast cancer cell lines: Hs587T and MCF-7 were purchased from ATCC; MDA-MB-231 was purchased from Xenogen and cultured in non-selecting media. All lines are banked as low passage stock from which working banks are periodically renewed. All lines were verified by gene microarrays twice in the past 7 years. Morphology, in vitro growth rate and in vivo growth rate were the same as the original lines. The mycoplasma test was performed regularly and the cell lines were mycoplasma-free for each in vitro and in vivo experiment.
Cell lines were cultured in RPMI (plus glutamine 2mM, penicillin 50 U/ml, streptomycin 50 μg/ml, MEM non-essential amino acids 0.1 mM, HEPES buffer 10 mM, sodium pyruvate 0.1 mM) and 10% fetal calf serum in T150 flasks at seed density of 2×106 cells/25ml. At 90% confluence fresh medium was added and cells were cultured for an additional 48 hours. Supernatant was centrifuged and stored at −80 °C.
Peripheral blood mononuclear cells (PBMC) were obtained by leukapheresis from healthy donors (Institutional Review Board (IRB) approved). Primary tissues from patients were obtained from the BUMC Tissue Bank and are exempt. Animal experiments were carried out with permission from the IACUC.
β-glucan, curdlan (Wako Pure Chemical Industries, Japan), was in PBS at a working concentration of 100μg/ml. The working concentrations of the neutralization antibodies were: 20μg/ml for anti-dectin-1 (clone 259931, R&D; Minneapolis, MN), 10μg/ml anti-IL-12 (clone 20C2, Thermo Scientific; Waltham, MA), 100μg/ml anti-TGF-β (clone 1D11, R&D), 50μg/ml anti-CD103 (Clone Ber-ACT8, Biolegend; San Diego, CA) and 100μg/ml anti-β8 (clone 37E1). Curdlan was labeled with Aminofluorescein (5-DTAF) (Molecular Probes-Invitrogen; Carlsbad, CA).
Dendritic cells
DC were enriched from PBMC obtained after Ficoll-Paque Plus density gradient centrifugation (Stemcell Technologies; Vancouver, BC, Canada) by negative selection with monoclonal antibodies (mAb) to CD3, CD9, CD14, CD16, CD19, CD34, CD56, CD66b, and glycophorin A (Human pan-DC pre-enrichment kit; Stemcell Technologies). Cells were labeled with anti-human lineage cocktail-FITC (CD3, CD14, CD16, CD19, CD20 and CD56); CD123-PE (9F5) and CD11c-APC (S-HCL-3) (BD Biosciences; Franklin Lakes, NJ), and HLA-DR-APC-eflour780 (LN3) (Sigma-Aldrich; St. Louis, MO); lin−CD123−HLA-DR+CD11c+ DC were sorted with FACS Aria (BD Bioscience). DC were seeded at 100 × 103 cells/well in 200 μl of RPMI with 10% human AB serum, and cultured with medium alone or in the presence of 20 ng/ml of rhTSLP (R&D), or tumor-derived products. After 48 hours DC were harvested, washed, and analyzed or used in experiments.
Immunofluorescence
OCT-embedded (Sakura Finetek U.S.A., Torrance, CA), snap-frozen tissues were cut at 6 μm and air-dried on Superfrost slides (Cardinal Health, Dublin, OH). Frozen sections were fixed with cold acetone for 10 minutes. Dectin-1 was stained with mAb prepared in-house (clone12.2D8.2D4) followed by Alexa Flour488 or Alexa Flour568 goat anti-mouse IgG1 (Invitrogen). Cytokeratin 19 was labeled with clone A53-BA2 (Abcam; Cambridge, CA) followed by Alexa Fluor568 goat anti-mouse IgG2a (Invitrogen). CD83 was stained with clone HB15a (Immunotech; Irvine, CA) followed by Alexa Fluor568 goat anti-mouse IgG2b (Invitrogen). CD20 was stained with clone L26 (Dako; Carpinteria, CA) followed by Alexa Fluor488 goat anti-mouse IgG2a (Invitrogen). Directly labeled antibodies used were FITC anti-HLA-DR (clone L243, BD biosciences), and FITC anti-CD11c (clone KB 90, Dako). Finally, sections were counterstained for 2 minutes with the nuclear stain DAPI (3 μM in PBS; Invitrogen-Molecular Probes).
Flow Cytometry
mAb to human OX40L-PE (clone Ik-1), HLA-DR (clone L243), lineage cocktail-FITC (CD3, CD14, CD16, CD19, CD20 and CD56), CD11c-APC (clone S-HCL-3), CD3-PerCP (clone SK7), CD4-PE-Cy7 (clone SK3), CD8-APC-Cy7 (clone SK1), CD80–PE (L307.4), CD86–FITC (clone 2331(FUN-1); CD70-PE (Ki-24); CD83-FITC (HB15e); IL-13-PE (JES10-5A2); TNF-α-PECy7(mAb11); IFN-γ-Alexa Flour700 (B27); pSTAT4-FITC (38/p-stat4); pSTAT6-PE (J91-99358.11); pSTAT3-AF647 (4/pStat3); and pSTAT5-AF647 (47) were obtained from BD (Franklin Lakes, NJ). mAb to MHC class I-PE (W6/32) was from DAKO. IL-10-Pacific blue (JES3-9D7) and Perforin-PE (dG9) were from eBioscience (San Diego, CA). mAb to IL-17A-PerCP Cy5.5 (BL168); CD103-Alexa Flour647 (Ber-act8); Granzyme A-Pacific blue (GB9) and Granzyme B-Alexa Flour700 (GB11) were from Biolegend; anti-integrin β8 (14E5) was conjugated with AF488 in-house.
For surface staining, cells were incubated with antibodies for 30 minutes at 4 °C in the dark, washed and fixed with 1% paraformaldehyde (PFA), events of stained cells were acquired with FACS Canto or LSR-II (BD), and analyzed with the FlowJo software (Tree Star, Ashland, OR). For intracellular cytokines, cells were stained using the BD cytofix/cytoperm fixation/permeabilization kit according to the manufacturer’s directions. For pSTATs staining, cells were fixed with 2–4% formaldehyde for 10 minutes at 37°C and permeabilized with iced-cold methanol for 30 minutes at 4 °C. Cells were washed and stained with mAb to pSTAT3, pSTAT4, pSTAT5 and pSTAT6 for 30 minutes at room temperature.
Cytokines
T cells from DC-T co-cultures were resuspended at 106 cells/ml in medium and activated for 5 h with PMA and ionomycin. Brefeldin A (Golgiplug, BD Biosciences) and monensin (Golgistop, BD Biosciences) were added for the last 2.5 h. BD cytofix/cytoperm fixation/permeabilization kit was used according to the manufacturer’s directions. Labeled samples were acquired with FACS Canto or LSR-II (BD). Whole-tissue fragments of tumors from humanized mice (4 × 4 × 4 mm, 0.015–0.030 g, approximately) were placed in culture medium with 50 ng/ml of PMA (Sigma-Aldrich) and 1 μg/ml of ionomycin (Sigma-Aldrich) for 18 h. Cytokine production was analyzed in the culture supernatant by Luminex.
DC-T cell co-cultures
Total T cells were enriched from apheresis using magnetic depletion of other leukocytes (EasySep® Human T Cell Enrichment Kit, Stemcell Technologies). Blood DC cultured with medium, TSLP or tumor-derived factors were co-cultured with naïve allogeneic T cells in a ratio of 1:5. For curdlan treatment, DC were pre-incubated with curdlan for 3 min at room temperature.
Humanized mice
NOD/SCID/β2m−/− mice were irradiated the day before tumor implantation. Tumors were injected with 1×106 monocyte-derived DC, and with autologous T cells: 10×106 CD4+ T cells admixed with 10×106 CD8+ T cells. Monocyte-derived DC were generated by culturing the adherent fraction of PBMC with 100 ng/ml GM-CSF (Genzyme, Cambridge, MA) and 10 ng/ml IL-4 (R&D). CD4+ and CD8+ T cells from the same donor as DC were positively selected from thawed PBMC according to the manufacturer’s instructions (Miltenyi Biotec; Auburn, CA) to >90% purity. Tumor volume was monitored every 2–3 days: [(short diameter) 2 × long diameter]/2. Tumors were injected with 100 μg/ml of curdlan at days 3, 6, and 9 post-implantation.
Stamper-Woodruff binding assay
20,000 CD8+ T cells sorted from DC-T co-cultures and labeled with CFSE were put on acetone-fixed breast tumor sections and incubated at 37°C. After 1hr, the slides were washed to remove the unbound cells, fixed with 4% PFA for 10 min, treated with background buster for 30min at RT, stained with cytokeratin, and finally counterstained for 2 minutes with the nuclear stain DAPI.
T-cell retention in vivo
NOD/SCID/β2m−/− mice were subcutaneously injected with 10×106 MDA-MB231 cells. 500,000 CD8+T cells sorted from DC-T co-cultures and labeled with CFSE were injected into the tumors. After 3 days, the tumors were harvested and frozen with OCT or digested with collagenase (2.5 mg/ml; Roche Diagnostics, Indianapolis, IN), and processed to single cell suspension. Some groups of mice were left for tumor growth monitoring.
Microarrays
Total RNA was purified using mirVana miRNA Isolation Kit (Invitrogen). RNA integrity was assessed using the Bioanalyzer 2000 (Agilent). Target labeling was carried out using the TargetAmp Nano-g Biotin-aRNA Labeling Kit for the Illumina System (Epicentre). Labeled RNA was hybridized onto HumanHT-12 v4 Expression BeadChips (Illumina). Illumina GenomeStudio version 1.9.0 software was used to subtract background and scale samples to the global average signal intensity. Ingenuity Pathway Analysis (IPA) was applied to reveal transcriptional networks as described (16).
RESULTS
Curdlan inhibits the generation of iTh2 cells and breast tumor development
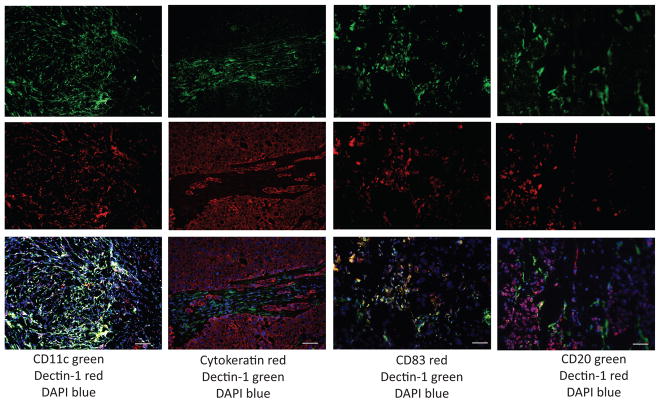
Immunofluorescence analysis of tissues from 27 primary breast cancers (Supplement Table 1) revealed the presence of β-glucan receptor dectin-1 in all samples with CD11c+CD20−HLA-DR+CD83+ mature DC; dectin-1-positive cells were found in the peri-tumoral areas (Figure 1).
Figure 1. Dectin-1-expressing cells infiltrate primary breast tumors.
Immunofluorescence staining on frozen tissue sections from patients’ primary cancers. Columns left to right: CD11c (green)/Dectin-1 (red); Cytokeratin (red)/Dectin-1 (green); CD83 (red)/Dectin-1(green); CD20 (red)/Dectin-1 (green). Top to bottom: single fluorescence for each indicated antibody and overlay. Blue: nuclear staining with DAPI. Representative of 27 tumors analyzed. Bar: 90μm.
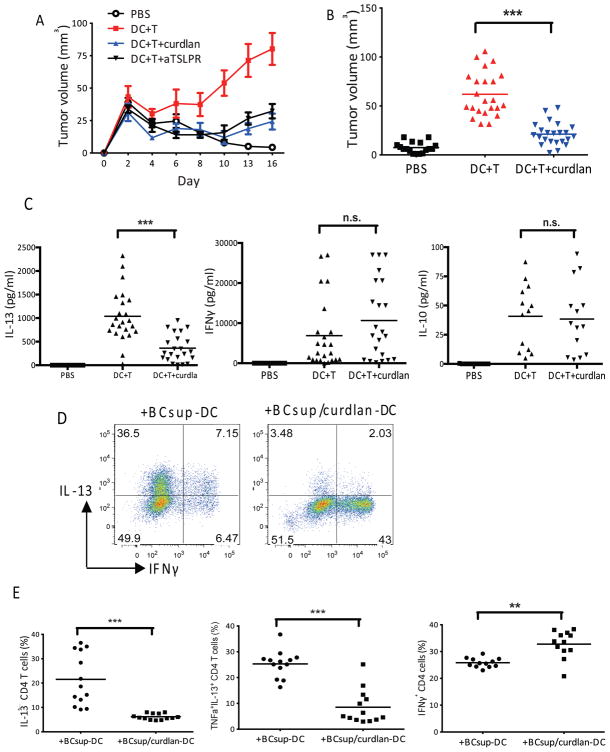
To establish whether the ligation of dectin-1 by curdlan in the tumor microenvironment might impact breast cancer progression in vivo, we used a humanized mouse model of human breast cancer that we have described earlier (2, 3). Intratumoral administration of 10 μg of curdlan prevented breast cancer progression (Hs578T breast cancer cell line) and was as effective as the neutralizing anti-TSLP receptor antibody (Figure 2A). The antitumor effect of curdlan has been observed in five independent experiments with a total of 23 mice that had been grafted with monocyte-derived DC and autologous T cells obtained from several donors (Figure 2B). Breast tumor progression in this model is dependent on IL-13, as tumors do not grow in the absence of IL-13 or in the PBS control (2, 3), we analyzed IL-13 production by breast cancers that were harvested from humanized mice and activated with PMA and ionomycin (PMA/Iono). When compared to controls, curdlan-treated tumors produced significantly less IL-13 (DC+T: 1038±115 pg/ml; DC+T+curdlan: 361±62 pg/ml; n=23; p<0.0001) but similar levels of IFN-γ (DC+T: 6880±1796 pg/ml; DC+T+curdlan:10669±2081 pg/ml; n=23; p=0.17) and IL-10 (DC+T: 41±7.7 pg/ml; DC+T+curdlan:38±8 pg/ml; n=23; p=0.83) (Figure 2C). We have shown earlier that blood DC as well as monocyte-derived DC exposed to breast cancer cell supernatants (BCsups) such as MDA-MB231, Hs578T and MCF-7 (Supplement Table 2), which express and secrete TSLP, can induce the differentiation of naïve T cells into iTh2 cells (2, 3). To determine whether curdlan prevents the breast cancer-induced polarization of DC, purified blood LinnegCD123lowHLA-DR+CD11c+ DC were exposed for 48 hours to BCsups with and without curdlan, and subsequently co-cultured in vitro with naïve allogeneic CD4+T cells for seven days. Thereafter, T cells were activated for 5 hrs with PMA/Iono and analyzed using intracellular cytokine staining (ICS) and flow cytometry (Figure 2D). As expected, CD4+ T cells exposed to DC that had been pretreated with BCsups alone produced both IL-13 and TNF-α (22±3% of CD4+ T cells). In contrast, T cells exposed to DC treated with both BCsups and curdlan produced less IL-13 (6±0.3% of CD4+ T cells; n=13; p<0.0001) (Figures 2E). In both cases, CD4+ T cells produced IFN-γ (+BCsup-DC: 26±0.5%; and +BCsup/curdlan-DC: 33±1.5% of CD4+ T cells, respectively; n=13; p=0.0002) (Figures 2E). Thus, curdlan inhibits the progression of human breast cancer by preventing the generation of protumor iTh2 cells.
Figure 2. Curdlan blocks iTh2 in human breast cancer.
(A) Hs578T breast tumor-bearing NOD/SCID/β2m−/− mice were reconstituted with monocyte-derived DC and autologous T cells isolated from the same donor, with or without treatment with curdlan (100μg/ml) or anti-TSLPR antibody (200μg/mouse). Empty circles: PBS; red: DC+T; blue: DC+T+curdlan; black: DC+T+anti-TSLPR. (B) Mean value from five independent experiments, 23 mice per group. (C) Cytokines in the activated tumor supernatant measured by Luminex. Single points indicate individual mouse. (D) Sorted blood DC were pre-treated with 100μg/ml curdlan, incubated with supernatant of MDA-MB231 breast cancer cell line (BCsup) for 48 hrs and co-cultured with allogeneic naive CD4+T cells. Cells were re-stimulated for ICS at day 7. (E) Summary of different experiments. Single points represent the percentage from individual experiment with blood DC from 13 different healthy donors.
Ligation of dectin-1 with curdlan results in reprogramming of breast cancer-DC maturation
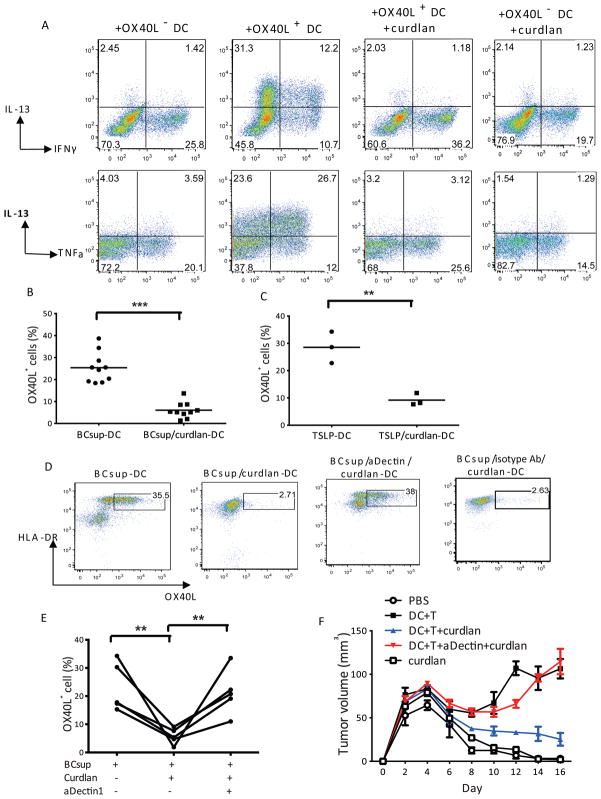
To determine whether curdlan can reprogram the function of tumor-conditioned DC, we sorted OX40L+ and OX40L− DC that arise in response of blood DC to BCsups. The sorted DC were then exposed to curdlan for 24 hours, washed and co-cultured with naïve allogeneic T cells. As expected, OX40L+ DC induced T cells to express IL-13 while OX40L− DC did not. Treatment of OX40L+ DC with curdlan altered their T cell polarization capacity as no IL-13 was induced (Figure 3A). Adding curdlan to DC also prevented the induction of OX40L by BCsups (BCsups DC=25±2%; n=10; BCsups DC+curdlan=6±1.1%, n=10; P<0.0001 (Figures 3B). The inhibition of OX40L expression by curdlan was also observed when DC were treated with human recombinant TSLP (Figure 3C). Conversely, the addition of anti-dectin-1 antibodies, which block the binding of curdlan to DC (13), before curdlan treatment allowed OX40L expression by DC exposed to BCsups in several independent experiments (Figures 3D and 3E), demonstrating that curdlan does indeed engage dectin-1. In vivo the administration of anti-dectin-1 antibodies to developing breast cancer tumors prevented the protective effect of curdlan (Figure 3F). These results confirm that curdlan acts through the dectin-1 expressed by tumor-infiltrating DC in breast cancer.
Figure 3. Curdlan reprograms the function of DC in breast cancer via dectin-1.
(A) Sorted blood DC were exposed to BCsup for 48 hours, and OX40L+ or OX40L− DC were sorted by FACS. OX40L+ DC were re-cultured with or without curdlan for 24 hrs and then co-cultured with naïve allogeneic total T cells. After 7 day culture, cells were collected and re-stimulated for ICS. (B) Sorted blood DC were exposed to BCsup for 48 hours. The curdlan (100μg/ml) treatment is 3 min at room temperature prior to adding BCsup. Each point indicates the percentage of OX40L+ DC from 10 independent experiments analyzed by flow cytometry. (C) Sorted blood DC were exposed to recombinant human TSLP (20ng/ml) for 48 hours with or without 3 min pre-treatment with curdlan (100μg/ml) and analyzed by flow cytometry. (D) DC were pre-treated with anti-dectin-1 neutralizing antibody, followed by curdlan and BCsup. OX40L expression on DC by flow cytometry. Representative of four independent experiments. (E) Summary of the four experiments. Each line indicates an independent experiment. (F) Hs578T-bearing NOD/SCID/β2m−/− mice were reconstituted with monocyte-derived DC and autologous T cells isolated from the same donor. β-glucan (Curdlan) (100μg/ml) or anti-dectin 1 mAb plus curdlan were co-injected with DC and T cells. Tumor size (ordinate) was monitored at indicated days (abscissa). White circle: PBS, black square: DC+T, blue triangle: DC+T+curdlan, red triangle: DC+T+anti-Dectin+curdlan, white square: curdlan.
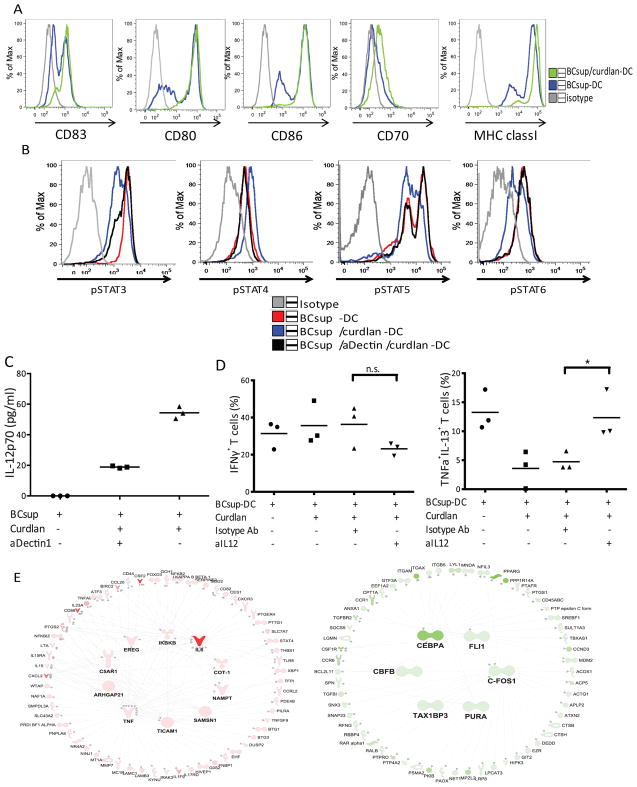
We then observed that DC treated with BCsups and curdlan showed high levels of CD83, CD80, CD86, CD70 and MHC class I indicating that curdlan is able to induce DC maturation in the presence of breast cancer-derived factors (17) (Figure 4A). Thus, curdlan blocks specifically OX40L expression without interfering with the other components of the DC maturation program. OX40L transcription in DC depends upon the phosphorylation of STAT5 and STAT6 (18). As we showed earlier, STAT6 in both the leukocyte infiltrate and the cancer cells is activated in the breast cancer microenvironment (13). Exposure of BCsups-DC to curdlan led to enhanced phosphorylation of STAT4 and decreased phosphorylation of STAT6 (Figure 4B) thereby resulting in an increased in the pSTAT4/pSTAT6 ratio. This switch in the activation pattern of STATs was associated with increased secretion of IL-12p70 by curdlan-treated DC (Figure 4C). Adding IL-12 neutralizing antibodies to co-cultures of naïve allogeneic T cells with curdlan-treated BCsups-DC restored the generation of iTh2 cells (Figure 4D). Thus, curdlan enables STAT4 activation in BCsups-DC, which is associated with increased IL-12 production and subsequent Th1 response.
Figure 4. Curdlan modulates DC maturation in breast cancer.
(A) Sorted blood DC were harvested after 48 hours of incubation under the indicated conditions, and the expression of CD83, CD80, CD86, CD70, and MHC class I were analyzed. Grey: isotype control; blue: BCsup-DC; green: BCsup/curdlan-DC. Representative histograms of 3 independent experiments. (B) Sorted blood DC were harvested after 1 hour incubation under the indicated conditions, and the expression of pSTAT3, pSTAT4, pSTAT5, and pSTAT6 were analyzed by intracellular staining and flow cytometry. Grey: isotype control; red: BCsup-DC; blue: BCsup/curdlan-DC; black: BCsup/aDectin/curdlan-DC. (C) The supernatant from DC culture was collected for IL-12p70 examination. Single points indicate samples from independent experiments. (D) Sorted blood DC were pretreated with curdlan and exposed to BCsup. The anti-IL-12 neutralizing Ab was used to pretreat the DC, which were harvested and co-cultured with allogeneic naïve T cells. The percentage of IFN-γ+ T cells and TNF-α+IL-13+ T cells defined at day 7 by ICS from 3 independent experiments. (E) Transcriptional profiles of BCsup-DC from 3 donors cultured for 6 hours in vitro in the presence of LPS-induced TLR4-activation inhibitor Polymyxin B (PMB); PMB+Curdlan or BCsup alone. 314 transcripts over-expressed 1.5 fold in Curdlan + PMB treatment compared to BCsup alone (Welch T-Test 0.05) were identified. 873 transcripts under-expressed 1.5 fold in Curdlan + PMB treatment compared to BCsup alone (Welch T-Test 0.05). Samples were normalized to each donor’s untreated reference sample. IPA of the 314 transcripts identified in the right panel. The color scale represents the fold-change of the molecules selected in the average of PMB+curdlan-treated DC as compared to untreated reference samples. The major over-expressed regulators are represented in the center of the network. Edges represent literature-based connections between molecules (full: direct connection, dashed: indirect connection). IPA of the 873 transcripts identified in the left panel.
Transcriptome analysis revealed the over-expression of 314 transcripts and the under-expression of 873 transcripts by curdlan-treated BCsup-DC (Figure 4E). Ingenuity pathway analysis (IPA) of the over-expressed transcripts revealed networks centered on NFκB, IL-6 and TNF (Figure 4E). The under-expressed transcripts formed networks centered on several transcription factors (Figure 4E). Curdlan-exposed DC showed abundant transcription of DC maturation markers such as CD86 and TNFSF9 (4-1BBL); cytokines such as GM-CSF, TNF, IL-6, IL-12, IL-15, and IL-23; integrins including ITGB8 that is involved in the activation of TGF-β (19); and several molecules that might facilitate migration including matrix metalloproteinase 7 (MMP7) (Supplement Table 3). The latter might facilitate DC migration to the draining lymph nodes, a feature that appears blocked in breast cancer-infiltrating DC (20). Conversely, curdlan-exposed DC under-expressed CD14, CD68, and CSF1R, all of which are associated with an immature DC phenotype. Consistent with DC maturation, CCR6, which contributes to immature DC retention at the tumor site by binding to MIP3-α (20), was also under-expressed. Thus, curdlan prevents the polarization of DC induced by soluble tumor factors and TSLP.
Dectin-1 signal blocks Tc2 differentiation and enables generation of effector CD8+T cells
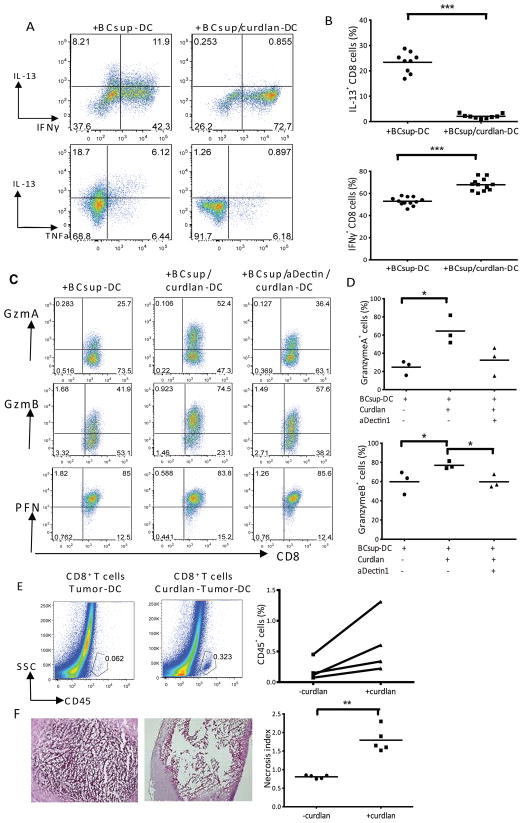
As CD8+T cells are essential effectors of antitumor immunity, naïve allogeneic CD8+T cells were co-cultured with BCsup-DC, exposed or not exposed to curdlan. ICS at day 7 revealed that upon PMA/Iono restimulation, CD8+T cells cultured with BCsup-DC produce IL-13 (+BCsup-DC: 23±1.3%; n=9), IFN-γ and TNF (Figure 5A and 5B), indicating a partial Type 2-polarization. However, CD8+T cells cultured with curdlan-treated BCsup-DC displayed a Type 1 phenotype with few IL-13-producing CD8+T cells (+BCsup-DC: 23±1.3%; +BCsup/curdlan-DC: 2±1%; n=9; p<0.0001), and predominantly IFN-γ-producing CD8+T cells (+BCsup-DC: 53±1%; +BCsup/curdlan-DC: 68±1.6%; n=9; p<0.001) (Figure 5A and 5B).
Figure 5. Curdlan enables generation of CTL able to induce tumor necrosis.
(A) Curdlan/BCsup-DC were co-cultured with allogeneic naïve T cells. ICS at day 7; gated AquanegCD3+CD8+ T cells. (B) Summary of 9 independent experiments. (C) Granzyme A, Granzyme B and perforin in CD8+T cells elicited by DC pre-treated under the indicated conditions. (D) Summary of 3 independent experiments. (E) CD8+T cells were sorted from in vitro culture and injected into MDA-MB231 breast tumors in NOD-SCID mice. Single cell suspensions from tumors harvested at day 3 were stained with anti-human-CD45 mAb and analyzed by flow cytometry. The percentage of CD45+ cells from 4 independent experiments. (F) H&E staining of tumor sections. The necrosis level is rated and calculated in each region. Summary of Points x areas/total area in each section=necrosis index. Necrosis index from 5 sections.
CD8+T cells cultured with BCsup-DC expressed high levels of perforin but low levels of granzymes (Gzm) A and B (Figure 5C and 5D). Similarly to monocyte-derived DC (13,21), curdlan-exposed BCsup-DC allowed the generation of CD8+T cells expressing high levels of Gzm A and B (Figures 5C and 5D). To test their effector function, CD8+T cells were labeled with CFSE and cultured with BCsup-DC with or without curdlan treatment for 6 days. Then, proliferating CFSE-negative CD8+T cells were sorted and injected into breast cancer tumors established in immunodeficient mice. At day 3 post-injection, CD8+T cells generated with curdlan-treated BCsup-DC persisted in the breast cancer microenvironment better than CD8+T cells generated by BCsup-DC (Figure 5E). CD8+T cell persistence within the tumor was associated with tumor necrosis (Figure 5F). Curdlan exposure of BCsup-DC resulted in the enhanced transcription of IL-15, IL15-RA and 4-1BBL (Supplement Table 3), molecules that are known to play important roles in the generation of high avidity CD8+ effector T cells facilitating cancer rejection (22–24).
Dectin-1-signal enables breast cancer-DC to promote generation of mucosal CD8+T cells via TGF-β
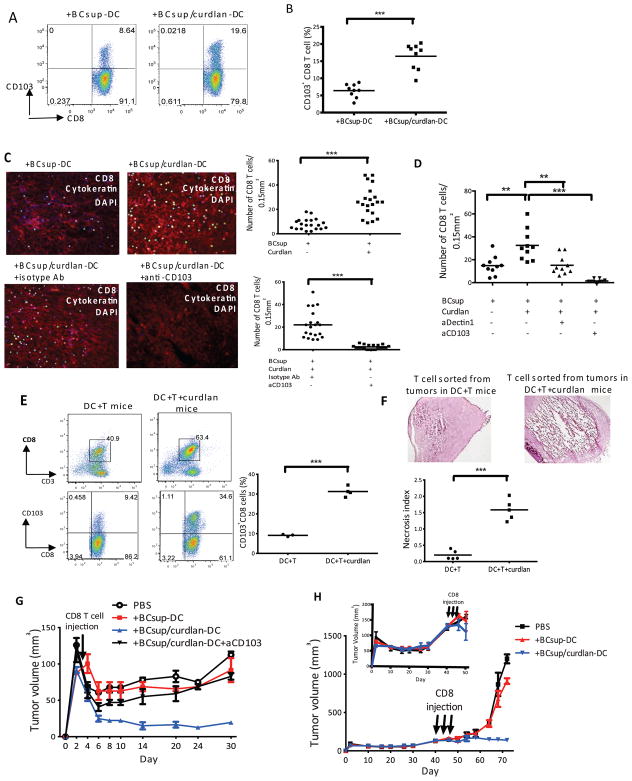
The accumulation and persistence of CD8+T cells in cancer nests is critical for cancer rejection. CD103 integrin allows the retention of effector and memory T cells (25) via binding to E-cadherin expressed on epithelial cells (26–29). DC exposed to curdlan showed an increased ability to induce CD103 on CD8+T cells (Figure 6A and 6B). To assess whether these CD103+CD8+T cells adhered to breast cancer, we used a modified Stamper-Woodruff tissue binding assay (20). Proliferating CFSE-negative allogeneic CD8+T cells were sorted from co-cultures with DC, re-labeled with CFSE (green), and overlaid on frozen breast cancer tissue sections to allow adherence. After 60-minutes, tissue sections were washed and counter-stained with anti-cytokeratin mAb (red) to visualize cancer cells. Numbers of bound T cells per 0.15 mm2 cytokeratin+ areas were counted on a series of consecutive tissue sections. CD8+T cells exposed to curdlan-treated DC adhered significantly more to frozen breast cancer tissue sections (Figure 6C) (+BCsup-DC: 7±1; +BCsup/curdlan-DC: 26±2; n=20; p<0.0001) and blocking CD103 with a mAb decreased their numbers (Figure 6C) (+BCsup/curdlan-DC+isotype Ab: 22±3; +BCsup/curdlan-DC+aCD103: 2±0.5; n=20; p<0.0001). The binding of CD8+T cells to breast cancer tissue sections was also decreased when BCsup-DC were pre-treated with anti-dectin-1 antibody prior to curdlan treatment (Figure 6D) (+BCsup/curdlan-DC:32±4; +BCsup/aDectin/curdlan-DC: 15±3; n=10; p=0.003). Thus, curdlan exposure enables DC to induce the differentiation of CD103+CD8+T cells in a dectin-1-dependent manner.
Figure 6. Curdlan enables generation of CD103+CD8+ T cells with enhanced binding to cancer cells and reject established breast cancer.
(A) Curdlan/BCsup-DC were co-cultured with allogeneic naïve T cells. CD103 expression on CD8+T cells at day 6. (B) Summary of 7 independent experiments. (C) CD8+T cells were sorted from DC-T co-cultures, labeled with CFSE, and used to overlay on frozen breast tumor sections. The sections were fixed and stained with cytokeratin (red). The CD8+T cells (green) were counted in each 0.15 mm2 cytokeratin+ area. Twenty fields were counted from two individual breast tumor sections. The T cells were pre-incubated with anti-CD103 or isotype control antibodies and then overlaid on breast tumor sections and processed as above. (D) CD8+T cells were co-cultured with DC that were pretreated with anti-dectin-1 neutralizing antibody, followed by curdlan and BCsup. CD8+T cells were sorted from DC-T co-culture and labeled with CFSE for Stamper-Woodruff assay as described above. Ten fields were counted from two individual breast tumor sections. (E) Hs578T-bearing NOD/SCID/β2m−/− mice were reconstituted with monocyte-derived DC and autologous T cells with or without Curdlan (100μg/ml). Gating of single cell suspension for human CD45+ cells and CD103+CD8+ T cells. Single dots represent the percentage of CD103+CD8+ T cells from each mouse. (F) CD8+T cells sorted from the tumor cell suspensions were injected into MDA-MB231 tumors in NOD-SCID mice. Frozen sections from the tumors at day 3, H&E staining. The necrosis level is rated and calculated as in Figure 6. (G) The NOD/SCID/β2m−/− mice were injected subcutaneously with 10×106 MDA-MB231 cells. 500,000 sorted CD8+T cells were injected into tumors. Black circles: PBS, n=6; Red squares: CD8+T cells from BCsup-DC-T co-culture, n=6; Blue triangles: CD8+T cells from BCsup/curdlan-DC-T co-culture, n=7; Green triangles: CD8+T cells from BCsup/curdlan-DC-T co-culture with pre-treatment with anti-CD103 mAb, n=8. (H) NOD/SCID/β2m−/− mice were injected subcutaneously with 10×106 MDA-MB231 cells. 500,000 sorted CD8+T cells were injected into tumors starting at day 40 after tumor implantation (insert plot: tumor size around 150mm3) every other day for a total of 3 injections. Black squares: PBS, n=6; Red triangles: CD8+T cells from BCsup-DC-T co-culture, n=6; Blue triangles: CD8+T cells from BCsup/curdlan-DC-T co-culture, n=7
Intratumoral injection of curdlan increased the frequency of CD103+CD8+T cells in breast cancer tumors in vivo (Figure 6E) (DC+T: 9±0.3 % of CD8+ T cells; n=3; DC+T+curdlan: 31±1.2 of CD8+T cells; n=4; p<0.0001). When sorted, these CD8+T cells triggered tumor necrosis upon transfer into tumors established in immunodeficient mice (Figure 6F). To establish whether the activated CD8+T cells can inhibit the development of highly proliferative tumors, we used MDA-MB231 breast cancer cells that can grow in immune microenvironment-independent manner. A single injection of CD8+T cells elicited by BCsup-DC treated with curdlan completely inhibited breast cancer progression in a manner dependent upon the expression of CD103 (Figure 6G). Indeed, breast cancer tumors only grew in mice that received control CD8+T cells or in the presence of CD103-blocking Ab (Figure 6G). Finally, CD8+T cells elicited by curdlan-treated DC were able to reject established breast cancers in vivo upon repeated adoptive transfer of as few as 0.5 × 106 T cells (Figure 6H).
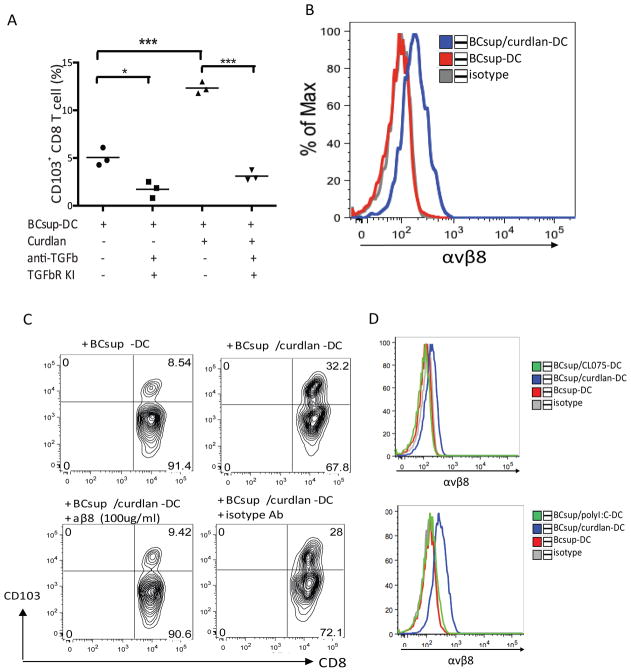
To determine the mechanism by which DC enabled the induction of CD103 expression in CD8+T cells, we analyzed the role of TGF-β1 as it induces CD103 expression on T cells (30,31). Using TGF-β1-neutralizing antibodies and pharmacologic blockade of TGF-β1 by the TGF-β RI kinase inhibitor II (32), the ability of curdlan-treated DC to induce the differentiation of CD103+CD8+T cells was substantially reduced (Figure 7A). Transcriptional profiling revealed that curdlan exposure enables the over-expression of ITGB8 in DC (Supplement Table 3). The product of this gene is a cell-surface receptor for the latent domain (LAP) of TGF-β (33). The binding to the integrin αvβ8 with subsequent metalloproteolytic cleavage of LAP represents a major mechanism of TGF-β activation in vivo (34). Consistent with RNA expression, curdlan-treated BCsup-DC showed increased cell-surface expression of αvβ8 (Figure 7B). Adding antibodies that neutralize αvβ8 to CD8+T cell co-cultures with curdlan-treated BCsup-DC resulted in the complete inhibition of CD103 expression by CD8+T cells triggered as the result of DC exposure to curdlan (Figure 7C). Thus, curdlan-treated DC activate TGF-β1 through αvβ8 to induce CD103+CD8+T cells that reject breast cancer cells. The impact of curdlan on DC is unique as DC activated with the TLR8 ligand or poly IC do not express αvβ8 (Figure 7D).
Figure 7. CD103+CD8+ T cells induction is TGFβ-dependent and mediated by αvβ8.
(A) DC pretreated with anti-TGF-β1 and TGF-β1 receptor kinase inhibitor for 30 min were co-cultured with T cells. Single points represent independent experiments. (B) αvβ8 staining. Grey: isotype control; red: BCsup-DC; blue: curdlan/BCsup-DC. (C) Sorted blood DC were pretreated with curdlan, activated by BCsup for 48 hours, pretreated with anti-β8 mAb, and co-cultured with allogeneic naïve T cells. Single point indicates the percentage of CD103+CD8+ T cells from 3 individual DC donors at day 6. (D) αvβ8 staining. Grey: isotype control; red: BCsup-DC; blue: poly-I:C or CL075/BCsup-DC.
Thus, reprogramming tumor-infiltrating DC via dectin-1 ligation enables the simultaneous blockade of Th2 inflammation and induction of mucosal CD8+T cells that are able to reject established cancers in vivo. This opens a novel avenue for immunotherapy of breast and pancreatic cancer, where links between type 2 inflammation and poor prognosis have been demonstrated.
DISCUSSION
Our previous studies have established the roles of tumor cells, DC, and iTh2 cells in the progression of breast cancer (2, 3). Here, we show an immunotherapy strategy for breast cancer based on the reprogramming of tumor-infiltrating DC in situ by targeting pattern-recognition receptor dectin-1. Indeed, the direct engagement of dectin-1 via intratumoral delivery of its ligand (β-glucan) initiated the reprogramming of DC maturation resulting in the broad modulation of tumor-infiltrating CD4+ and CD8+ T-cell function leading to breast cancer rejection. The key principle is a simultaneous blockade of protumor iTh2 response, a switch to Th1 immunity, and an amplification of a potent antitumor CD8+ T-cell immunity. The direct binding of β-glucan to tumor-infiltrating DC allows the reprogramming of their function including the blockade of iTh2 cells secreting IL-4 and IL-13 in favor of the generation of IFN-γ-secreting CD4+T cells, thus corroborating results from earlier studies (35,36). β-glucan-exposed DC induced the generation of CD8+T cells expressing CD103, a ligand for E-cadherin, with superior capacity to accumulate in and to reject breast cancer in vivo.
Suppression of type 2 responses is linked with enhanced IL-12 production by DC. Interestingly, the blockade of IL-12 in DC-T cell co-cultures restored iTh2 differentiation even though we have shown earlier that iTh2 differentiation is dependent on OX40L (3). A possible explanation is that when IL-12 is blocked and the CD40L signal is provided by T cells, the OX40L can be expressed and drive T-cell polarization (37). Whereas the abundance of IL-12 upon curdlan exposure was expected, genomic profiling revealed several intriguing transcripts including Notch 2 and IL1F9 (IL-36γ). In the mouse, DC-specific deletion of the Notch2 receptor caused a reduction of DC populations in the spleen, and was associated with the loss of CD11b+CD103+ DC in the intestinal lamina propria and a corresponding decrease of IL-17-producing CD4+T cells in the intestine (38). The IL-36 receptor pathway has been suggested in the regulation of IFN-γ secretion by murine CD4+T cells (39, 40). Furthermore, IL-36γ has been shown as downstream of the dectin-1/Syk signaling pathway upon exposure to Aspergillus Fumigatus (41). Thus, curdlan exposure in the presence of tumor-derived factors leads to phenotype-switch, and enables DC commitment to induce IFN-γ-secreting CD4+T cells. Although assessment of the global IFN-γ-secretion at the tumor level does not reveal a difference between curdlan-treated and -untreated tumors, we observe a clear difference at the level of CD4+T cells. These results suggest that other cells might contribute to IFN-γ secretion in untreated tumors.
The impact on mucosal CD8+T cell differentiation was specific to curdlan-dectin-1 signaling and could not be induced by exposure of DC to TLR8 ligand CL075 or to poly IC (42). Dectin-1-dependent mucosal marker of CD8+T cells is a CD103 integrin αE, which forms a heterodimer with the integrin β7 allowing peripheral CD8+T cells to be retained in the epithelial compartments (43, 44). CD103 specifically binds E-cadherin that is expressed on murine and human epithelial cancer cells (26,27). The expression of CD103 on CD8+T cells appears to depend mostly upon TGF-β1 (30,31). Studies on graft-versus-host disease in mice lacking TGF-β receptor signaling demonstrated that the effector CD8+T cells infiltrating the intestinal epithelium did not express CD103 and were less pathogenic (45). We have shown previously that human CD1c+ DC utilize activated membrane-bound TGF-β1 to induce CD103 expression on proliferating CD8+T cells, both in the allogeneic and autologous influenza-specific T-cell responses (46). Herein, we find that in the breast cancer environment, CD1c+ DC are largely inhibited by TSLP in their capacity to generate CD103-expressing CD8+T cells. However, dectin-1 engagement enables DC to express integrin β8 thereby facilitating TGF-β1 activation. Accordingly, whereas in the influenza model CD103 expression was contact-dependent (46), here the activity could be transferred by exposure to DC supernatant.
In the context of cancer, CD103 expression by cytotoxic T-lymphocytes (CTL) mediates adherence to E-cadherin resulting in cancer rejection (28). Indeed, mucosal-homing and retention of CD8+T cells is important for mucosal cancer vaccines (47). For example, only intranasal vaccination elicited mucosal-specific CD8+T cells expressing the mucosal integrin CD49a (47). These results confirm the critical role of the route of immunization for the trafficking of effector T cells (48, 49) and the critical role of tissue DC in imprinting the trafficking patterns of elicited T cells (50). Here, we provide another mechanism by which CD8+T cells can be equipped with molecules allowing mucosal retention.
In summary, our studies have identified a number of targets generated by tumor-infiltrating DC and T cells, the ligation of which results in tumor destruction in vivo by the human immune system in humanized mice. These include OX40L, IL-13, and now dectin-1; these agents act in a unique pathway that we have characterized. Whereas we need to characterize the impact of dectin-1 engagement on other cells present in the tumor microenvironment, in diseases where the iTh2 signature is associated with poor outcomes as is the case in breast (4, 5) and in pancreatic (6) cancer, the prevention of cancer-promoting effects combined with the expansion of potent CD8+ T-cell immunity might represent a novel option for these patients.
Supplementary Material
Acknowledgments
We thank the patients and the volunteers; Luz S. Muniz, Joseph Fay, and the Cores at BIIR including: Clinical, Apheresis, Flow Cytometry, and Imaging Core and the Animal Facility; and Jennifer L. Smith for the help provided. K.P. acknowledges the support from the BIIR, Baylor University Medical Center foundation, Cancer Prevention Research Institute of Texas, and NIH/NCI.
Footnotes
Conflict of interest:
Dr. Nishimura has a grant from Medimmune. Other authors do not report any conflict of interest.
References
- 1.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29(2):309–16. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204(5):1037–47. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208(3):479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teschendorff AE, Gomez S, Arenas A, El-Ashry D, Schmidt M, Gehrmann M, et al. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. doi: 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109(8):2802–7. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16(2):157–62. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WJ, Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42(1):39–47. doi: 10.1016/j.cyto.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Denardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011;186(10):5656–62. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10(2):203–13. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 14.Baran J, Allendorf DJ, Hong F, Ross GD. Oral beta-glucan adjuvant therapy converts nonprotective Th2 response to protective Th1 cell-mediated immune response in mammary tumor-bearing mice. Folia Histochem Cytobiol. 2007;45(2):107–14. [PubMed] [Google Scholar]
- 15.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–5. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E, et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38(4):831–44. doi: 10.1016/j.immuni.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min L, Isa SA, Fam WN, Sze SK, Beretta O, Mortellaro A, et al. Synergism between curdlan and GM-CSF confers a strong inflammatory signature to dendritic cells. Journal of immunology. 2012;188(4):1789–98. doi: 10.4049/jimmunol.1101755. [DOI] [PubMed] [Google Scholar]
- 18.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Science signaling. 2010;3(105):ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markovics JA, Araya J, Cambier S, Jablons D, Hill A, Wolters PJ, et al. Transcription of the transforming growth factor beta activating integrin beta8 subunit is regulated by SP3, AP-1, and the p38 pathway. J Biol Chem. 2010;285(32):24695–706. doi: 10.1074/jbc.M110.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190(10):1417–26. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112(13):4971–80. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 22.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37(6):1678–90. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 23.Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, et al. Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood. 2012;119(22):5182–90. doi: 10.1182/blood-2011-09-382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201–10. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372(6502):190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 26.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12(5):563–8. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 27.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162(11):6641–9. [PubMed] [Google Scholar]
- 28.Le Floc’h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204(3):559–70. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Floc’h A, Jalil A, Franciszkiewicz K, Validire P, Vergnon I, Mami-Chouaib F. Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cgamma-dependent pathway. Cancer Res. 2011;71(2):328–38. doi: 10.1158/0008-5472.CAN-10-2457. [DOI] [PubMed] [Google Scholar]
- 30.Parker CM, Cepek KL, Russell GJ, Shaw SK, Posnett DN, Schwarting R, et al. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992;89(5):1924–8. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rihs S, Walker C, Virchow JC, Jr, Boer C, Kroegel C, Giri SN, et al. Differential expression of alpha E beta 7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by alpha 4 beta 1-integrin crosslinking and TGF-beta. Am J Respir Cell Mol Biol. 1996;15(5):600–10. doi: 10.1165/ajrcmb.15.5.8918367. [DOI] [PubMed] [Google Scholar]
- 32.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28(6):870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J Clin Invest. 2011;121(7):2863–75. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134(3):392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragicevic A, Dzopalic T, Vasilijic S, Vucevic D, Tomic S, Bozic B, et al. Signaling through Toll-like receptor 3 and Dectin-1 potentiates the capability of human monocyte-derived dendritic cells to promote T-helper 1 and T-helper 17 immune responses. Cytotherapy. 2012;14(5):598–607. doi: 10.3109/14653249.2012.667873. [DOI] [PubMed] [Google Scholar]
- 36.Lin JY, Chen JS, Chen PC, Chung MH, Liu CY, Miaw SC, et al. Concurrent exposure to a dectin-1 agonist suppresses the Th2 response to epicutaneously introduced antigen in mice. Journal of biomedical science. 2013 Jan 3;20:1. doi: 10.1186/1423-0127-20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159(8):3838–48. [PubMed] [Google Scholar]
- 38.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35(5):780–91. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118(22):5813–23. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 40.Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120(17):3478–87. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- 41.Gresnigt MS, Rosler B, Jacobs CW, Becker KL, Joosten LA, van der Meer JW, et al. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur J Immunol. 2012;43(2):416–26. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- 42.Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PloS one. 2010;5(10):e13418. doi: 10.1371/journal.pone.0013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7(5):335–42. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12(6):485–91. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201(10):1647–57. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, et al. Human CD1c(+) Dendritic Cells Drive the Differentiation of CD103(+) CD8(+) Mucosal Effector T Cells via the Cytokine TGF-beta. Immunity. 2013;38(4):818–30. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci Transl Med. 2013;5(172):172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198(7):1023–34. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheasley-O’neill SL, Brinkman CC, Ferguson AR, Dispenza MC, Engelhard VH. Dendritic cell immunization route determines integrin expression and lymphoid and nonlymphoid tissue distribution of CD8 T cells. J Immunol. 2007;178:1512–22. doi: 10.4049/jimmunol.178.3.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.