Abstract
Antibody-mediated lymphocyte depletion is frequently used as induction therapy in sensitized transplant patients. Although T cells with an effector/memory phenotype remain detectable after lymphoablative therapies in human transplant recipients, the role of pre-existing donor-reactive memory in reconstitution of the T cell repertoire and induction of alloimmune responses following lymphoablation is poorly understood. We show in a mouse cardiac transplantation model that anti-donor immune responses following treatment with rabbit anti-mouse thymocyte globulin (mATG) were dominated by T cells derived from the pre-existing memory compartment. Administration of mATG one week prior to transplantation (pre-TP) was more efficient in targeting pre-existing donor-reactive memory T cells, inhibiting overall anti-donor T cell responses, and prolonging heart allograft survival than the commonly used treatment at the time of transplantation (peri-TP). The failure of peri-TP mATG to control anti-donor memory responses was due to faster recovery of pre-existing memory T cells rather than their inefficient depletion. This rapid recovery did not depend on T cell specificity for donor alloantigens suggesting an important role for posttransplant inflammation in this process. Our findings provide insights into the components of the alloimmune response remaining after lymphoablation and may help guide the future use of ATG in sensitized transplant recipients.
Keywords: memory T cells, lymphoablation, antithymocyte globulin
Introduction
The presence of donor-reactive memory T cells prior to transplantation results in robust immune responses to transplanted organs leading to poor graft outcome in humans and accelerated allograft rejection in animal models (1-5). Activated donor-specific memory T cell subsets mediate allograft injury through a variety of mechanisms. We have previously reported that memory CD4 T cells provide efficient help for activation of naïve donor-reactive CD4 and CD8 T cells and alloantibody production that in turn mediate allograft destruction (6-8). In addition, the initial contact of endogenous memory CD8 T cells with the donor endothelium early posttransplant promotes the infiltration of recipient leukocytes into the graft via activation of endothelial cells and up-regulation of adhesion molecules and chemokines (9, 10).
Lymphoablative agents such as polyclonal anti-thymocyte globulin (ATG) or monoclonal anti-CD52 antibody are commonly used as induction therapy in transplantation, particularly in highly sensitized patients and in patients receiving cadaveric donor and other marginal grafts (11-14). A problem with the use of these agents is that memory T cells are less susceptible to depletion strategies (15-18). Studies in non-human primates and human transplant recipients demonstrate that T cells with an effector/memory phenotype are detectable after anti-CD52 mAb or ATG induction and are associated with acute rejection episodes (19, 20). These activated T cells could arise from pre-existing memory T cells resistant to depletion therapy or from residual naïve T cells in response to the allograft and during homeostatic proliferation in the lymphopenic host. However, the origin of these cells, as well as their numbers, anatomical distribution, and effects on graft survival cannot be rigorously tested in human patients.
A rabbit anti-mouse thymocyte globulin (mATG), comparable with the commercial product used in clinical transplantation, was recently generated (21). Similar to the use of other depletion strategies, memory T cells are not entirely depleted in naïve non-transplanted mice even by high doses of the mATG (21, 22). A recent study of non-transplanted mice treated with mATG suggested that memory T cells do not have an advantage in proliferation over naïve T cells in ATG-generated lymphopenic conditions (22). However, the susceptibility of distinct donor-reactive memory T cell subsets to mATG depletion and the potential homeostatic or antigen-driven proliferation of the remaining memory T cells in the presence of an allograft have not been previously determined. The goals of our current study were to address these questions in a murine model of cardiac transplantation and to test whether the pathogenic functions of preexisting alloreactive memory T cells can be diminished by altering the timing of mATG administration.
Our results show that anti-donor immune responses after mATG depletion were dominated by T cells derived from the pre-existing memory compartment. Strikingly, administration of mATG one week prior to transplantation (pre-TP) was substantially more efficient in targeting pre-existing donor-reactive memory T cells, inhibiting overall anti-donor T cell responses, and prolonging heart allograft survival than the commonly used mATG treatment at the time of transplantation (peri-TP). The inferior ability of peri-TP mATG to control anti-donor memory responses was due to faster recovery of pre-existing memory T cells rather than their inefficient depletion. This rapid recovery did not depend on T cell specificity for donor alloantigens suggesting an important role for posttransplant inflammation in this process.
Materials and methods
Animals
The following mice, aged 6-8 weeks, were purchased from the Jackson Laboratories (Bar Harbor, ME): male C57BlL/6J (H-2b) [B6], congenic male B6.PL-Thy1a/Cy (H2b) [Thy1.1] and female B6.SJL-Ptprca Pep3b/ByJ [CD45.1], male BALB/cJ (H-2d) [BALB/c], female C3H/HeJ MMTV (H2k) [C3H] and female SJL/J-Pde6brd1 (H2s) [SJL]. 2C TCR transgenic mice on B6 background (H2b) were provided by Dr. Robert Fairchild (Cleveland Clinic). All animals were maintained and bred in the pathogen-free facility at the Cleveland Clinic. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Heart transplantation and recipient treatment
Vascularized heterotopic cardiac allografts were placed in the abdomen of recipient mice using standard techniques and monitored as previously described (6, 7, 23). Rejection was defined as the absence of a palpable heart beat and confirmed by laparotomy. Grafts were harvested at the time of rejection or at indicated time points, embedded in paraffin and stained with H&E and anti-C4d antibody as previously published (24). Rabbit anti-mouse thymocyte globulin (mATG) and control rabbit IgG were generated by Genzyme as previously described (25). Heart allograft recipients were treated with mATG or control rabbit IgG (0.5 mg/injection in BALB/c recipients and 0.5 mg or 1mg/injection in B6 recipients) either on days -7 and -4 (pre-TP mATG) or on days 0 and 4 (peri-TP mATG) relative to the transplantation.
Flow cytometry
Phycoerythrin (PE)-conjugated anti-mouse CD4, fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4, allophycocyanin (APC)-conjugated anti-mouse CD8, PE-conjugated anti-mouse anti CD44, peridinin-chlorophyll protein (PerCP)-conjugated anti-mouse CD44, FITC-conjugated anti-mouse CD45.1, APC-conjugated anti-mouse CD45.1, FITC-conjugated anti-mouse CD45.2, PE-conjugated anti-mouse Thy1.1, FITC-conjugated anti-mouse Thy1.2, PE-conjugated anti-mouse CD62L were purchased from BD Pharmingen (San Diego, CA) or from eBioscience (San Diego, CA). Cells were isolated from peripheral blood, spleen, bone marrow, lung and liver and stained with indicated reagents as previously described (6, 7, 23). For intracellular staining, cells initially stained for cell surface markers were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), washed and incubated for 1 hour at room temperature with PE-conjugated anti-mouse Ki-67 antibody (eBioscience) in PBS plus 0.1% bovine serum albumin and 0.1% saponin. The labeled cells were washed in PBS plus 0.1% BSA and 0.02% NaN3 and resuspended in PBS. At least 200,000 events/sample were acquired on a BD Bioscience FACSCalibur (BD Biosciences, San Diego, CA) followed by data analysis using FlowJo software (Tree Star Inc., Ashland, OR).
Adoptive transfer experiments
To generate alloreactive memory T cells, BALB/c skin allograft were placed onto B6 or 2C TCR transgenic recipients. Four weeks after transplantation, total T cells were isolated from recipient spleens by negative selection using commercially available murine T cell isolation columns from R&D Systems (Minneapolis, MN) or EasySep magnetic bead particles from STEMCELL Technologies (Vancouver, BC). After T cell enrichment, cells were resuspended at 10 × 106/ml in PBS+ 2% Fetal Bovine Serum, and labeled using antibodies against CD4, CD8 and CD44 for 30 minutes on ice followed by two washes. CD4+CD44hi T cells and CD8+CD44hi T cells were then sorted on a FACSAria II sorter (BD Biosciences). Analogously, CD4+CD44lo and CD8+CD44lo T cells were sorted from spleens of naive congenic B6 mice. Then, 2 × 106 sorted naive or memory CD4 and CD8 T cells were intravenously injected into congenic Thy1 or CD45 disparate B6 mice (see Figure 4A for detailed experimental design). In some experiments, isolated memory T cells were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFSE) as previously published (26). Briefly, cells were resuspended at 20 × 106/ml in PBS and incubated with 1.25μM CFSE for 8 minutes at room temperature. The staining was stopped by the addition of equal volume of fetal calf serum (FCS, Atlanta Biologicals, Lawrenceville, GA) followed by 1 minute incubation. Stained cells were washed twice with RPMI1640 (Gibco Life Technologies, Grand Island, NY) plus 10% FCS, counted and used for adoptive transfer experiments.
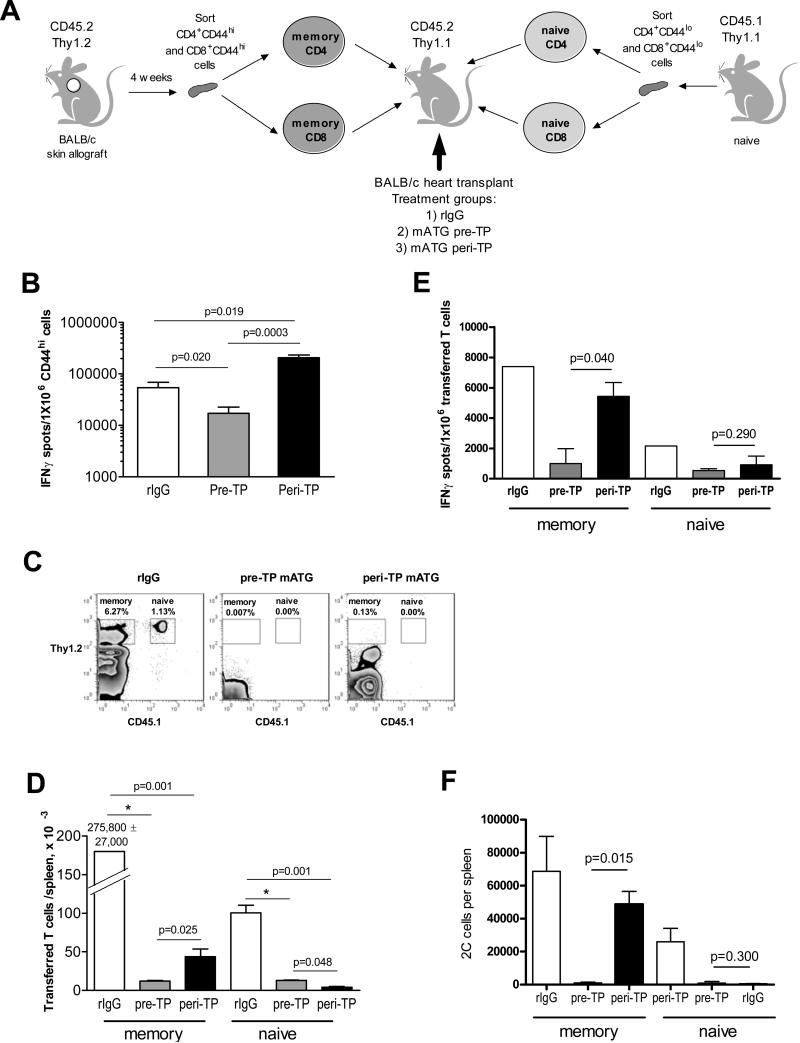
Figure 4.
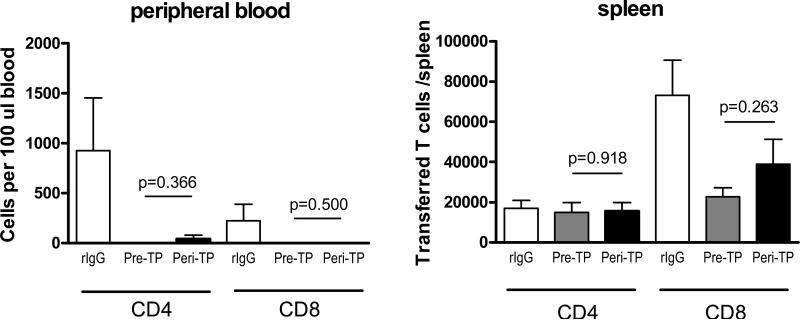
Pre-TP mATG results in reduced recovery of pre-existing donor-reactive memory T cells compared to peri-TP treatment. A. Experimental design. BALB/c-reactive memory CD4 and CD8 T cells were generated by placing BALB/c skin allografts onto CD45.2/Thy1.2 B6 recipients and isolated by flow sorting 5 weeks after skin transplantation. Naïve CD4 and CD8 T cells were isolated by flow sorting from CD45.1/Thy1.2 B6 mice. CD45.2/Thy1.1 B6 recipients were treated pre- or peri-TP with mATG or control rabbit IgG and sacrificed on day 10 after transplantation. B. Recipient spleen cells were tested in a recall IFNγ ELISPOT assay against donor BALB/c or third party SJL stimulator cells. The results are expressed as numbers of IFNγ spots per 1×106 CD44hi spleen T cells. C-D. Transferred CD45.2+Thy1.2+ memory and CD45.1+Thy1.1+ naïve T cells were enumerated in recipient spleens by flow cytometry. C. Representative flow cytometry dot plots showing gating strategy. The numbers show the percentages of target populations within live spleen cells. D. Cell numbers per spleen. E. Transferred CD45.2+Thy1.2+ and CD45.1+Thy1.2+ cells were re-isolated from recipient spleens by flow sorting and tested against donor BALB/c or third party SJL stimulator cells in a recall ELISPOT assay. The results are expressed as numbers of IFNγ spots per 1×106 purified memory or initially naïve T cells. N=5-10 recipients per group. F. 2C/Thy1.1 naïve and 2C/Thy1.2 memory T cells were isolated from 2C recipients of BALB/c skin allografts 5 weeks after skin transplantation and transferred into CD45.1 B6 recipients of BALB/c cardiac allografts treated with mATG or rIgG as indicated. Transferred T cell subsets were enumerated on d. 10 posttransplant by flow cytometry. Gating strategy was similar to that for polyclonal naïve and memory T cells. N=3-10 mice per group.
ELISPOT assay
Assays were performed as previously described using capture and detecting anti-mouse IFNγ antibody from BD Pharmingen (27). Recipient spleen cells were stimulated with mitomycin C-treated BALB/c, SJL or B6 spleen cells for 24 h. Responder cells were titrated from 400,000 to 20,000 per well with the addition of 400,000 stimulator cells per well. The resulting spots were analyzed using an ImmunoSpot Series 4 analyzer (Cellular Technology, Cleveland, OH).
Statistical Analysis
Heart allograft survival was compared between groups by Kaplan-Meier analysis. All other results were analyzed by non-parametric Mann-Whitney test. Total numbers of animals per experimental groups are indicated in figure legends. A p value < 0.05 was considered a significant difference. Unless noted otherwise, the data are represented as mean values ± SD.
Results
T cells with an effector/memory phenotype are more resistant to mATG-mediated depletion than naïve T cells
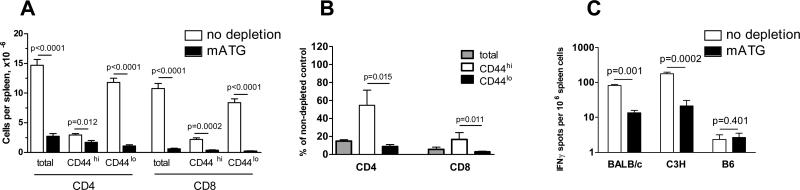
Naïve 4-6 week old B6 mice contain endogenous effector/memory CD44hi CD4 and CD8 T cells (3-5% of total splenocytes). Some of these cells are reactive to alloantigens and secrete the effector cytokine IFNγ in short-term ELISPOT assays (28, 29). In initial experiments, we evaluated the effects of mATG on endogenous memory T lymphocyte subsets in naïve non-transplanted B6 mice. Consistent with previous reports (21, 22), two injections of mATG (25mg/kg) depleted 95-98% of T cells in the circulation and up to 95% T cells in the spleen with more prominent effects on CD8 than on CD4 T cells (Figures S1 and 1A). The residual CD4 and CD8 T lymphocytes were enriched for cells with a CD44hi effector/memory phenotype (Figures S1 and 1B). IFNγ ELISPOT assays performed prior to and following depletion showed that mATG treatment significantly decreased the frequencies as well as the total numbers of alloreactive T cells (Figure 1C and not shown). Nevertheless, alloreactive memory T cells still remained detectable in the spleens of mATG-treated mice with a potential to undergo activation by donor antigens and contribute to allograft rejection after transplantation (Figure 1C).
Figure 1.
T cells with an effector/memory phenotype are more resistant to mATG depletion than naïve T cells. Non-transplanted B6 mice were injected with 25 mg/kg mATG on days 0 and 4. The numbers of spleen CD44hi and CD44lo T cells were determined by flow cytometry 2 days after the last mATG injection and are represented as total numbers (A) or percent of corresponding T cell populations in non-depleted B6 mice (B). C. The frequencies of spleen cells secreting IFNγ in response to BALB/c and C3H alloantigens and to self B6 antigens were determined by ELISPOT assay prior to and on day 6 after the last mATG injection. N=4-5 mice per group.
Pre-transplant administration of mATG is more efficient in prolonging heart allograft survival and inhibiting anti-donor T cell responses than treatment at the time of transplantation
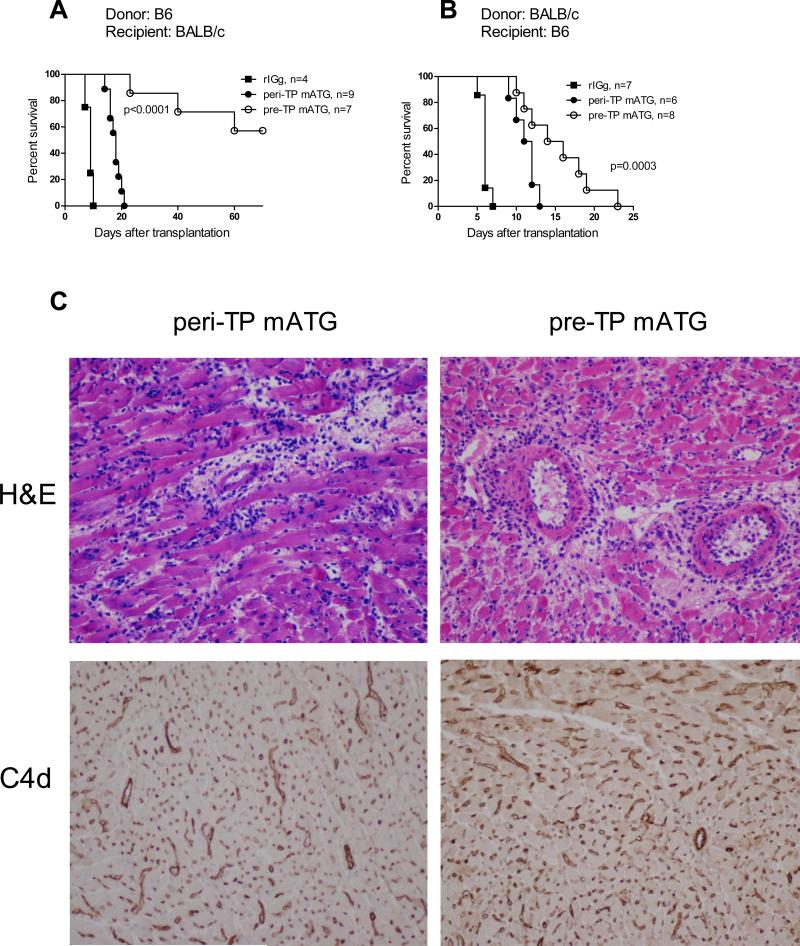
We next tested whether administration of mATG prior to re-activation with alloantigen will be more efficient in depleting donor-reactive memory T cells and in prolonging heart allograft survival than peri-transplant treatment. BALB/c (H-2d) recipients of B6 (H-2b) heart allografts and B6 recipients of BALB/c heart allografts were treated with mATG either on days -7 and -4 before transplantation (pre-TP) or on days 0 and 4 after transplantation (peri-TP). Two pre- or peri-TP injections of 0.5 mg mATG prolonged cardiac allograft survival in BALB/c recipients compared to control IgG treated mice (Figure 2A). However, the same mATG treatments had more modest effects in B6 recipients of BALB/c heart allografts (MST of 6.0 ± 0.6, 8.0 ± 0.6 and 11.5 ± 0.3 days for control IgG, peri-TP and pre-TP treatments, respectively) consistent with previous data on the resistance of the B6 strain to graft-prolonging therapies (30, 31). Increasing the dose of mATG to 1 mg/injection resulted in improved graft survival in B6 recipients (Figure 2B). The differences in allograft survival time between mATG treated B6 and BALB/c recipients was likely to result from higher immunogenicity of BALB/c heart allografts rather than from different numbers of preexisting memory T cells or to the different T cell susceptibility to mATG depletion between two strains (Figure S2). Importantly, regardless of the donor/recipient strain combination or mATG dose, pre-TP mATG resulted in better allograft outcome than peri-TP treatment.
Figure 2.
Pre-TP administration of mATG is more efficient in prolonging heart allograft survival than peri-TP treatment. Recipients of fully MHC-mismatched heart allografts were treated with mATG either on days 0 and 4 after transplantation (peri-TP) or 7 and 4 days prior to transplantation (pre-TP). Control groups were treated with rabbit IgG on days -7 and -4 prior to transplantation. A. Survival of B6 heart allografts in BALB/c recipient treated with 25 mg/kg mATG per injection. B. Survival of BALB/c heart allografts in B6 recipients treated with 50 mg/kg mATG per injection. C. Heart allografts were harvested from B6 recipients at the time of rejection. H&E staining and immunohistochemical staining for C4d were performed on paraffin-embedded tissue sections. The images are representative of 4-5 rejecting heart allografts analyzed in each group. Original magnification 200x.
In both mATG-treated groups, the rejecting heart allografts were heavily infiltrated with mononuclear cells characteristic of acute cellular rejection. In addition, immunohistochemical staining revealed diffuse deposition of the complement split product C4d on graft capillaries (Figure 2C). Consistent with these findings, comparable titers of donor-reactive IgG alloantibodies were detected in serum of recipients treated with mATG pre- or peri-TP at the time of rejection (data not shown). Thus, neither pre- nor peri-TP mATG treatment entirely eliminates CD4 T cell help for alloantibody production and the grafts rejected by mATG treated recipients demonstrate signs of antibody-mediated injury.
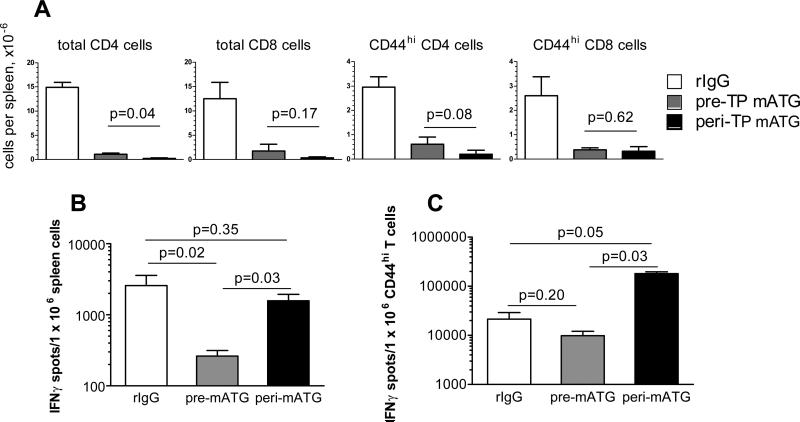
We next tested whether prolonged heart allograft survival in the pre-TP mATG treated group was associated with more efficient depletion of recipient T lymphocytes. Both mATG treatment regimens resulted in prominent depletion of CD44lo as well as CD44hi T cells compared to rIgG treatment. At the time of rejection, recipients treated with mATG either pre- or peri-TP had comparable percentages and numbers of total CD4 and CD8 T cells and CD44hi CD4 and CD8 T cells in the spleen (Figure 3A) and in the peripheral blood (not shown). Despite the massive T cell depletion by peri-TP mATG, however, the frequencies of spleen cells secreting IFNγ in response to donor antigens were similar to those in non-depleted mice (Figure 3B). Furthermore, the residual CD44hi population was enriched for donor-reactive IFNγ-producing T cells compared to non-depleted recipients (Figure 3C). In contrast, pre-TP administration of mATG resulted in significantly lower frequencies of donor-specific IFNγ-secreting T cells compared to peri-TP mATG and to the control rIgG treatment and did not lead to the accumulation of donor-reactive T cells with an effector/memory phenotype.
Figure 3.
Pre-transplantation (pre-TP) administration of mATG is more effective in inhibiting anti-donor responses. B6 recipients of BALB/c heart allografts were treated with mATG either pre- or peri-TP and sacrificed at the time of rejection (d. 5, 6×5, 7 for rIgG treated group, d. 9, 10, 11, 12×2, 13 for peri-TP mATG treated group, and d. 10, 11, 12, 14, 16, 18, 19, 23 for pre-TP mATG treated group). A. The numbers of total, CD44hi and CD44lo CD4 and CD8T cells in the spleen were determined by flow cytometry. B-C. Spleen cells were tested against donor BALB/c or third party SJL stimulator cells in a recall ELISPOT assay at the time of rejection. The results are expressed as numbers of IFNγ spots per 1×106 spleen cells (B) or per 1×106 CD44hi spleen T cells (C). The numbers of cells producing IFNγ in response to the third party SLJ stimulators were < 20/1×106 spleen cells and < 500/1×106 CD44hi spleen T cells in all groups. N=3-4 recipients per group. The experiment was performed twice with similar results observed each time.
Pre-TP mATG results in greater inhibition of pre-existing donor-reactive memory T cell responses than peri-TP treatment
To dissect the effects of mATG treatment on pre-existing memory versus naïve T cells, we generated B6 mice containing congenic populations of naïve CD4 and CD8 and BALB/c-reactive memory CD4 and CD8 T cells so as to track each subset separately within the same recipient (Figure 4A). These recipients were transplanted with BALB/c heart allografts and treated with mATG or with control rabbit IgG either pre- or peri-TP. The numbers of transferred cells in spleen and peripheral blood were determined by flow cytometry on day 10 after transplantation. Recall IFNγ production by recipient spleen cells was comparable to that in the absence of T cell transfer (Figures 4B and 3C) indicating that injected tracer T cell subsets did not significantly alter overall anti-donor responses. Whereas both mATG regimens resulted in prominent reduction in numbers of T cells derived from naïve precursors, pre-TP mATG was significantly more efficient than peri-TP treatment in decreasing numbers of CD4 and CD8 T cells derived from preexisting memory populations (Figure 4C-D, Figure S3). Furthermore, pre-TP mATG inhibited donor-specific IFNγ production by residual memory T cells to a greater degree than peri-TP administration (Figure 4E). Similar reduction in tracer memory T cell numbers in pre- versus peri-TP mATG treated recipients was observed on day 7 after transplantation (20,645 ± 12,466 vs.187,573 ± 82,229 transferred memory T cells per spleen, respectively).
Unequal precursor frequencies of polyclonal alloreactive T cells within naïve and memory populations could potentially influence the accumulation of these cells in mATG treated allograft recipients. To rule out this possibility, we performed analogous experiments using 2C TCR transgenic CD8 T cells reactive to Ld class I MHC molecule expressed by H-2d BALB/c mice. The results were similar to those observed with polyclonal CD4 and CD8 T cells confirming the superior control of pre-existing memory T cells by pre-TP mATG (Figure 4F).
In contrast to pre-TP mATG, peri-TP lymphoablation results in faster recovery of pre existing memory T cells regardless of their antigen specificity
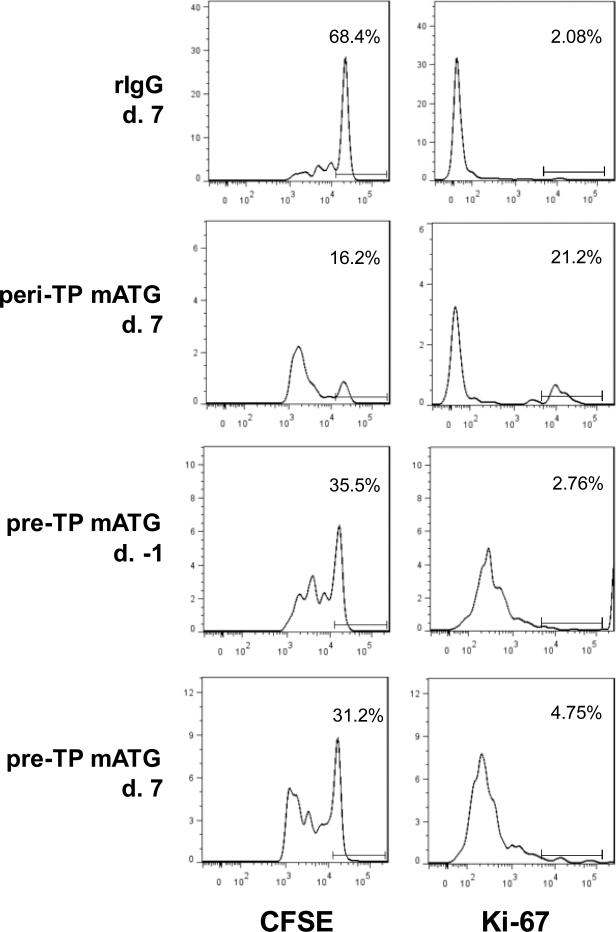
In our adoptive transfer experiments, both the efficiency of memory T cell depletion and their homeostatic and antigen-driven expansion can contribute to cell numbers observed on day 10 after transplantation. We next tested whether the presence of specific antigens and rapid reactivation of memory T cells undermines their depletion by peri-TP mATG. Groups of B6 recipients containing tracer subsets of donor-reactive memory T cells received a single injection of 1 mg mATG pre- or peri-TP and were sacrificed 2 days later (prior to graft placement in the pre-TP treated group and on day 2 post transplant in the peri-TP treated group) to eliminate the contribution of T cell expansion. Unexpectedly, the numbers of residual memory T cells were similar in both groups regardless of the presence of an allograft (Figure 5). These findings suggested that accelerated expansion rather than inefficient depletion of donor-specific memory T cells accounts for their increased accumulation in peri-TP treated recipients. To examine this possibility, donor-reactive memory T cells were labeled with CFSE prior to the adoptive transfer into heart allograft recipients treated with mATG or control rIgG. The proliferation history and ongoing cell division were assessed at d. 7 posttransplant by CFSE dilution and intracellular staining for nuclear protein Ki-67, respectively. Both approaches showed that the proliferation of tracer memory T cells was increased in recipients treated with mATG peri-TP compared to pre-TP treated group (Figure 6). Notably, memory T cell in pre-TP treated recipients mostly expanded prior to transplantation and did not proliferate further after allograft placement (d. -1 vs. d. 7 time point in Figure 6).
Figure 5.
Pre-TP and peri-TP mATG administration deplete preexisting memory T cells with equal efficiency. Groups of CD45.1 B6 mice were injected with BALB/c-reactive CD45.2+ memory CD4 and CD8 T cells and treated with a single dose of 1 mg mATG or rabbit IgG either simultaneously with BALB/c heart allograft placement (peri-TP) or in the absence of transplantation (pre-TP). The numbers of transferred CD4 and CD8 T cells in peripheral blood and spleen were determined 2 days after the mATG injection. N=4 mice per group.
Figure 6.
Pre-TP mATG administration results in decreased proliferation of preexisting memory T cells compared to peri-TP treatment. BALB/c-reactive CD45.2+ memory T cells were labeled with CFSE and adoptively transferred into CD45.1 B6 mice followed by BALB/c heart allograft placement and rIgG or mATG treatment. CFSE dilution and Ki-67 expression were assessed on d. 7 posttransplant for IgG and peri-TP mATG treated groups and on d. -1 and 7 posttransplant for pre-TP mATG treated group. The histograms are shown after gating on transferred cells and are representative of 3-4 mice per group. The numbers within histograms represent % of non-dividing CFSEhi cells and % of Ki-67hi cells.
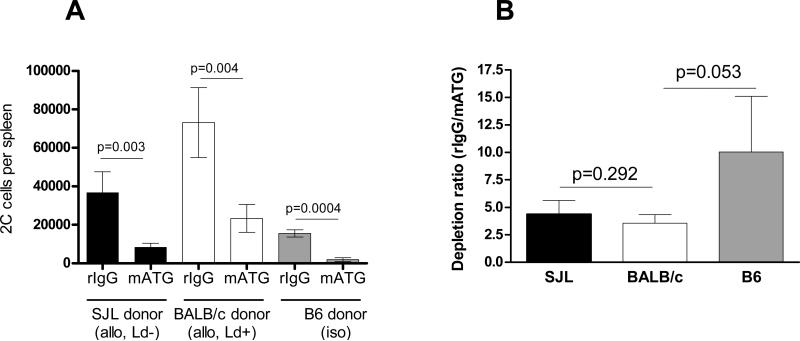
We next tested whether memory T cells specific for donor alloantigens preferentially expand and accumulate in lymphopenic host after peri-TP mATG treatment. B6 recipients injected with congenic 2C memory T cells were transplanted with either Ld+ BALB/c or Ld- SJL heart allografts or with B6 heart isografts and treated with mATG or control rabbit IgG at the time of transplantation. As anticipated, reactivation with Ld alloantigen led to increased numbers of transferred 2C cells in control-treated recipients compared to the control SJL allograft or B6 isograft recipients (Figure 7A). In allograft recipients, peri-mATG was equally potent in reducing the numbers of 2C cells in comparison to control-treated mice regardless of donor Ld expression (Figure 7B). Thus, the faster recovery of memory T cells observed after peri-TP mATG administration does not depend on their specificity for donor alloantigens. The recovery of 2C cells in isograft recipients was compromised compared to both types of allograft recipients.
Figure 7.
Recovery of pre-existing memory T cells after peri-TP mATG treatment does not depend on their antigen specificity. Memory 2C cells were adoptively transferred into B6 mice followed by placement of Ld+ BALB/c or Ld- SJL heart allografts or B6 isografts and mATG or rabbit IgG treatment at the time of transplantation (peri-TP). The numbers of transferred 2C cells were determined by flow cytometry on day 10 after transplantation. The results are presented as absolute numbers per spleen and as ratios of 2C cell numbers detected in rIgG treated versus mATG treated recipients. N=5-12 mice per group.
Discussion
Lymphoablation is commonly used as an induction therapy in transplant patients (11-14). Previous studies in non-human primates and in human transplant patients demonstrated that T cells with an activated/memory phenotype are detectable in the circulation after lymphoablative therapies and are associated with the recovery of anti-donor cellular reactivity and acute rejection episodes (15, 20, 26). In theory, these cells can arise from pre-existing memory T cells resistant to depletion therapy, from residual naïve T cells in response to the allograft and during homeostatic proliferation in the lymphopenic host or from naïve T cells newly generated in the thymus. This is highly relevant to clinical practice as one indication for the use of T cell depleting agents is the recipient's previous sensitization to donor or third party alloantigens. However, the effects of depletion therapies on different arms of the memory immune response have not been previously assessed. In this study, we evaluated whether ATG treatment controls pre-existing donor-reactive memory T cells and investigated the origin of anti-donor alloreactivity in ATG-treated murine heart allograft recipients. We realize that the polyclonal anitbodies raised against rodent cells may have different spectra of specificities and biological effects from anti-human thymoglobulin. One well-documented example of such differences is the depletion of B lymphocytes by anti-human thymoglobulin but not by mATG (14, 21). Nevertheless, both anti-human and anti-murine ATG have high potency in depleting T lymphocytes thus justifying the use of mATG in mouse model of transplantation to study T cell depletion and recovery following ATG induction.
Consistent with previous studies using various depletion agents, T cells remaining in the periphery of non-transplanted mice after mATG treatment were enriched for the effector/memory phenotype (16, 17, 21, 22, 32, 33). Despite prominent T lymphocyte depletion in the peripheral blood and spleen, memory T cells within the bone marrow and non-lymphoid tissues such as lung and liver were more resistant to the effects of mATG (data not shown). Ongoing studies in our laboratory will investigate whether this resistance is due to anatomical location and inaccessibility to circulating mATG or due to intrinsic properties of memory T cells from specific tissue compartments. Furthermore, the contributions of residual T cells from different compartments to host T cell repertoire reconstitution and to subsequent responses against transplanted organ remain to be determined.
In the absence of other immunosuppression, peri-TP mATG treatment resulted in modest prolongation of cardiac allograft survival in two different recipient strains. Despite initial depletion of both naïve and memory T cells, anti-donor responses in recipients treated with peri-TP mATG were either comparable to or exceeded those in control non-depleted recipients on day 10 post transplant and at the time of rejection (Figures 3B-C and Figure 4B). Using the adoptive transfer system that allowed simultaneous tracing of both naïve and memory T cell populations within the same recipient, we demonstrated for the first time that pre-existing donor-reactive memory T cells contribute significantly to the anti-donor immune responses observed in mATG treated heart allograft recipients. The presence of an allograft may play an important role in the recovery of different T cell subsets as Sener and colleagues previously reported that memory T cells do not have an advantage in proliferation over naïve T cells in ATG-treated non-transplanted mice (22).
We reasoned that memory T cell depletion in the absence of donor antigens and inflammation inflicted by the surgery may be more prominent and thereby beneficial for graft outcome and tested this by administering mATG prior to transplantation (pre-TP). Pre-TP mATG was much more efficient than peri-TP treatment in inhibiting overall anti-donor T cell responses, reducing the numbers of pre-existing CD4 and CD8 donor-reactive memory T cells and prolonging heart allograft survival (Figures 2-4, Figure S3). Unexpectedly, the presence of an allograft at the time of mATG administration did not result in inferior depletion of memory or naïve T cells (Figure 5). Instead, the higher numbers of donor-reactive T cells derived from memory precursors were likely to be due to the more efficient expansion of memory T cells in peri-TP treated recipients (Figure 6). The superior homeostatic proliferation of memory over naïve T cells after lymphoablation in naïve mice has been reported (17), however, the influence of an allograft on this process has not been previously assessed. While donor alloantigens played a role in the overall accumulation of donor-reactive memory T cells, non-specific memory T cells transferred into heart allograft recipients demonstrated similar rates of recovery suggesting that posttransplant inflammation at the time of depletion enhances their homeostatic expansion. Conversely, the recovery of donor-reactive memory T cells may be impeded by currently used immunosuppression as suggested by the kinetics of T cell repopulation in human transplant patients after lymphodepletion (20, 26). Notably, the recovery of memory T cells in isograft recipients was compromised compared to the recipients of both specific antigen expressing and non-expressing allografts (Figure 7). These findings are consistent with previously reported lower levels of early and late posttransplant inflammation in iso- versus allografts (10, 34, 35). Our laboratory is currently investigating how transplantation-induced inflammatory cytokines such as TNFα and IL-1 and the timing of the exposure to these factors affect homeostatic and antigen-driven expansion of memory T cells.
The contribution of depletion-resistant memory T cell subsets and their descendants to graft rejection may vary at different stages after transplantation. For example, circulating donor-reactive memory CD8 T cells infiltrate the vascularized cardiac allograft within hours after transplantation and amplify early intragraft inflammation and subsequent recruitment of newly generated effector T cells (9, 10). This mechanisms is operational in peri-TP mATG treated recipients as the greatest level of T cell depletion is not achieved until 4-5 days after the mATG injection ((21) and data not shown). In contrast, preemptive lymphoablation significantly reduces the numbers of circulating T cells prior to transplantation thus alleviating early intragraft inflammation.
Our results strongly suggest that even in non-sensitized mice, endogenous memory T cells are a prominent component of anti-donor immune responses after peri-TP lymphoablation and that targeting these memory T cells with pre-TP mATG administration significantly improves allograft outcome. In addition to effects on preexisting memory T cells, peri-TP mATG can trigger several other mechanisms of graft prolongation compared to pre-TP treatment. In addition to T cell depletion, mATG is likely to affect other types of recipient cells (14, 36-39). For example, mATG injected at the time of transplantation can directly react with graft endothelial cells and augment their activation, cytokine and chemokine release as well as early complement deposition in the graft. This possibility is corroborated by clinical reports that horse ATG therapy was associated with the deposition of horse IgG and activation of complement in renal transplants and, in a few cases, induction with ATG led to hyperacute antibody-mediated rejection (34, 35). In contrast, administration of mATG several days prior to the surgery is likely to result in significantly diminished binding of the rabbit antibody directly to the graft, as >95% of mATG is absorbed or cleared within 24 hours after injection (21). We are currently evaluating the contribution of early ATG deposition in the graft to overall intragraft inflammation including cytokine and chemokine influx, donor alloantigens release and presentation and subsequent T cell recruitment.
Another possible factor modulating the efficacy of different mATG regimens is the kinetics of recipient and donor antigen presenting cell (APC) depletion. While pre-TP mATG reduced the number of peripheral CD11c+ cells (data not shown), depletion at the time of transplantation may allow both donor and recipient dendritic cells to present donor antigens prior to depletion by mATG. This early antigen presentation by professional antigen presenting cells may be especially important in enhancing the proliferation of antigen-experienced memory T cell subsets.
Among several proposed mechanisms of graft prolongation by ATG in human patients as well as in animal models is the expansion and function of T regulatory cells (32, 40, 41). The absolute numbers of Foxp3+ T cells in the recipient spleen and heart allograft in our study were similar in all experimental groups arguing against the possibility that pre-TP mATG results in superior expansion of Tregs compared to peri-TP mATG administration. However, due to more prominent inhibition of memory T cell recovery and anti-donor effector cell generation by pre-TP mATG, the decreased Teff/Treg ratio may influence allograft outcome in these recipients.
In summary, our results in a mouse cardiac allograft model strongly suggest that memory T cells can be a prominent component of ensuing anti-donor immune responses in recipients undergoing lymphoablative therapy at the time of transplantation. Most remarkably, the graft-prolonging effects of ATG therapy and its ability to target preexisting memory T cells can be significantly increased by a pre-transplant administration regimen. These findings provide insights into the components of the alloimmune response after lymphoablation and may guide the future use of ATG in sensitized transplant recipients.
Supplementary Material
Acknowledgments
We thank Nina Volokh and Earla Biekert for expert technical assistance.
This work was supported by a 1P01 AI087586 grant and by a R01 AI058088 from the NIH (AV).
Abbreviations
- APC
antigen presenting cell
- ATG
antithymocyte globulin
- BSA
bovine serum albumin
- FCS
fetal calf serum
- mATG
rabbit anti-mouse thymocyte globulin
- MFI
mean fluorescence intensity
- MST
mean survival time
Footnotes
Disclosure
Rabbit anti-mouse thymocyte globulin (mATG) and control rabbit IgG were provided by Genzyme.
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. Am J Transplant. 2004;4(6):846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 2.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82(1):1–9. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 3.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6(4):647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 4.Valujskikh A. Targeting T-cell memory: where do we stand? Curr Opin Organ Transplant. 2008;13(4):344–349. doi: 10.1097/MOT.0b013e3283061126. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol. 2007;18(8):2252–2261. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176(2):770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 8.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8(3):497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 9.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9(1):64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8(8):1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13(2):117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Golshayan D, Pascual M. Tolerance-inducing immunosuppressive strategies in clinical transplantation: an overview. Drugs. 2008;68(15):2113–2130. doi: 10.2165/00003495-200868150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Haudebourg T, Poirier N, Vanhove B. Depleting T-cell subpopulations in organ transplantation. Transpl Int. 2009;22(5):509–518. doi: 10.1111/j.1432-2277.2008.00788.x. [DOI] [PubMed] [Google Scholar]
- 14.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 15.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7(5):1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer A, Xiao X, Vu MD, Gao W, Minamimura K, Chen M, et al. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol. 2007;179(8):5584–5591. doi: 10.4049/jimmunol.179.8.5584. [DOI] [PubMed] [Google Scholar]
- 17.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176(8):4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 18.Zeevi A, Husain S, Spichty KJ, Raza K, Woodcock JB, Zaldonis D, et al. Recovery of functional memory T cells in lung transplant recipients following induction therapy with alemtuzumab. Am J Transplant. 2007;7(2):471–475. doi: 10.1111/j.1600-6143.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 19.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, t Hart BA, et al. No synergy between ATG induction and costimulation blockade induced kidney allograft survival in rhesus monkeys. Transplantation. 2006;82(9):1194–1201. doi: 10.1097/01.tp.0000235910.47214.67. [DOI] [PubMed] [Google Scholar]
- 20.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruzek MC, Neff KS, Luong M, Smith KA, Culm-Merdek K, Richards SM, et al. In vivo characterization of rabbit anti-mouse thymocyte globulin: a surrogate for rabbit anti-human thymocyte globulin. Transplantation. 2009;88(2):170–179. doi: 10.1097/TP.0b013e3181abc061. [DOI] [PubMed] [Google Scholar]
- 22.Sener A, Tang AL, Farber DL. Memory T-cell predominance following T-cell depletional therapy derives from homeostatic expansion of naive T cells. Am J Transplant. 2009;9(11):2615–2623. doi: 10.1111/j.1600-6143.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 23.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 24.Murata K, Fox-Talbot K, Qian Z, Takahashi K, Stahl GL, Baldwin WM, 3rd, et al. Synergistic deposition of C4d by complement-activating and non-activating antibodies in cardiac transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(11):2605–2614. doi: 10.1111/j.1600-6143.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 25.Ruzek MC, Waire JS, Hopkins D, Lacorcia G, Sullivan J, Roberts BL, et al. Characterization of in vitro antimurine thymocyte globulin-induced regulatory T cells that inhibit graft-versus-host disease in vivo. Blood. 2008;111(3):1726–1734. doi: 10.1182/blood-2007-08-106526. [DOI] [PubMed] [Google Scholar]
- 26.Do JS, Valujskikh A, Vignali DA, Fairchild RL, Min B. Unexpected role for MHC II-peptide complexes in shaping CD8 T-cell expansion and differentiation in vivo. Proc Natl Acad Sci U S A. 2012;109(31):12698–12703. doi: 10.1073/pnas.1207219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valujskikh A, Heeger P. Enzyme linked immunosorbent spot (ELISPOT) assay for detection of alloreactive cytokine-secreting cells - detailed methods. Graft. 2000;3(5):250–258. [Google Scholar]
- 28.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352–358. [PubMed] [Google Scholar]
- 29.Matesic D, Lehmann PV, Heeger PS. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation. 1998;65(7):906–914. doi: 10.1097/00007890-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams MA, Trambley J, Ha J, Adams AB, Durham MM, Rees P, et al. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165(12):6849–6857. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 32.D'Addio F, Yuan X, Habicht A, Williams J, Ruzek M, Iacomini J, et al. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation. 2010;90(3):260–269. doi: 10.1097/tp.0b013e3181e64217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neff KS, Richards SM, Williams JM, Garman RD, Ruzek MC. Murine antithymocyte globulin T-cell depletion is mediated predominantly by macrophages, but the Fas/FasL pathway selectively targets regulatory T cells. Transplantation. 2011;92(5):523–528. doi: 10.1097/TP.0b013e31822923f7. [DOI] [PubMed] [Google Scholar]
- 34.Fairchild RL, VanBuskirk AM, Kondo T, Wakely ME, Orosz CG. Expression of chemokine genes during rejection and long-term acceptance of cardiac allografts. Transplantation. 1997;63(12):1807–1812. doi: 10.1097/00007890-199706270-00018. [DOI] [PubMed] [Google Scholar]
- 35.Kondo T, Novick AC, Toma H, Fairchild RL. Induction of chemokine gene expression during allogeneic skin graft rejection. Transplantation. 1996;61(12):1750–1757. doi: 10.1097/00007890-199606270-00015. [DOI] [PubMed] [Google Scholar]
- 36.Bonnefoy-Berard N, Vincent C, Revillard JP. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation. 1991;51(3):669–673. doi: 10.1097/00007890-199103000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Michallet MC, Preville X, Flacher M, Fournel S, Genestier L, Revillard JP. Functional antibodies to leukocyte adhesion molecules in antithymocyte globulins. Transplantation. 2003;75(5):657–662. doi: 10.1097/01.TP.0000053198.99206.E6. [DOI] [PubMed] [Google Scholar]
- 38.Naujokat C, Berges C, Fuchs D, Sadeghi M, Opelz G, Daniel V. Antithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation. 2007;83(4):485–497. doi: 10.1097/01.tp.0000251975.81281.22. [DOI] [PubMed] [Google Scholar]
- 39.Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59(8):1194–1200. [PubMed] [Google Scholar]
- 40.Gao W, Richard J, Waire J, Nambiar P, Descarreaux A, Weber W, et al. Increased intra-graft FoxP3+ T cells and cardiac allograft survival with pre-transplant administration of ATG. Am J Transplant. 2010;10(Suppl. 4):217. [Google Scholar]
- 41.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.